Abstract
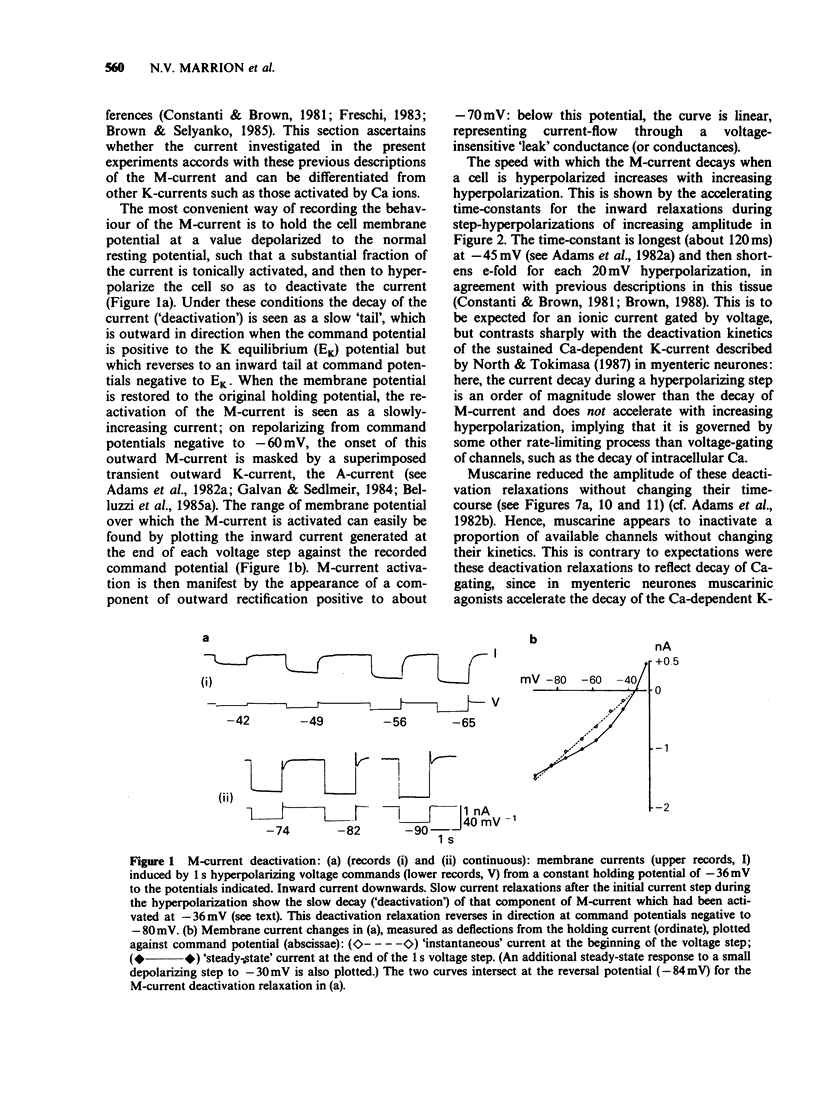
