Abstract
1. The potency of a series of selective muscarinic antagonists has been measured on two functional isolated tissue preparations (rat ileum and atria) and these compared with their potency on a range of binding preparations in order to determine whether the subtypes of M2 receptor measured functionally are the same as those measured in binding studies. 2. On the functional preparations pirenzepine, hexahydrosiladiphenidol (HSD) and 4-diphenylacetoxy-N-methylpiperidine (4-DAMP) were more potent on the ileum than on the atrium (3 fold, 29 fold and 5 fold respectively), whereas himbacine, AF-DX 116 and methoctramine showed the opposite selectivity (5 fold, 3 fold and 56 fold respectively). Atropine had a similar potency on the ileum and atrium. 3. [3H]-N-methyl scopolamine was used to study M2 binding sites on membranes from rat heart and rat submandibular gland. Each preparation appeared to contain a homogeneous binding site population. The potencies of the five M2 selective antagonists (and pirenzepine) in binding studies to heart membranes were very similar to those observed in functional studies of rat atria (correlation coefficient = 0.98). Similarly the binding to submandibular gland membranes was very similar to that observed in functional studies on rat ileum (correlation coefficient = 0.97). 4. [3H]-pirenzepine was used to examine the binding of these antagonists to M1 binding sites on membranes from rat cerebral cortex. The affinities of 4-DAMP, HSD, AF-DX116 and himbacine at M1 sites were similar to their affinities on the gland. Only pirenzepine and methoctramine had higher affinity on M1 sites than on the gland.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


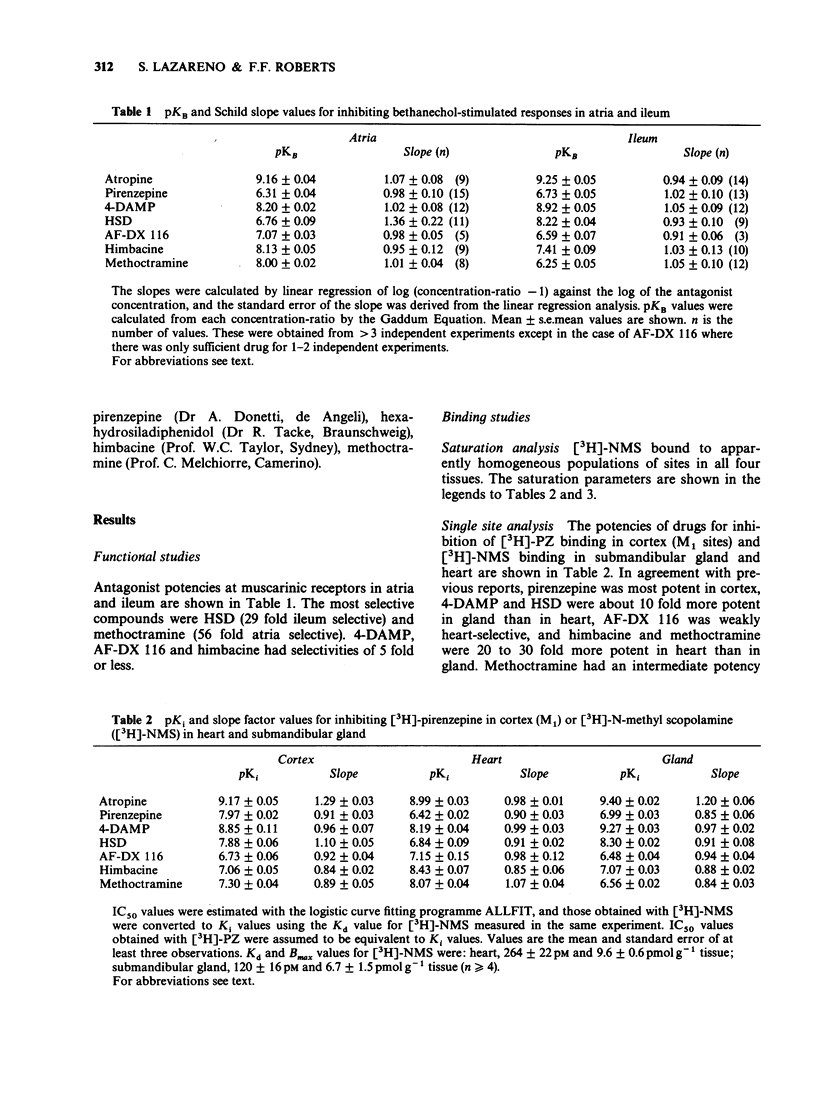
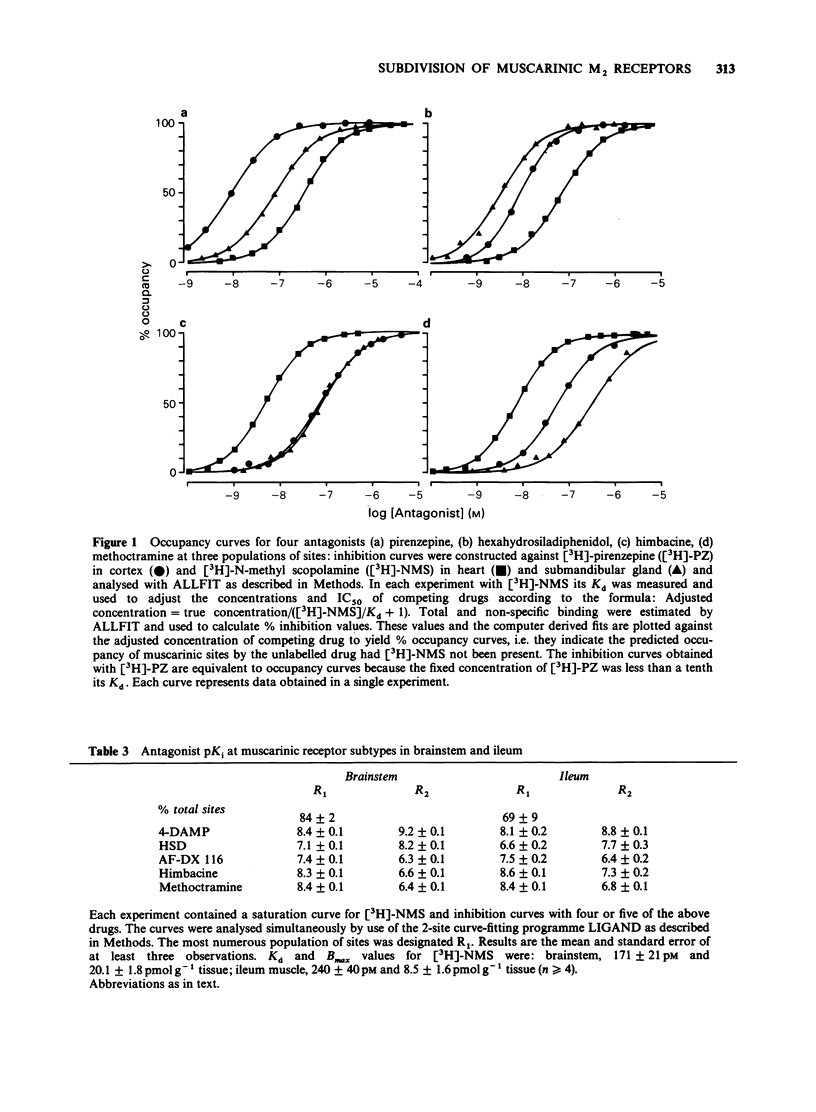
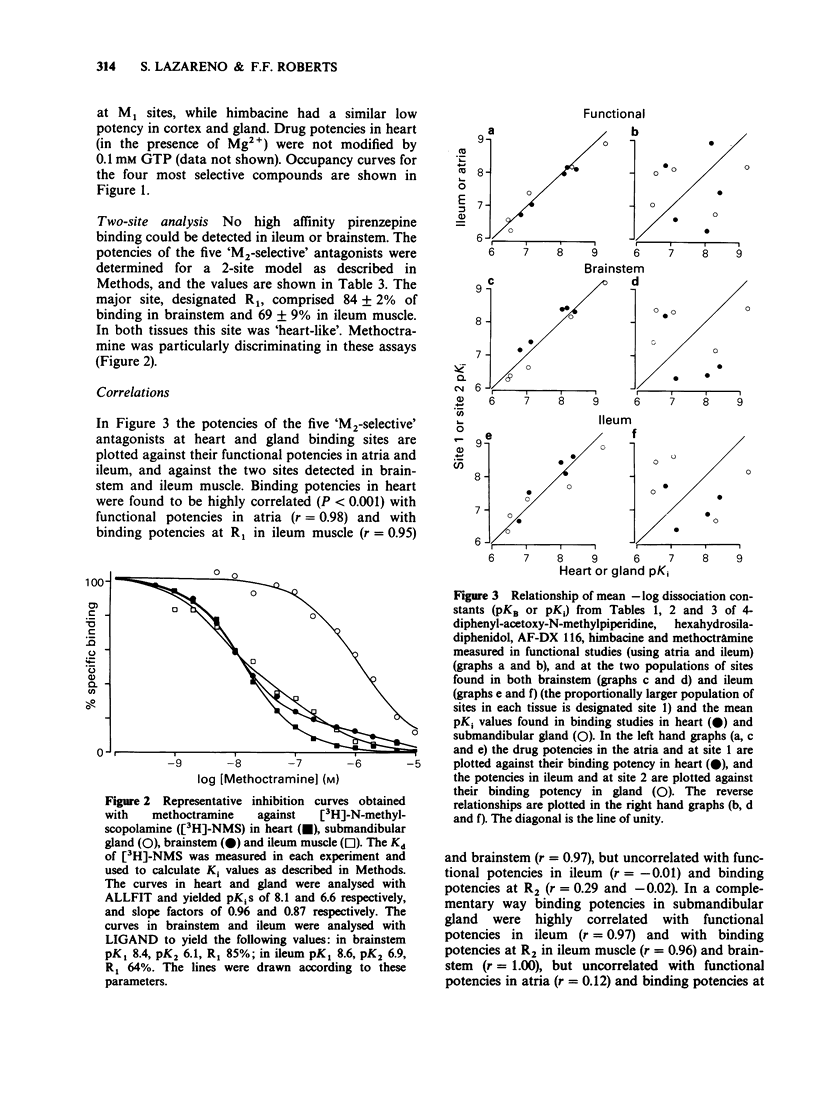



