Abstract
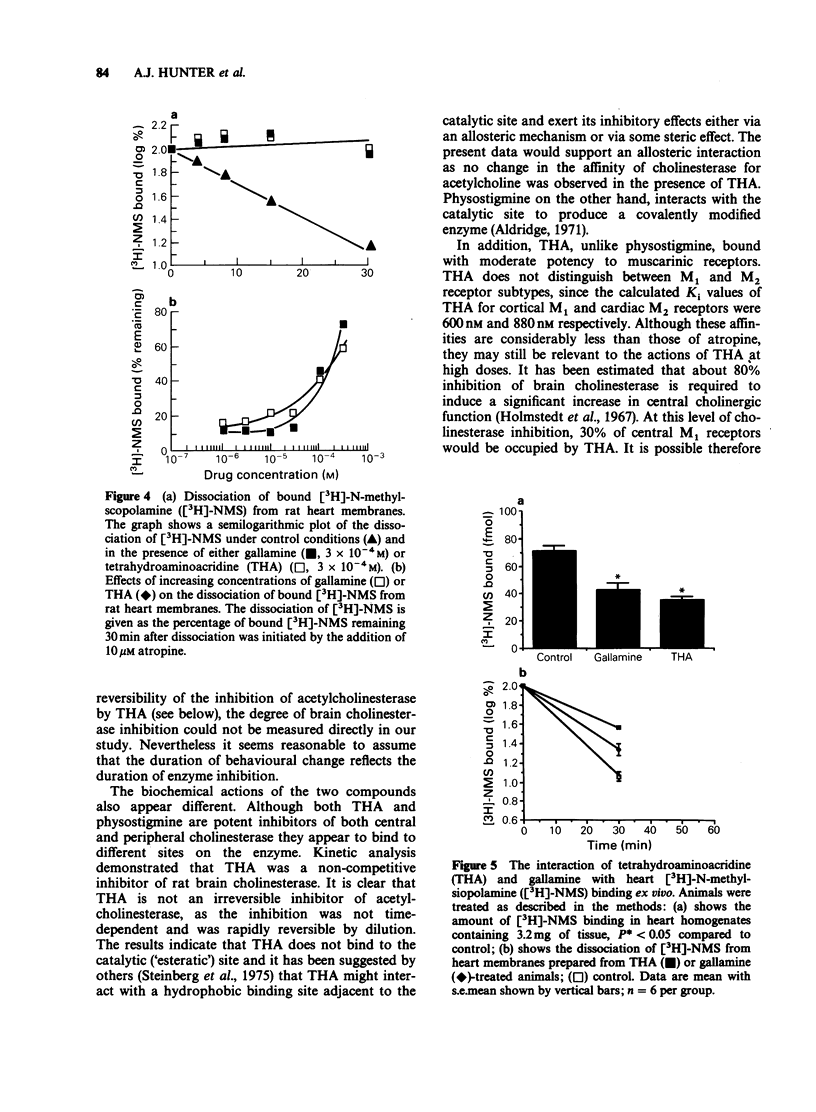
1. The effect of tetrahydroaminoacridine (THA) on cholinergically mediated behaviour in the rat and mouse has been investigated. In addition the actions of this compound on cholinesterase activity and on muscarinic and nicotinic receptors has also been examined. 2. Administration of THA (5-20 mg kg-1, i.p.) produced a dose-dependent increase in tremor, hypothermia and salivation in both rats and mice. A similar profile of activity was seen following physostigmine (0.1-0.6 mg kg-1) administration. 3. THA was approximately fifty fold less potent than physostigmine in inducing behavioural change but its effects persisted for over twice as long as those of physostigmine. For example THA-induced hypothermia was still present at 4 h in the mouse and 8 h in the rat. 4. In vitro THA was a potent non-competitive inhibitor of rat brain cholinesterase (IC50: 57 +/- 6 nM) and bovine erythrocyte acetylcholinesterase (IC50: 50 +/- 10 nM) but was a more potent inhibitor of horse serum butyrylcholinesterase (IC50: 7.2 +/- 1.4 nM). 5. Radioligand binding studies indicated that THA binds non-selectively but with moderate potency to both M1 (Ki: 600 nM) and M2 (Ki: 880 nM) muscarinic receptors. THA also interacted with the allosteric site present on cardiac M2 receptors. 6. It is concluded that THA is a reversible non-competitive inhibitor of cholinesterase with a long half life (compared with physostigmine). It also may antagonize muscarinic receptors at high doses. The long half life may account for its reported efficacy in the treatment of Alzheimer's disease.