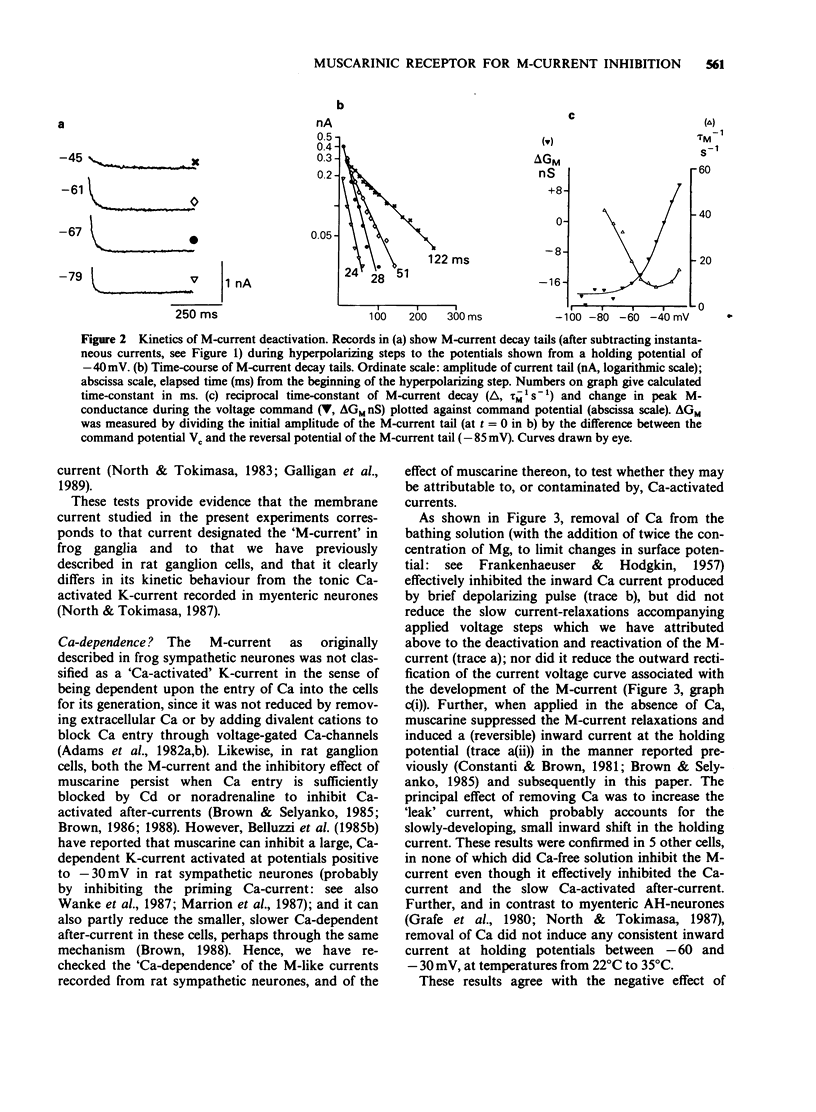
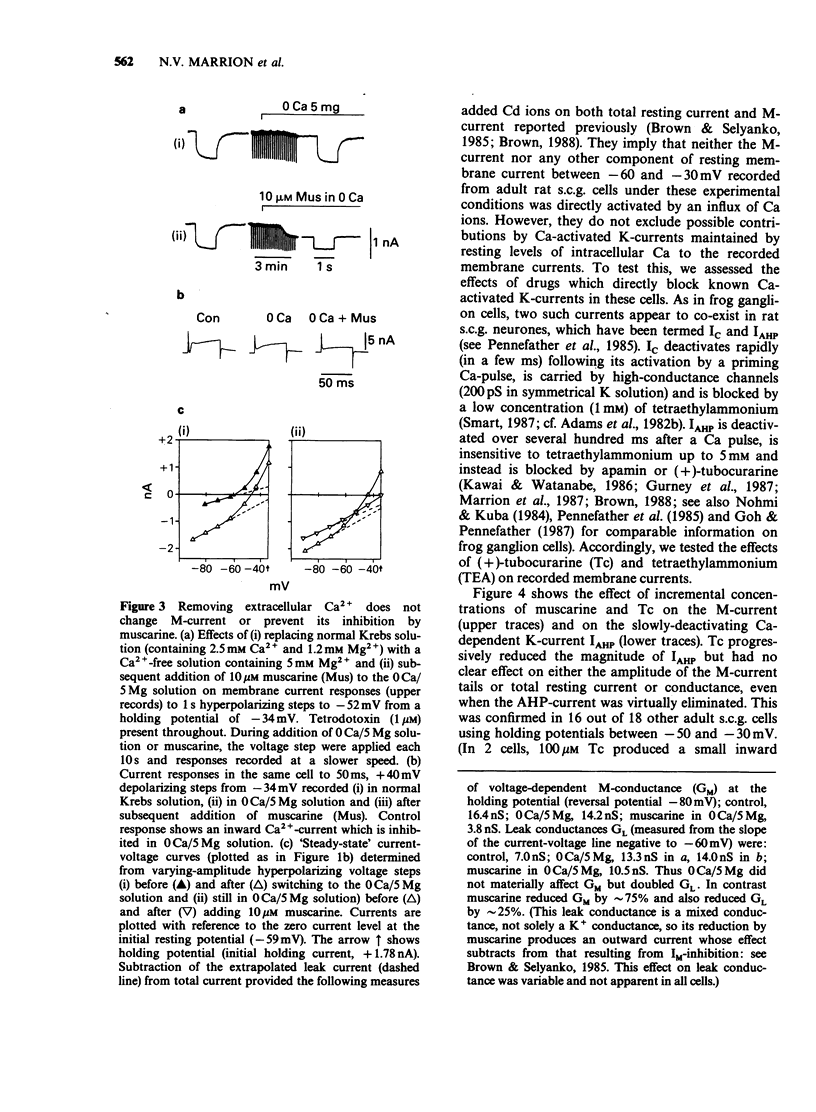
