Abstract
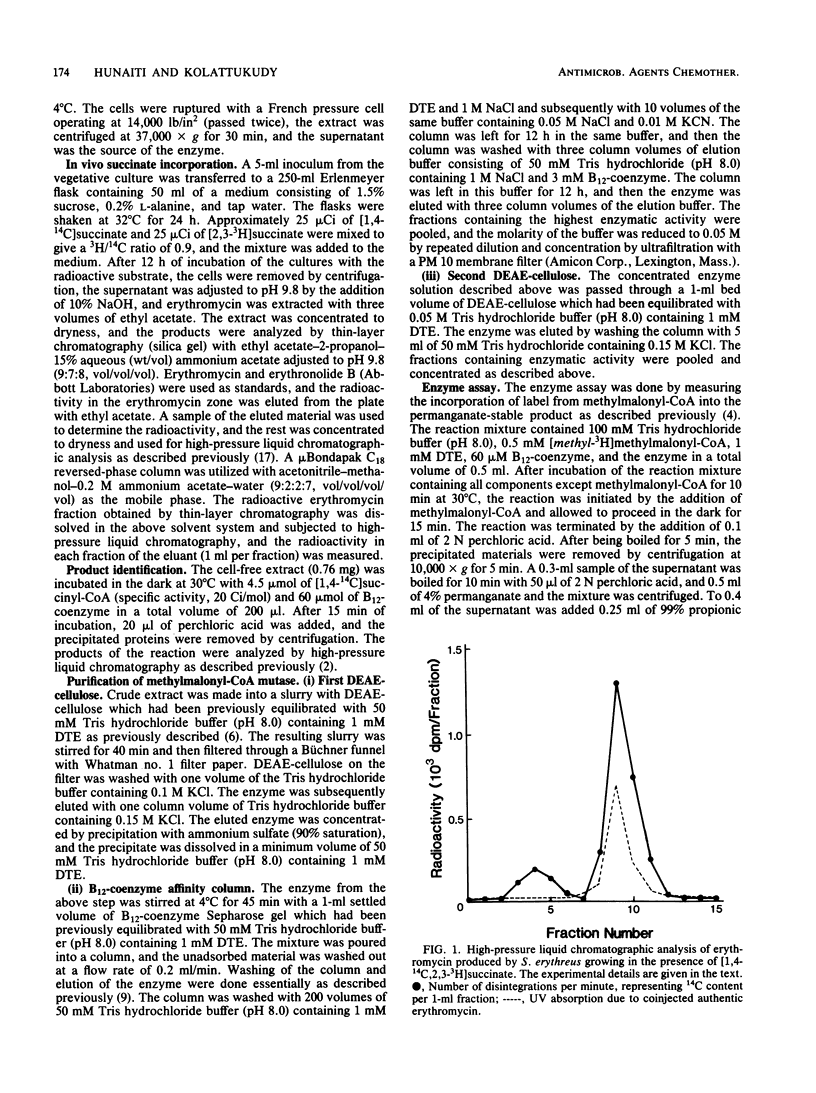
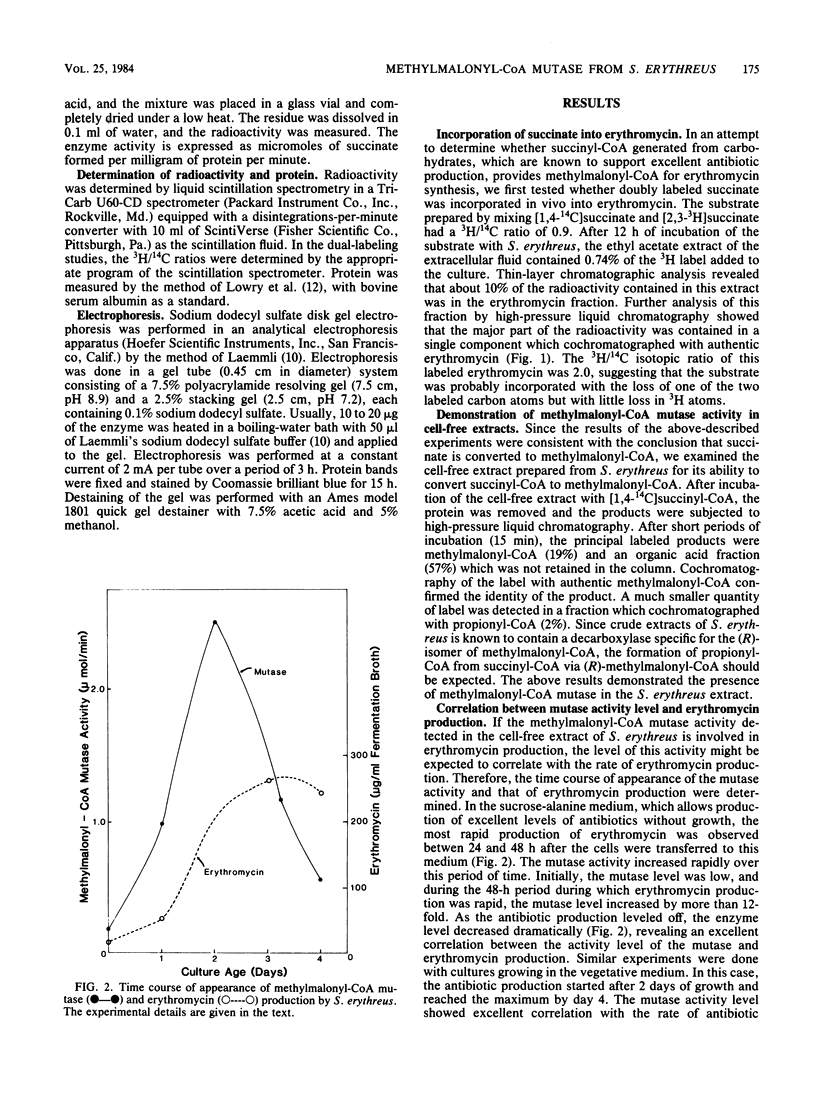
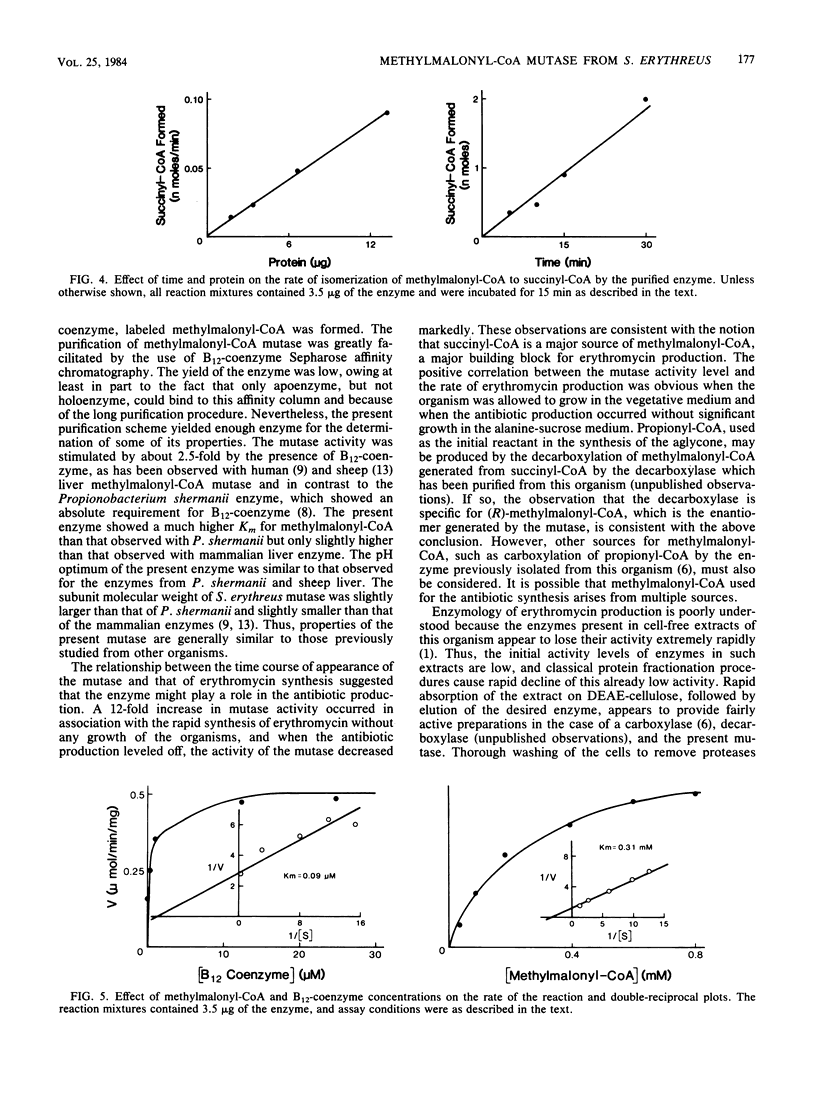
Streptomyces erythreus produces erythromycin, presumably from methylmalonyl-coenzyme A (CoA), which could be generated by the isomerization of succinyl-CoA. In S. erythreus cultures, [1,4-14C,2,3-3H]succinate was incorporated into erythromycin with a doubling of the 3H/14C ratio. This result is consistent with the hypothesis that succinyl-CoA is isomerized to methylmalonyl-CoA before incorporation into the macrocyclic lactone of erythromycin. The presence of methylmalonyl-CoA mutase, which catalyzes this isomerization, was demonstrated in cell-free extracts prepared from this organism. Consistent with the suggested role for this enzyme, methylmalonyl-CoA mutase activity increased over 12-fold at the time of the most rapid antibiotic production, and the activity level drastically declined when the antibiotic production ceased. The mutase was partially purified from this organism with DEAE-cellulose, ammonium sulfate precipitation, and affinity chromatography on a B12-coenzyme Sepharose column. The