Abstract
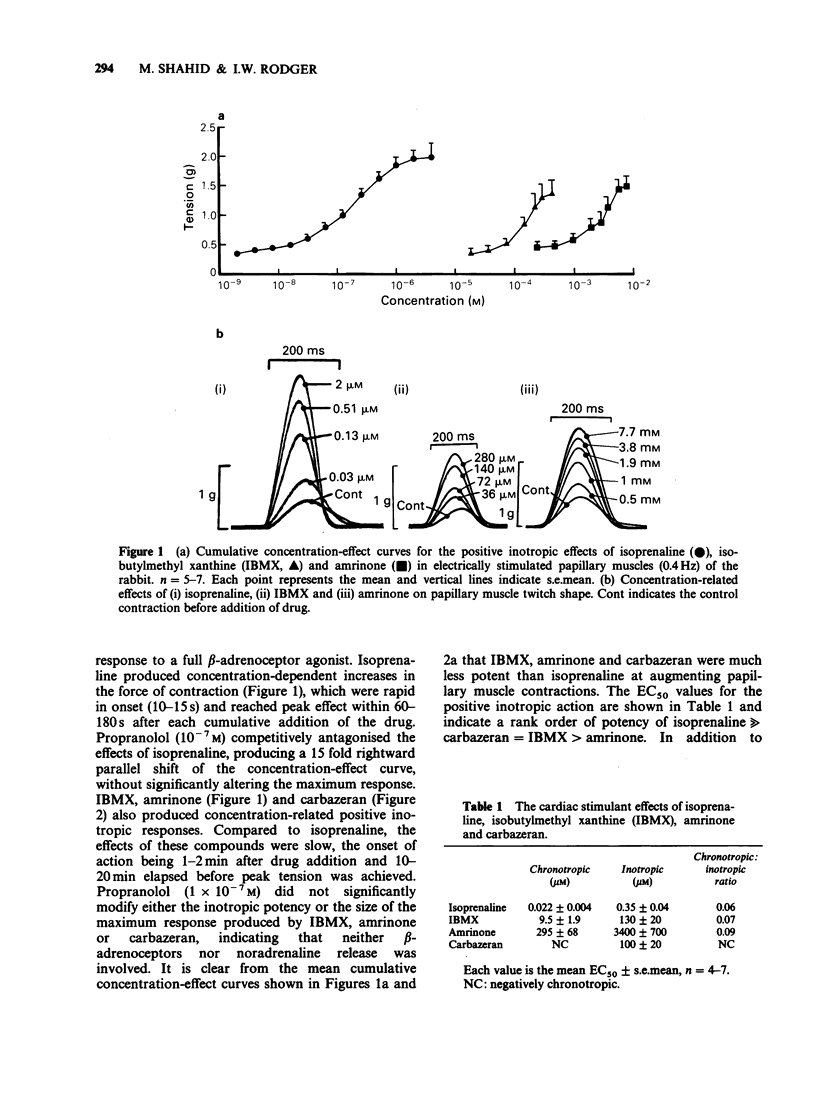
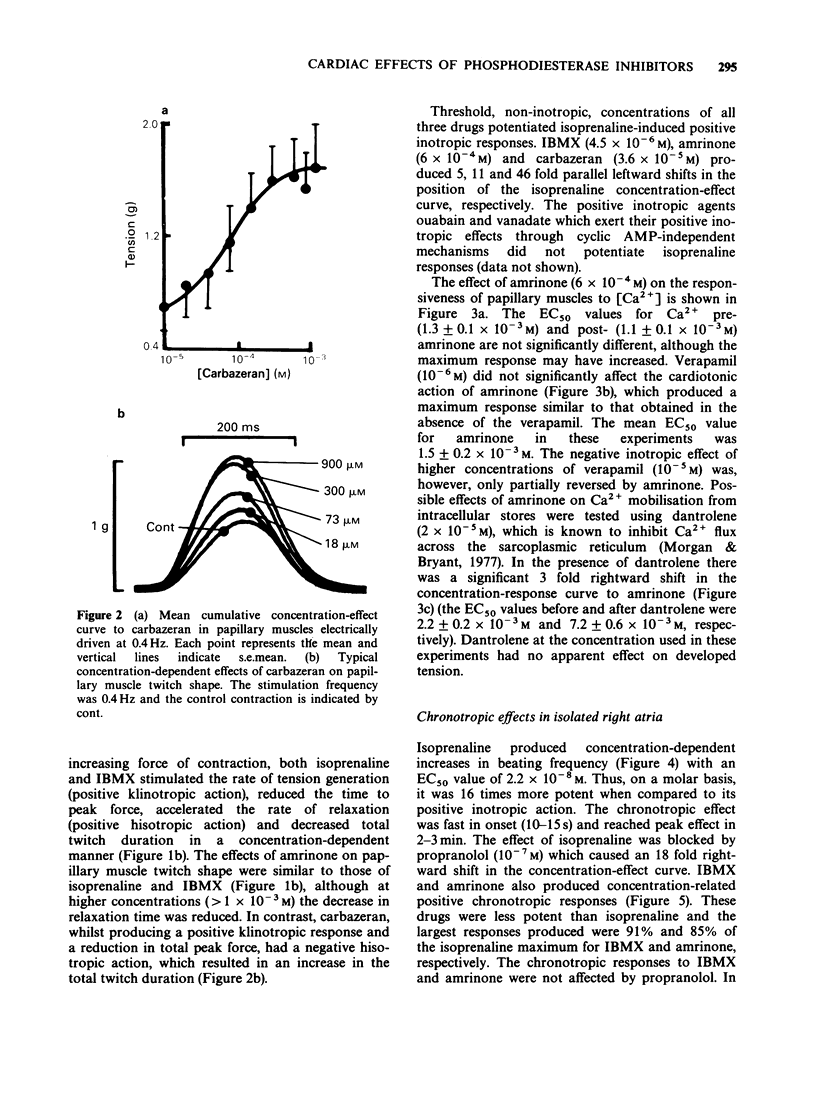
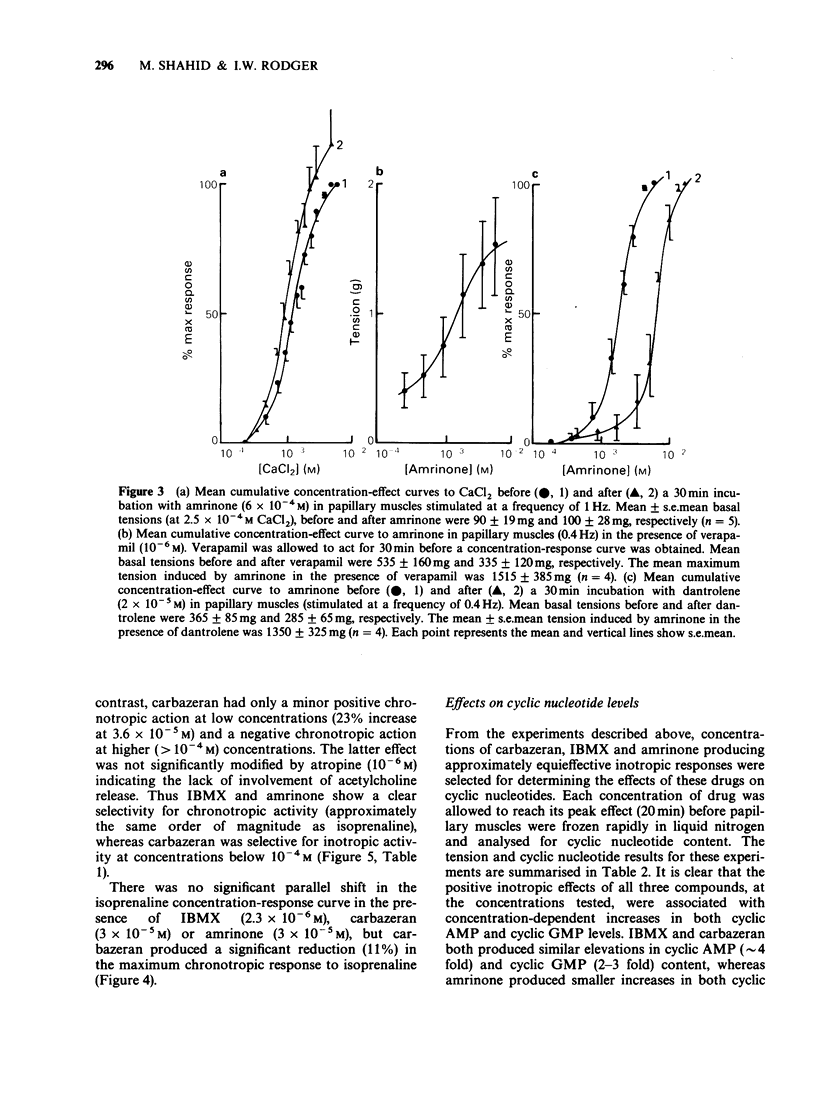
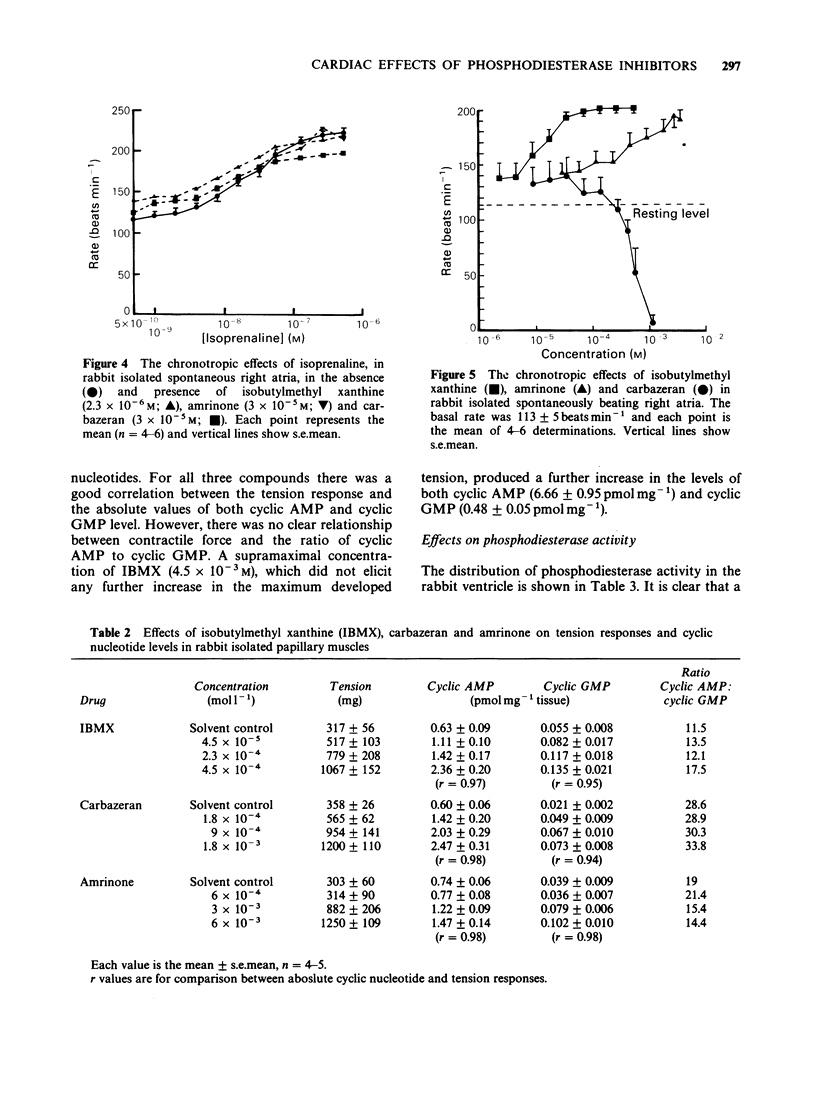
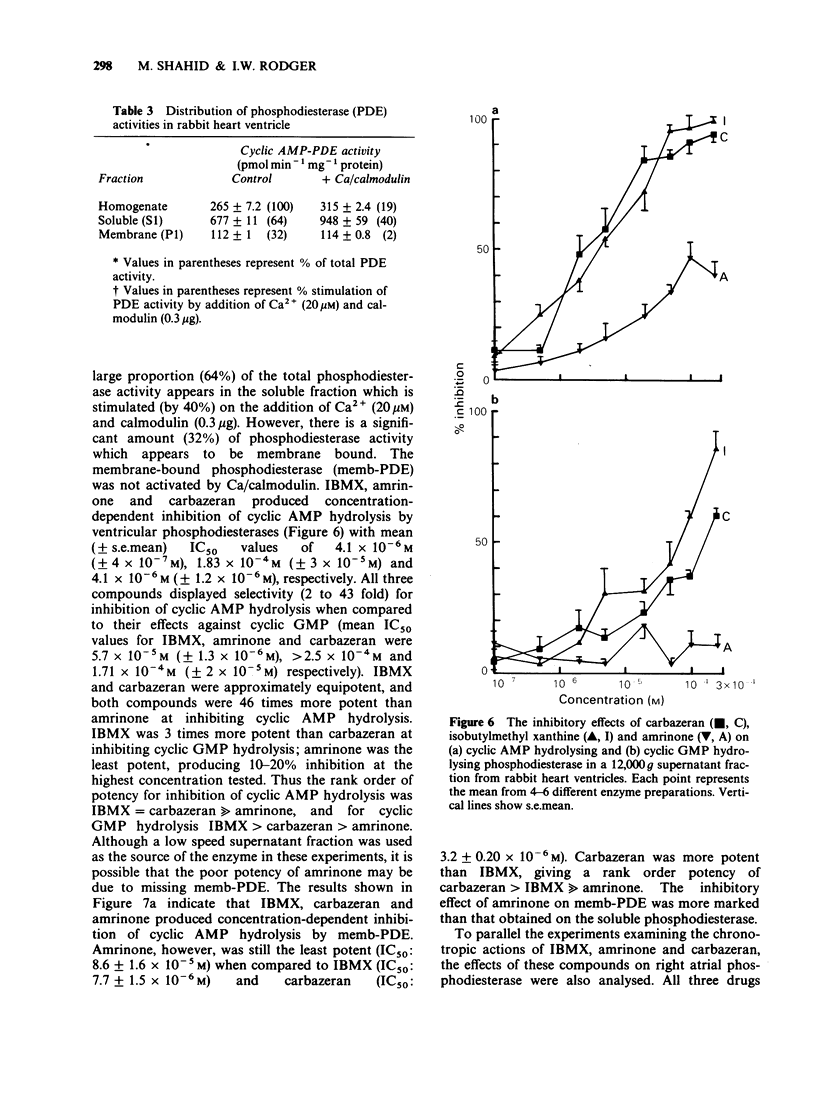
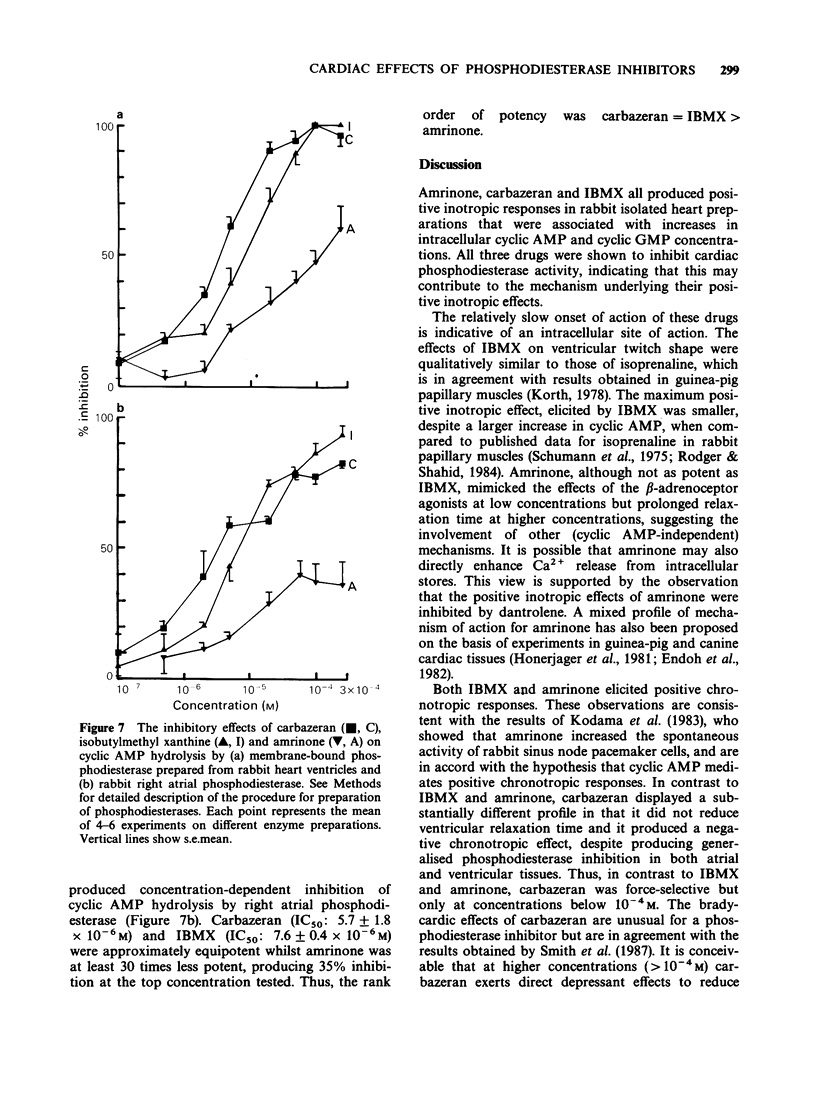
1. The chronotropic and inotropic effects of amrinone, carbazeran and 3-isobutyl-1-methyl xanthine (IBMX) were examined in isolated preparations of papillary muscle and right atria from rabbit heart. The effects of the drugs on cardiac phosphodiesterase and cyclic nucleotide content were also examined. 2. Amrinone (2.4 x 10(-4)M-2 x 10(-3) M), carbazeran (9.1 x 10(-6) M-1.2 x 10(-3) M), and IBMX (1.8 x 10(-5) M-4.5 x 10(-4) M) produced concentration-dependent positive inotropic responses of papillary muscle preparations, the rank order of potency being carbazeran = IBMX greater than amrinone. Sub-threshold positive inotropic concentrations of all three compounds potentiated the positive inotropic effects of isoprenaline; leftward shifts in the concentration-effect curves were 5 fold (IBMX), 11 fold (amrinone) and 46 fold (carbazeran). 3. Amrinone and IBMX produced concentration-dependent positive chronotropic responses in isolated right atria and showed a similar rate selectivity to isoprenaline, but carbazeran elicited a decrease in beating frequency. None of these drugs potentiated the positive chronotropic effects of isoprenaline. 