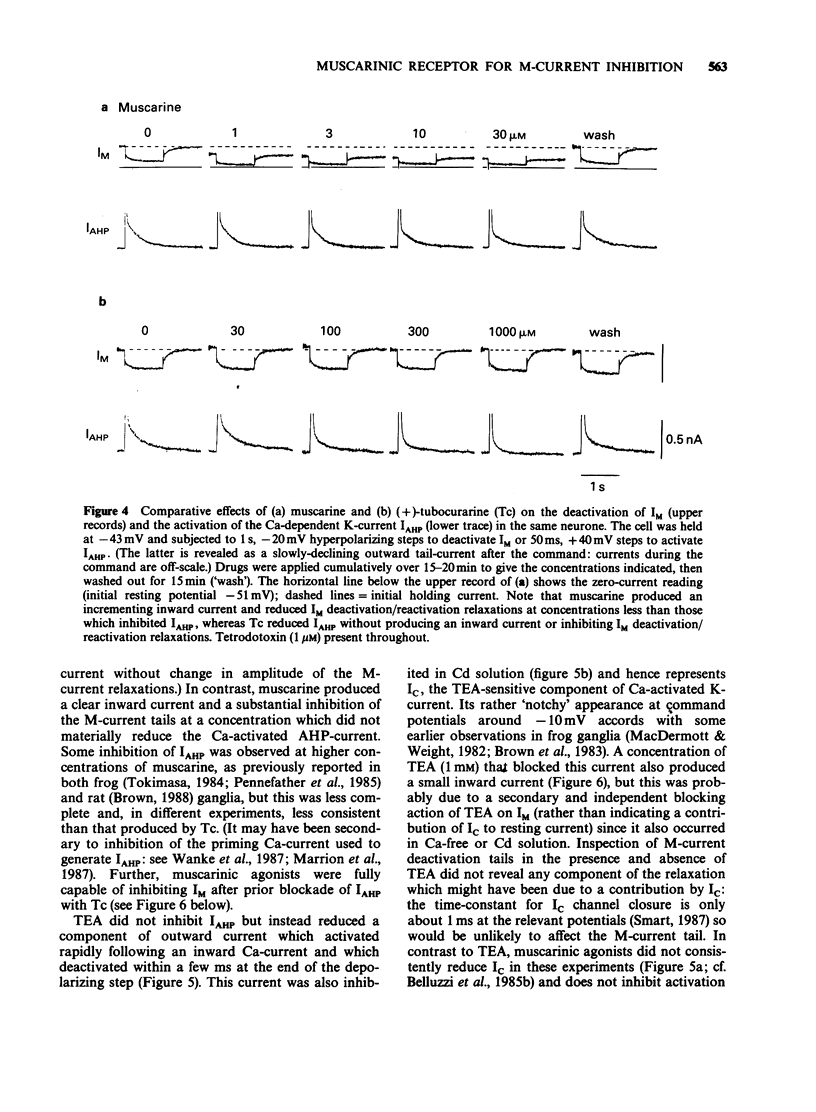