Full text
PDF


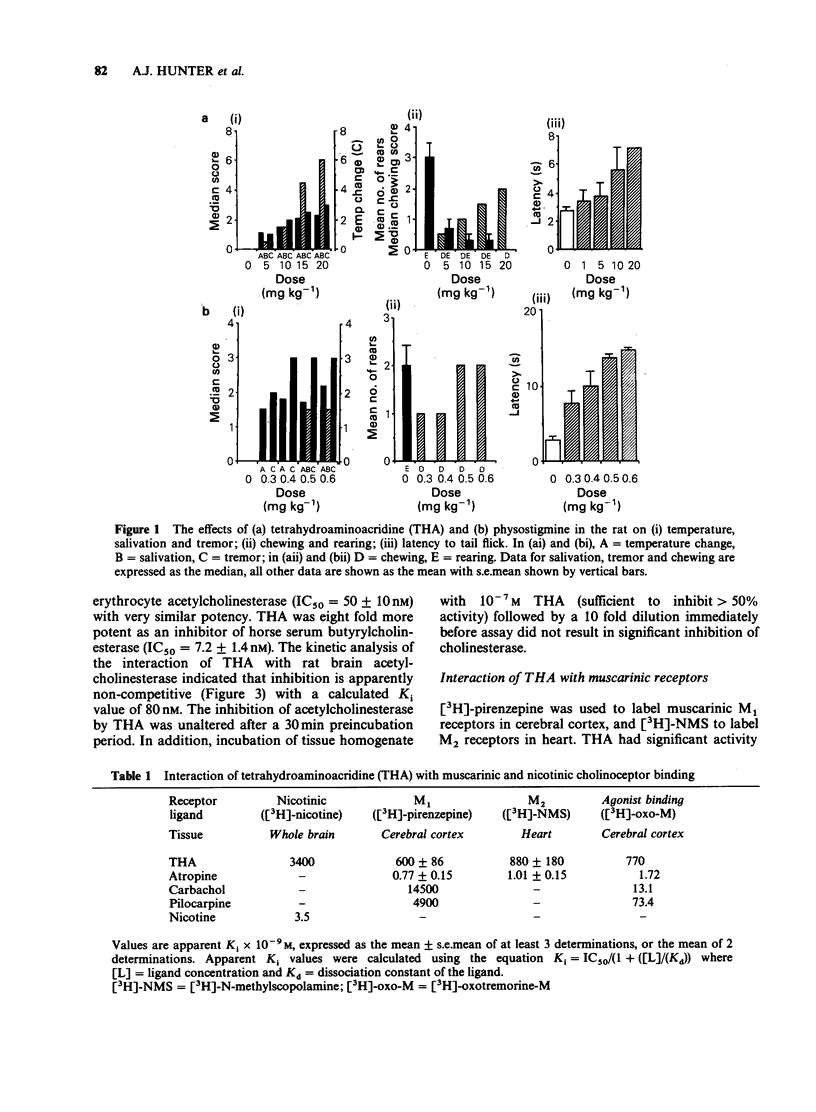
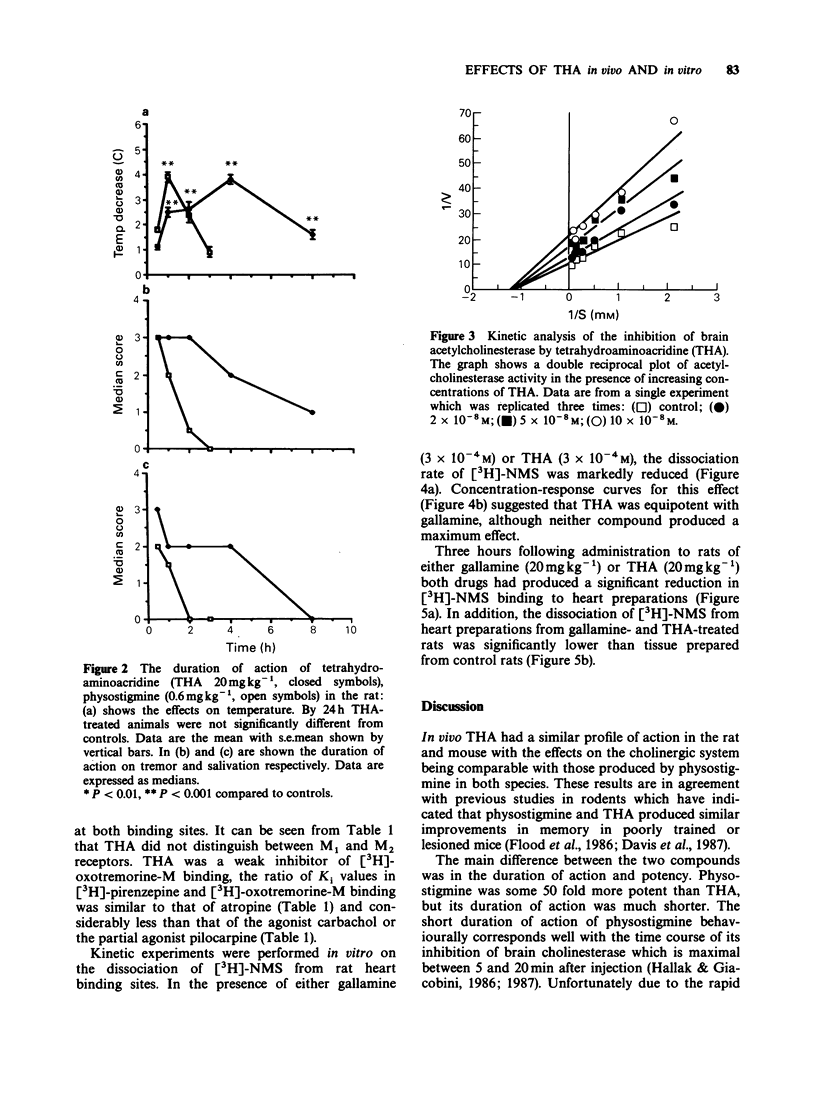


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge W. N. The nature of the reaction of organophosphorus compounds and carbamates with esterases. Bull World Health Organ. 1971;44(1-3):25–30. [PMC free article] [PubMed] [Google Scholar]
- Brinkman S. D., Gershon S. Measurement of cholinergic drug effects on memory in Alzheimer's disease. Neurobiol Aging. 1983 Summer;4(2):139–145. doi: 10.1016/0197-4580(83)90038-6. [DOI] [PubMed] [Google Scholar]
- Christie J. E., Shering A., Ferguson J., Glen A. I. Physostigmine and arecoline: effects of intravenous infusions in Alzheimer presenile dementia. Br J Psychiatry. 1981 Jan;138:46–50. doi: 10.1192/bjp.138.1.46. [DOI] [PubMed] [Google Scholar]
- Drukarch B., Leysen J. E., Stoof J. C. Further analysis of the neuropharmacological profile of 9-amino-1,2,3,4-tetrahydroacridine (THA), an alleged drug for the treatment of Alzheimer's disease. Life Sci. 1988;42(9):1011–1017. doi: 10.1016/0024-3205(88)90431-6. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Flood J. F., Smith G. E., Cherkin A. Memory enhancement: supra-additive effect of subcutaneous cholinergic drug combinations in mice. Psychopharmacology (Berl) 1985;86(1-2):61–67. doi: 10.1007/BF00431685. [DOI] [PubMed] [Google Scholar]
- Francis P. T., Palmer A. M., Sims N. R., Bowen D. M., Davison A. N., Esiri M. M., Neary D., Snowden J. S., Wilcock G. K. Neurochemical studies of early-onset Alzheimer's disease. Possible influence on treatment. N Engl J Med. 1985 Jul 4;313(1):7–11. doi: 10.1056/NEJM198507043130102. [DOI] [PubMed] [Google Scholar]