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar-ul S., Gilani H., Cobbin L. B. The cardio-selectivity of himbacine: a muscarine receptor antagonist. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):16–20. doi: 10.1007/BF00633191. [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Berry K. J., Glenton P. A., Nilolaou N. M., Soh K. S. A comparison of affinity constants for muscarine-sensitive acetylcholine receptors in guinea-pig atrial pacemaker cells at 29 degrees C and in ileum at 29 degrees C and 37 degrees C. Br J Pharmacol. 1976 Dec;58(4):613–620. doi: 10.1111/j.1476-5381.1976.tb08631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Brann M. R. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988 Dec;8(12):4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Choo L. K., Mitchelson F. J. Comparison of the affinity constant of some muscarinic receptor antagonists with their displacement of [3H]quinuclidinyl benzilate binding in atrial and ileal longitudinal muscle of the guinea-pig. J Pharm Pharmacol. 1985 Sep;37(9):656–658. doi: 10.1111/j.2042-7158.1985.tb05106.x. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Doods H. N., Mathy M. J., Davidesko D., van Charldorp K. J., de Jonge A., van Zwieten P. A. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther. 1987 Jul;242(1):257–262. [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Competitive and non-competitive antagonism exhibited by 'selective' antagonists at atrial and ileal muscarinic receptor subtypes. Br J Pharmacol. 1987 Apr;90(4):701–707. doi: 10.1111/j.1476-5381.1987.tb11223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachetti A., Micheletti R., Montagna E. Cardioselective profile of AF-DX 116, a muscarine M2 receptor antagonist. Life Sci. 1986 May 5;38(18):1663–1672. doi: 10.1016/0024-3205(86)90410-8. [DOI] [PubMed] [Google Scholar]
- Giraldo E., Hammer R., Ladinsky H. Distribution of muscarinic receptor subtypes in rat brain as determined in binding studies with AF-DX 116 and pirenzepine. Life Sci. 1987 Mar 2;40(9):833–840. doi: 10.1016/0024-3205(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Giraldo E., Micheletti R., Montagna E., Giachetti A., Viganò M. A., Ladinsky H., Melchiorre C. Binding and functional characterization of the cardioselective muscarinic antagonist methoctramine. J Pharmacol Exp Ther. 1988 Mar;244(3):1016–1020. [PubMed] [Google Scholar]