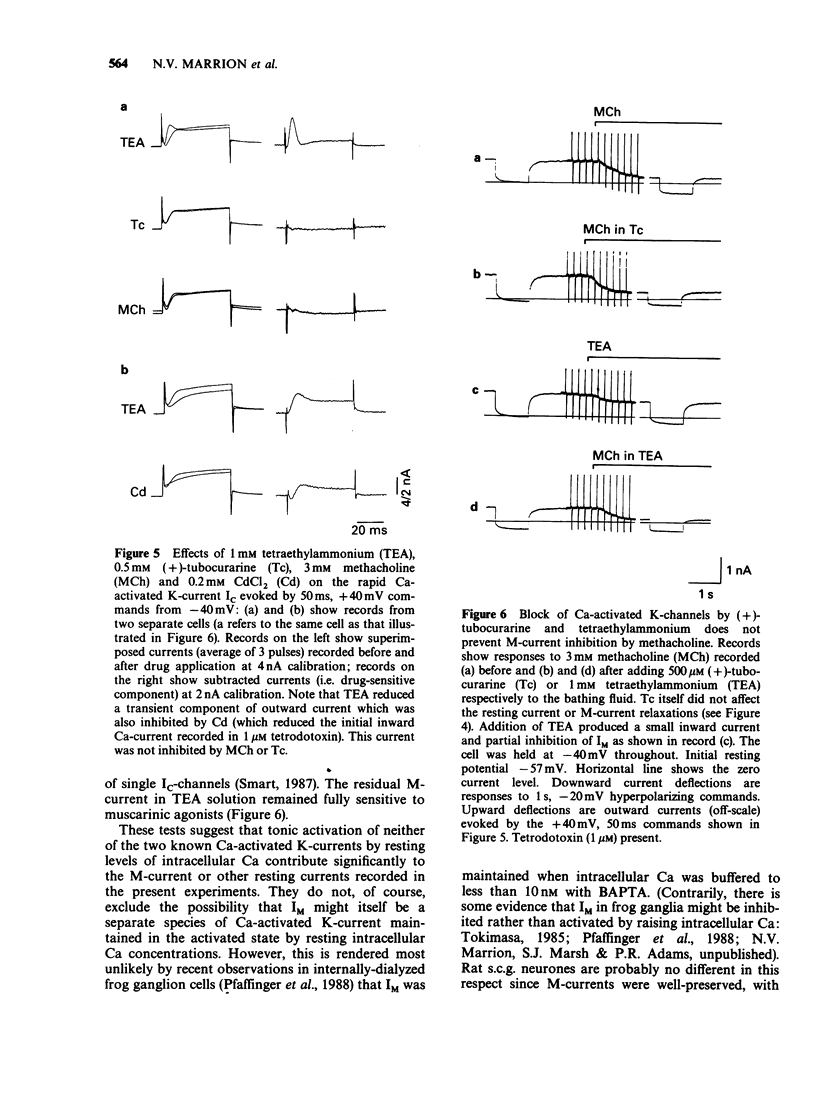