4. Concentrations of amrinone, carbazeran and IBMX that produced similar positive inotropic responses were associated with different increases in papillary muscle cyclic AMP and cyclic GMP concentrations. 5. All three compounds inhibited right atrial and ventricular phosphodiesterase, with amrinone being the least potent. There was, however, a marked difference between the IC50 and EC50 values for phosphodiesterase inhibition and positive inotropy. In contrast the positive chronotropic effects of amrinone and IBMX were observed in the same concentration ranges that produced phosphodiestrease inhibition. 6. The results indicate that amrinone possesses a similar rate/force selectivity to isoprenaline and IBMX. In contrast, carbazeran exerts both positive inotropic and negative chronotropic effects. Phosphodiesterase inhibition and elevation of intracellular cyclic AMP concentration may be involved, at least in part, in the cardiac effects of these drugs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Brunton L. L., Hayes J. S., Mayer S. E. Functional compartmentation of cyclic AMP and protein kinase in heart. Adv Cyclic Nucleotide Res. 1981;14:391–397. [PubMed] [Google Scholar]
- Clark S. J., Poyser R. H. Effect of tazolol on beta-adrenoceptors in isolated preparations of the guinea-pig and rat. J Pharm Pharmacol. 1977 Oct;29(10):630–632. doi: 10.1111/j.2042-7158.1977.tb11420.x. [DOI] [PubMed] [Google Scholar]
- Endoh M., Yamashita S., Taira N. Positive inotropic effect of amrinone in relation to cyclic nucleotide metabolism in the canine ventricular muscle. J Pharmacol Exp Ther. 1982 Jun;221(3):775–783. [PubMed] [Google Scholar]
- England P. J., Shahid M. Effects of forskolin on contractile responses and protein phosphorylation in the isolated perfused rat heart. Biochem J. 1987 Sep 15;246(3):687–695. doi: 10.1042/bj2460687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah A. E., Alousi A. A., Schwarz R. P., Jr Positive inotropic agents. Annu Rev Pharmacol Toxicol. 1984;24:275–328. doi: 10.1146/annurev.pa.24.040184.001423. [DOI] [PubMed] [Google Scholar]
- Honerjäger P., Schäfer-Korting M., Reiter M. Involvement of cyclic AMP in the direct inotropic action of amrinone. Biochemical and functional evidence. Naunyn Schmiedebergs Arch Pharmacol. 1981 Dec;318(2):112–120. doi: 10.1007/BF00508835. [DOI] [PubMed] [Google Scholar]
