Abstract
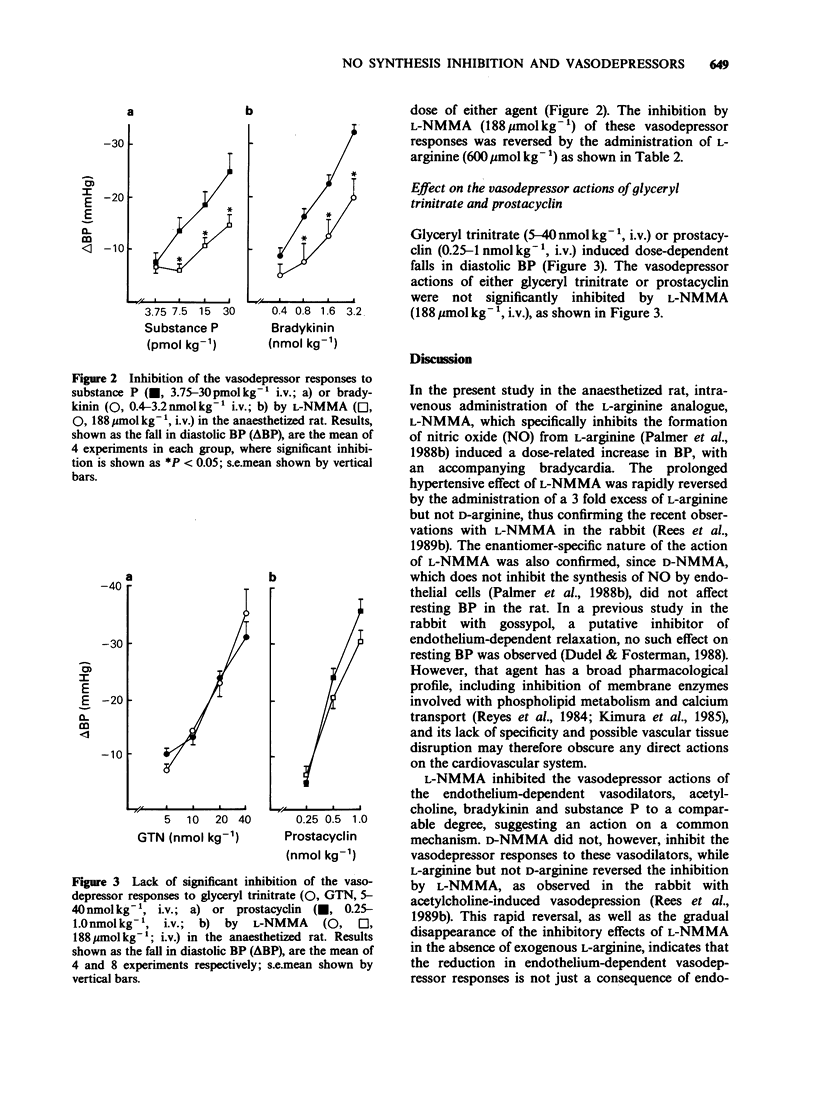
1. The effects of the specific inhibitor of nitric oxide (NO) formation, NG-monomethyl-L-arginine (L-NMMA), on resting systemic arterial blood pressure (BP) and on the actions of both endothelium-dependent and endothelium-independent vasodilators were investigated in the anaesthetized, normotensive rat. 2. Intravenous administration of L-NMMA (12.5-50 mg kg-1; 47-188 mumol kg-1) but not its enantiomer, D-NMMA, induced a dose-related increase in BP, which was reversed by the intravenous administration of L-arginine (150-600 mumol kg-1), but not D-arginine. 3. The vasodepressor responses to intravenous administration of the endothelium-dependent vasodilators, acetylcholine, bradykinin and substance P were significantly inhibited by L-NMMA (94 and 188 mumol kg-1 i.v.), but not by D-NMMA. 4. The inhibition by L-NMMA of these vasodepressor responses was reversed by administration of L-arginine, but not D-arginine. 5. Endothelin (ET-1) induced dose-related vasodepressor responses following bolus intravenous administration, which were significantly inhibited by L-NMMA but not by D-NMMA. This inhibition was reversed by administration of L-arginine. 6. The vasodepressor effects of the endothelium-independent vasodilators, glyceryl trinitrate or prostacyclin, were not significantly inhibited by L-NMMA. 7. These findings with L-NMMA suggest that resting blood pressure in the rat is modulated by endogenous NO biosynthesis and that endothelium-dependent vasodilators act through the formation of endogenous NO to exert their actions in vivo.