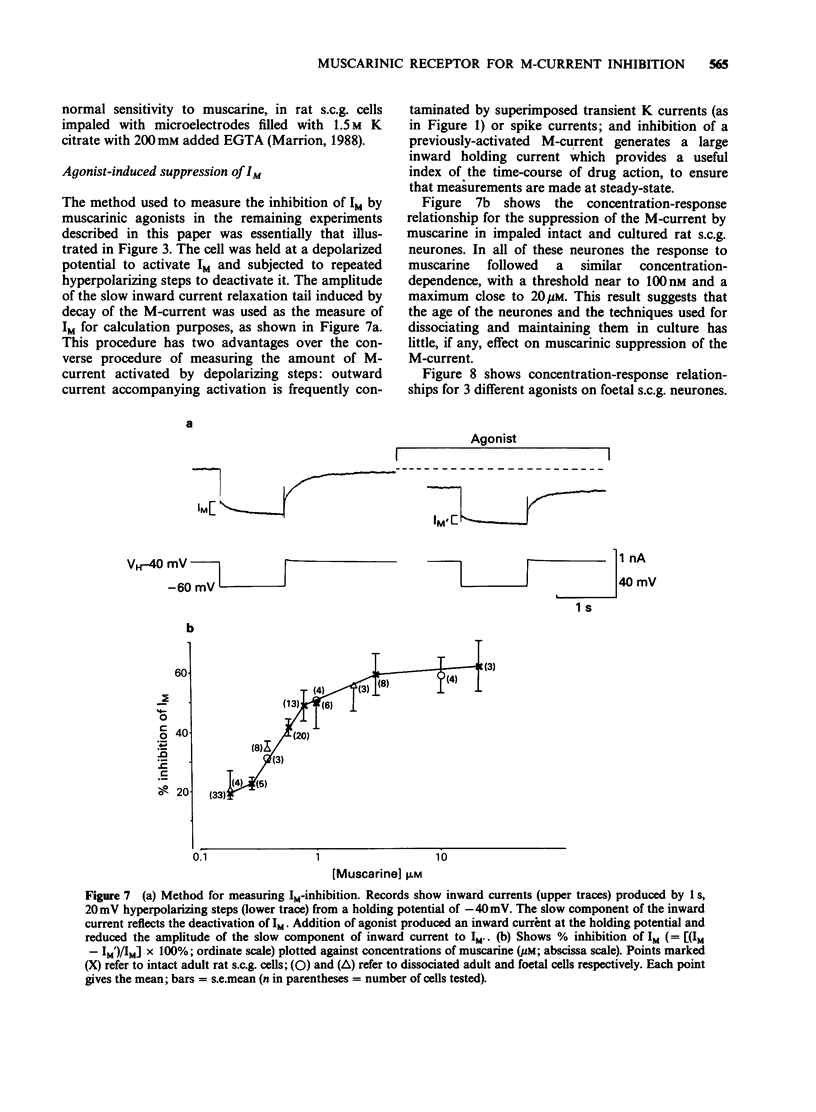
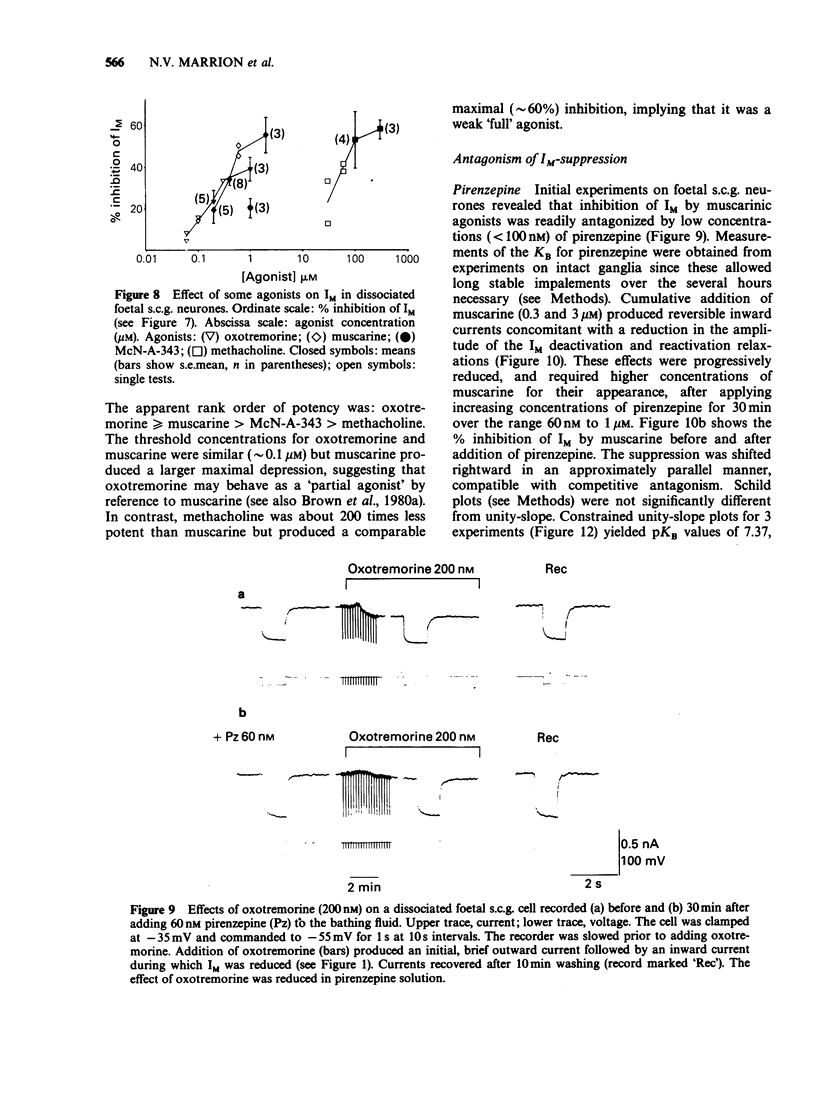