- Giraldo E., Monferini E., Ladinsky H., Hammer R. Muscarinic receptor heterogeneity in guinea pig intestinal smooth muscle: binding studies with AF-DX 116. Eur J Pharmacol. 1987 Sep 23;141(3):475–477. doi: 10.1016/0014-2999(87)90568-1. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Rattan S. Neurohumoral, hormonal, and drug receptors for the lower esophageal sphincter. Gastroenterology. 1978 Mar;74(3):598–619. [PubMed] [Google Scholar]
- Hammer R., Giraldo E., Schiavi G. B., Monferini E., Ladinsky H. Binding profile of a novel cardioselective muscarine receptor antagonist, AF-DX 116, to membranes of peripheral tissues and brain in the rat. Life Sci. 1986 May 5;38(18):1653–1662. doi: 10.1016/0024-3205(86)90409-1. [DOI] [PubMed] [Google Scholar]
- Ladinsky H., Giraldo E., Monferini E., Schiavi G. B., Viganò M. A., De Conti L., Micheletti R., Hammer R. Muscarinic receptor heterogeneity in smooth muscle: binding and functional studies with AF-DX 116. Trends Pharmacol Sci. 1988 Feb;Suppl:44–48. [PubMed] [Google Scholar]
- Maeda A., Kubo T., Mishina M., Numa S. Tissue distribution of mRNAs encoding muscarinic acetylcholine receptor subtypes. FEBS Lett. 1988 Nov 7;239(2):339–342. doi: 10.1016/0014-5793(88)80947-5. [DOI] [PubMed] [Google Scholar]
- Melchiorre C., Angeli P., Lambrecht G., Mutschler E., Picchio M. T., Wess J. Antimuscarinic action of methoctramine, a new cardioselective M-2 muscarinic receptor antagonist, alone and in combination with atropine and gallamine. Eur J Pharmacol. 1987 Dec 1;144(2):117–124. doi: 10.1016/0014-2999(87)90509-7. [DOI] [PubMed] [Google Scholar]
- Michel A. D., Whiting R. L. Direct binding studies on ileal and cardiac muscarinic receptors. Br J Pharmacol. 1987 Dec;92(4):755–767. doi: 10.1111/j.1476-5381.1987.tb11379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A. D., Whiting R. L. Methoctramine reveals heterogeneity of M2 muscarinic receptors in longitudinal ileal smooth muscle membranes. Eur J Pharmacol. 1988 Jan 19;145(3):305–311. doi: 10.1016/0014-2999(88)90434-7. [DOI] [PubMed] [Google Scholar]