Full text
PDF

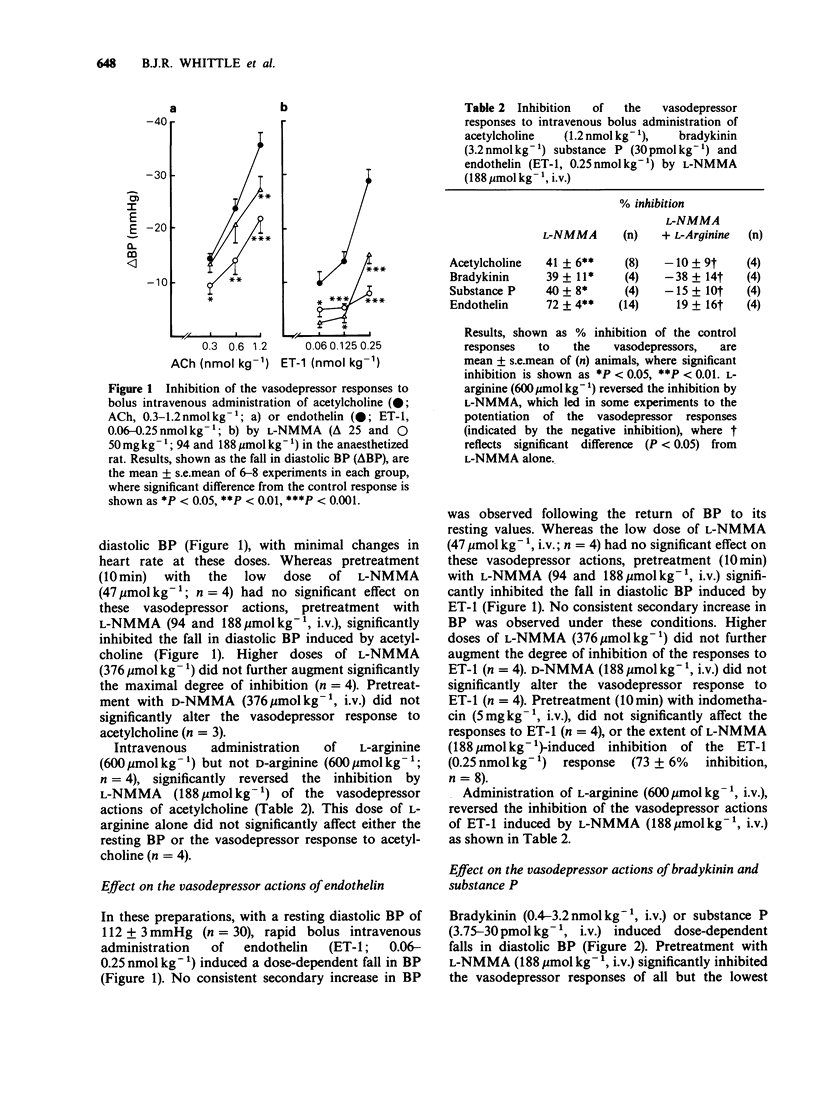



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A., Campbell G. R., Cocks T. M., Manderson J. A. Vasodilatation by acetylcholine is endothelium-dependent: a study by sonomicrometry in canine femoral artery in vivo. J Physiol. 1983 Nov;344:209–222. doi: 10.1113/jphysiol.1983.sp014934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H., Weston A. H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988 Dec;95(4):1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel C., Förstermann U. Gossypol attenuates selectively the blood pressure lowering effect of endothelium-dependent vasodilators in the rabbit in vivo. Eur J Pharmacol. 1988 Jan 12;145(2):217–221. doi: 10.1016/0014-2999(88)90234-8. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Hutchinson P. J., Palmer R. M., Moncada S. Comparative pharmacology of EDRF and nitric oxide on vascular strips. Eur J Pharmacol. 1987 Sep 23;141(3):445–451. doi: 10.1016/0014-2999(87)90563-2. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm M., Feelisch M., Spahr R., Piper H. M., Noack E., Schrader J. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem Biophys Res Commun. 1988 Jul 15;154(1):236–244. doi: 10.1016/0006-291x(88)90675-4. [DOI] [PubMed] [Google Scholar]
- Kimura K., Sakurada K., Katoh N. Inhibition by gossypol of phospholipid-sensitive Ca2+-dependent protein kinase from pig testis. Biochim Biophys Acta. 1985 May 8;839(3):276–280. doi: 10.1016/0304-4165(85)90009-1. [DOI] [PubMed] [Google Scholar]
- Martin W., Drazan K. M., Newby A. C. Methylene blue but not changes in cyclic GMP inhibits resting and bradykinin-stimulated production of prostacyclin by pig aortic endothelial cells. Br J Pharmacol. 1989 May;97(1):51–56. doi: 10.1111/j.1476-5381.1989.tb11922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Patthy A., Bajusz S., Patthy L. Preparation and characterization of Ng-mono-, di- and trimethylated arginines. Acta Biochim Biophys Acad Sci Hung. 1977;12(3):191–196. [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):281–296. [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes J., Allen J., Tanphaichitr N., Bellvé A. R., Benos D. J. Molecular mechanisms of gossypol action on lipid membranes. J Biol Chem. 1984 Aug 10;259(15):9607–9615. [PubMed] [Google Scholar]
- Rosenblum W. I., Nelson G. H., Povlishock J. T. Laser-induced endothelial damage inhibits endothelium-dependent relaxation in the cerebral microcirculation of the mouse. Circ Res. 1987 Feb;60(2):169–176. doi: 10.1161/01.res.60.2.169. [DOI] [PubMed] [Google Scholar]
- Sobey C. G., Woodman O. L., Dusting G. J. Inhibition of vasodilatation by methylene blue in large and small arteries of the dog hindlimb in vivo. Clin Exp Pharmacol Physiol. 1988 May;15(5):401–410. doi: 10.1111/j.1440-1681.1988.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Payne A. N., Esplugues J. V. Cardiopulmonary and gastric ulcerogenic actions of endothelin-1 in the guinea pig and rat. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S103–S123. doi: 10.1097/00005344-198900135-00026. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Fozard J. R. Regional vasodilation is a prominent feature of the haemodynamic response to endothelin in anaesthetized, spontaneously hypertensive rats. Eur J Pharmacol. 1988 Oct 11;155(1-2):201–203. doi: 10.1016/0014-2999(88)90425-6. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Inoue A., Ishikawa T., Kasuya Y., Kimura S., Kumagaye S., Nakajima K., Watanabe T. X., Sakakibara S., Goto K. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6964–6967. doi: 10.1073/pnas.85.18.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]