enzyme was stimulated 2.5-fold by the addition of B12-coenzyme. The enzyme showed a typical Michaelis-Menten type substrate saturation patterns, with KmS of 0.31 mM and 0.09 microM for methylmalonyl-CoA and B12-coenzyme, respectively, and a V of 0.5 mumol/min per mg. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the purified enzyme showed a major band with a molecular weight of 63,000. The properties of this enzyme appear to be fairly similar to those of the mutase previously obtained from other sources.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CORUM C. J., STARK W. M., WILD G. M., BIRD H. L., Jr Biochemical changes in a chemically defined medium by submerged cultures of Streptomyces erythreus. Appl Microbiol. 1954 Nov;2(6):326–329. doi: 10.1128/am.2.6.326-329.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkey B. E., Brandt M., Williams R. J., Williamson J. R. Assay of short-chain acyl coenzyme A intermediates in tissue extracts by high-pressure liquid chromatography. Anal Biochem. 1981 Nov 15;118(1):30–41. doi: 10.1016/0003-2697(81)90152-4. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN S. M., KANEDA T., CORCORAN J. W. ANTIBIOTIC GLYCOSIDES. V. A COMPARISON OF 2-METHYLMALONATE AND PROPIONATE AS PERCURSORS OF THE C21 BRANCHED CHAIN LACTONE IN ERYTHROMYCIN. J Biol Chem. 1964 Jul;239:2386–2391. [PubMed] [Google Scholar]
- Hunaiti A. R., Kolattukudy P. E. Isolation and characterization of an acyl-coenzyme A carboxylase from an erythromycin-producing Streptomyces erythreus. Arch Biochem Biophys. 1982 Jun;216(1):362–371. doi: 10.1016/0003-9861(82)90222-3. [DOI] [PubMed] [Google Scholar]
- Kolhouse J. F., Utley C., Allen R. H. Isolation and characterization of methylmalonyl-CoA mutase from human placenta. J Biol Chem. 1980 Apr 10;255(7):2708–2712. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rainwater D. L., Kolattukudy P. E. Synthesis of mycocerosic acids from methylmalonyl coenzyme A by cell-free extracts of Mycobacterium tuberculosis var. bovis BCG. J Biol Chem. 1983 Mar 10;258(5):2979–2985. [PubMed] [Google Scholar]
- Tsuji K., Goetz J. F. High-performance liquid chromatographic determination of erythromycin. J Chromatogr. 1978 Jan 11;147:359–367. doi: 10.1016/s0021-9673(00)85147-x. [DOI] [PubMed] [Google Scholar]