- Freedman S. B., Harley E. A., Iversen L. L. Relative affinities of drugs acting at cholinoceptors in displacing agonist and antagonist radioligands: the NMS/Oxo-M ratio as an index of efficacy at cortical muscarinic receptors. Br J Pharmacol. 1988 Feb;93(2):437–445. doi: 10.1111/j.1476-5381.1988.tb11451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak M., Giacobini E. A comparison of the effects of two inhibitors on brain cholinesterase. Neuropharmacology. 1987 Jun;26(6):521–530. doi: 10.1016/0028-3908(87)90143-2. [DOI] [PubMed] [Google Scholar]
- Hallak M., Giacobini E. Relation of brain regional physostigmine concentration to cholinesterase activity and acetylcholine and choline levels in rat. Neurochem Res. 1986 Jul;11(7):1037–1048. doi: 10.1007/BF00965592. [DOI] [PubMed] [Google Scholar]
- Holmstedt B., Härkönen M., Lundgren G., Sundwall A. Relationship between acetylcholine and cholinesterase activity in the brain following an organophosphorus cholinesterase inhibitor. Biochem Pharmacol. 1967 Feb;16(2):404–406. doi: 10.1016/0006-2952(67)90042-1. [DOI] [PubMed] [Google Scholar]
- Kopelman M. D. The cholinergic neurotransmitter system in human memory and dementia: a review. Q J Exp Psychol A. 1986 Nov;38(4):535–573. doi: 10.1080/14640748608401614. [DOI] [PubMed] [Google Scholar]
- Mohs R. C., Davis B. M., Greenwald B. S., Mathé A. A., Johns C. A., Horvath T. B., Davis K. L. Clinical studies of the cholinergic deficit in Alzheimer's disease. II. Psychopharmacologic studies. J Am Geriatr Soc. 1985 Nov;33(11):749–757. doi: 10.1111/j.1532-5415.1985.tb04185.x. [DOI] [PubMed] [Google Scholar]