- Kodama I., Kondo N., Shibata S. Effects of amrinone on the transmembrane action potential of rabbit sinus node pacemaker cells. Br J Pharmacol. 1983 Nov;80(3):511–517. doi: 10.1111/j.1476-5381.1983.tb10723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth M. Effects of several phosphodiesterase-inhibitors on guinea-pig myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1978 Mar;302(1):77–86. doi: 10.1007/BF00586601. [DOI] [PubMed] [Google Scholar]
- Manganiello V. C. Subcellular localization and biological function of specific cyclic nucleotide phosphodiesterases. J Mol Cell Cardiol. 1987 Oct;19(10):1037–1040. doi: 10.1016/s0022-2828(87)80575-8. [DOI] [PubMed] [Google Scholar]
- Methven P., Lemon M., Bhoola K. The metabolism of cyclic nucleotides in the guinea-pig pancreas. Cyclic AMP phosphodiesterase and cyclic GMP phosphodiesterase. Biochem J. 1980 Feb 15;186(2):491–498. doi: 10.1042/bj1860491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K. G., Bryant S. H. The mechanism of action of dantrolene sodium. J Pharmacol Exp Ther. 1977 Apr;201(1):138–147. [PubMed] [Google Scholar]
- Nawrath H., Gruschwitz P., Zong X. A negative inotropic effect of acetylcholine in the presence of several phosphodiesterase inhibitors. Experientia. 1981 Jul 15;37(7):755–756. doi: 10.1007/BF01967963. [DOI] [PubMed] [Google Scholar]
- Nicholson C. D., Broadley K. J. Irreversible beta-adrenoceptor blockade of atrial rate and tension responses. Eur J Pharmacol. 1978 Dec 1;52(3-4):259–269. doi: 10.1016/0014-2999(78)90278-9. [DOI] [PubMed] [Google Scholar]