- Michel A. D., Whiting R. L. Methoctramine, a polymethylene tetraamine, differentiates three subtypes of muscarinic receptor in direct binding studies. Eur J Pharmacol. 1988 Jan 5;145(1):61–66. doi: 10.1016/0014-2999(88)90349-4. [DOI] [PubMed] [Google Scholar]
- Micheletti R., Montagna E., Giachetti A. AF-DX 116, a cardioselective muscarinic antagonist. J Pharmacol Exp Ther. 1987 May;241(2):628–634. [PubMed] [Google Scholar]
- Mitchelson F. Muscarinic receptor differentiation. Pharmacol Ther. 1988;37(3):357–423. doi: 10.1016/0163-7258(88)90005-8. [DOI] [PubMed] [Google Scholar]
- Monferini E., Giraldo E., Ladinsky H. Characterization of the muscarinic receptor subtypes in the rat urinary bladder. Eur J Pharmacol. 1988 Mar 15;147(3):453–458. doi: 10.1016/0014-2999(88)90180-x. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffel A. F., in't Hout W. G., de Zeeuw R. A., Zaagsma J. The M2 selective antagonist AF-DX 116 shows high affinity for muscarine receptors in bovine tracheal membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 May;335(5):593–595. doi: 10.1007/BF00169130. [DOI] [PubMed] [Google Scholar]
- Stockton J. M., Birdsall N. J., Burgen A. S., Hulme E. C. Modification of the binding properties of muscarinic receptors by gallamine. Mol Pharmacol. 1983 May;23(3):551–557. [PubMed] [Google Scholar]
- Waelbroeck M., Gillard M., Robberecht P., Christophe J. Kinetic studies of [3H]-N-methylscopolamine binding to muscarinic receptors in the rat central nervous system: evidence for the existence of three classes of binding sites. Mol Pharmacol. 1986 Oct;30(4):305–314. [PubMed] [Google Scholar]
- Waelbroeck M., Gillard M., Robberecht P., Christophe J. Muscarinic receptor heterogeneity in rat central nervous system. I. Binding of four selective antagonists to three muscarinic receptor subclasses: a comparison with M2 cardiac muscarinic receptors of the C type. Mol Pharmacol. 1987 Jul;32(1):91–99. [PubMed] [Google Scholar]
- Wang J. X., Roeske W. R., Wang W., Yamamura H. I. Himbacine recognizes a high affinity subtype of M2 muscarinic cholinergic receptors in the rat cerebral cortex. Brain Res. 1988 Apr 12;446(1):155–158. doi: 10.1016/0006-8993(88)91306-6. [DOI] [PubMed] [Google Scholar]