- Osterrieder W. 9-Amino-1,2,3,4-tetrahydroacridine (THA) is a potent blocker of cardiac potassium channels. Br J Pharmacol. 1987 Nov;92(3):521–525. doi: 10.1111/j.1476-5381.1987.tb11352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Martin E. M., Wilson R. S., Fox J. H., Savoy S. M. Intraventricular bethanechol infusion for Alzheimer's disease: results of double-blind and escalating-dose trials. Neurology. 1988 Feb;38(2):219–222. doi: 10.1212/wnl.38.2.219. [DOI] [PubMed] [Google Scholar]
- Perry E. K., Tomlinson B. E., Blessed G., Bergmann K., Gibson P. H., Perry R. H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J. 1978 Nov 25;2(6150):1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. M., Swash M. Physostigmine in Alzheimer's disease. Lancet. 1979 Jan 6;1(8106):42–42. doi: 10.1016/s0140-6736(79)90479-3. [DOI] [PubMed] [Google Scholar]
- Steinberg G. M., Mednick M. L., Maddox J., Rice R. A hydrophobic binding site in acetylcholinesterase. J Med Chem. 1975 Nov;18(11):1057–1061. doi: 10.1021/jm00245a002. [DOI] [PubMed] [Google Scholar]
- Stevens D. R., Cotman C. W. Excitatory actions of tetrahydro-9-aminoacridine (THA) on hippocampal pyramidal neurons. Neurosci Lett. 1987 Aug 31;79(3):301–305. doi: 10.1016/0304-3940(87)90448-4. [DOI] [PubMed] [Google Scholar]
- Stockton J. M., Birdsall N. J., Burgen A. S., Hulme E. C. Modification of the binding properties of muscarinic receptors by gallamine. Mol Pharmacol. 1983 May;23(3):551–557. [PubMed] [Google Scholar]
- Summers W. K., Majovski L. V., Marsh G. M., Tachiki K., Kling A. Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N Engl J Med. 1986 Nov 13;315(20):1241–1245. doi: 10.1056/NEJM198611133152001. [DOI] [PubMed] [Google Scholar]
- Summers W. K., Viesselman J. O., Marsh G. M., Candelora K. Use of THA in treatment of Alzheimer-like dementia: pilot study in twelve patients. Biol Psychiatry. 1981 Feb;16(2):145–153. [PubMed] [Google Scholar]