- Reeves M. L., Leigh B. K., England P. J. The identification of a new cyclic nucleotide phosphodiesterase activity in human and guinea-pig cardiac ventricle. Implications for the mechanism of action of selective phosphodiesterase inhibitors. Biochem J. 1987 Jan 15;241(2):535–541. doi: 10.1042/bj2410535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger I. W., Shahid M. Forskolin, cyclic nucleotides and positive inotropism in isolated papillary muscles of the rabbit. Br J Pharmacol. 1984 Jan;81(1):151–159. doi: 10.1111/j.1476-5381.1984.tb10755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümann H. J., Endoh S., Brodde O. E. The time course of the effects of beta- and alpha-adrenoceptor stimulation by isoprenaline and methoxamine on the contractile force and cAMP level of the isolated rabbit papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(3):291–302. doi: 10.1007/BF00499982. [DOI] [PubMed] [Google Scholar]
- Sharpe D. N. Newer inotropic agents. Methods Find Exp Clin Pharmacol. 1984 Apr;6(4):215–218. [PubMed] [Google Scholar]
- Smith G. W., Hall J. C., West P. A. Lack of inotropic selectivity of phosphodiesterase enzyme inhibitors in-vitro. J Pharm Pharmacol. 1987 Sep;39(9):748–751. doi: 10.1111/j.2042-7158.1987.tb06985.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Fujiwara M., Ohsumi K. Possible involvement of cyclic adenosine 3':5'-monophosphate in the genesis of catecholamine-induced tachycardia in isolated rabbit sinoatrial node. J Pharmacol Exp Ther. 1977 Jun;201(3):678–688. [PubMed] [Google Scholar]
- Ward A., Brogden R. N., Heel R. C., Speight T. M., Avery G. S. Amrinone. A preliminary review of its pharmacological properties and therapeutic use. Drugs. 1983 Dec;26(6):468–502. doi: 10.2165/00003495-198326060-00002. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Burrows S. D., Kobylarz D. C., Quade M. M., Evans D. B. Multiple molecular forms of cyclic nucleotide phosphodiesterase in cardiac and smooth muscle and in platelets. Isolation, characterization, and effects of various reference phosphodiesterase inhibitors and cardiotonic agents. Biochem Pharmacol. 1986 Mar 1;35(5):787–800. doi: 10.1016/0006-2952(86)90247-9. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y., Fujiwara M., Toda N. Effects of intracellularly applied cyclic 3',5'-adenosine monophosphate and dibutyryl cyclic 3',5'-adenosine monophosphate on the electrical activity of sinoatrial nodal cells of the rabbit. J Pharmacol Exp Ther. 1974 Jul;190(1):15–20. [PubMed] [Google Scholar]