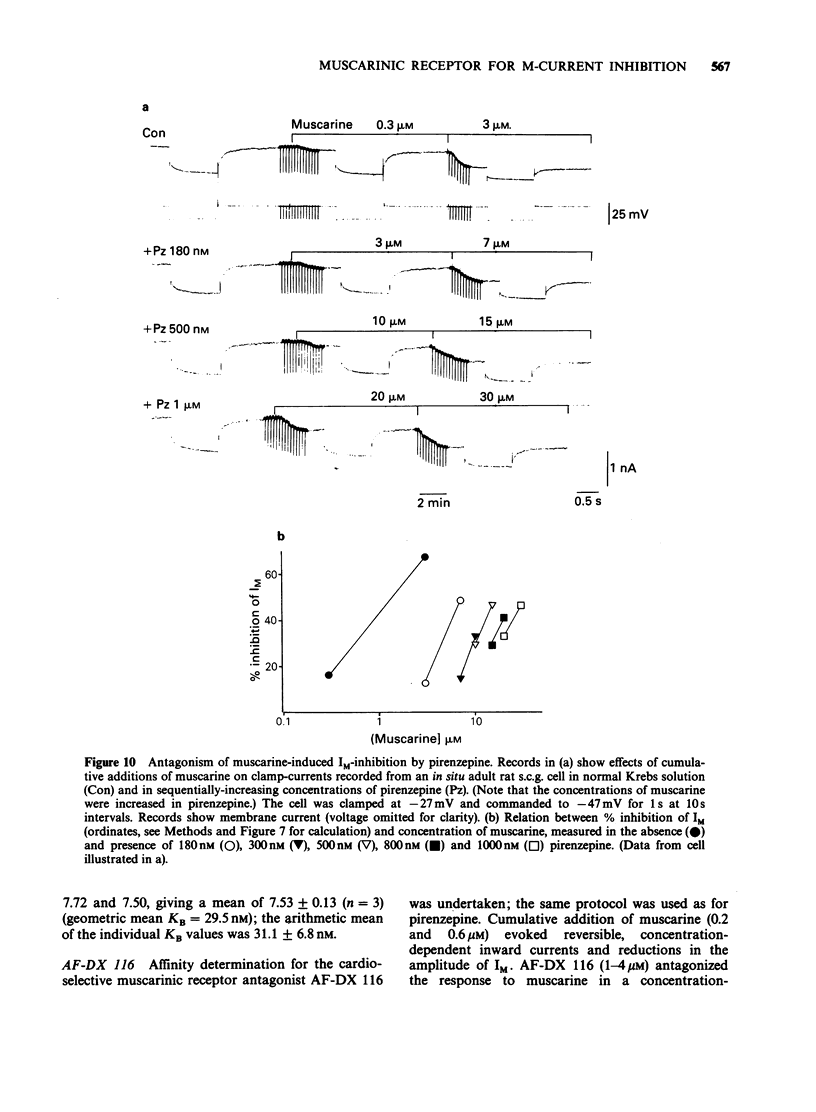
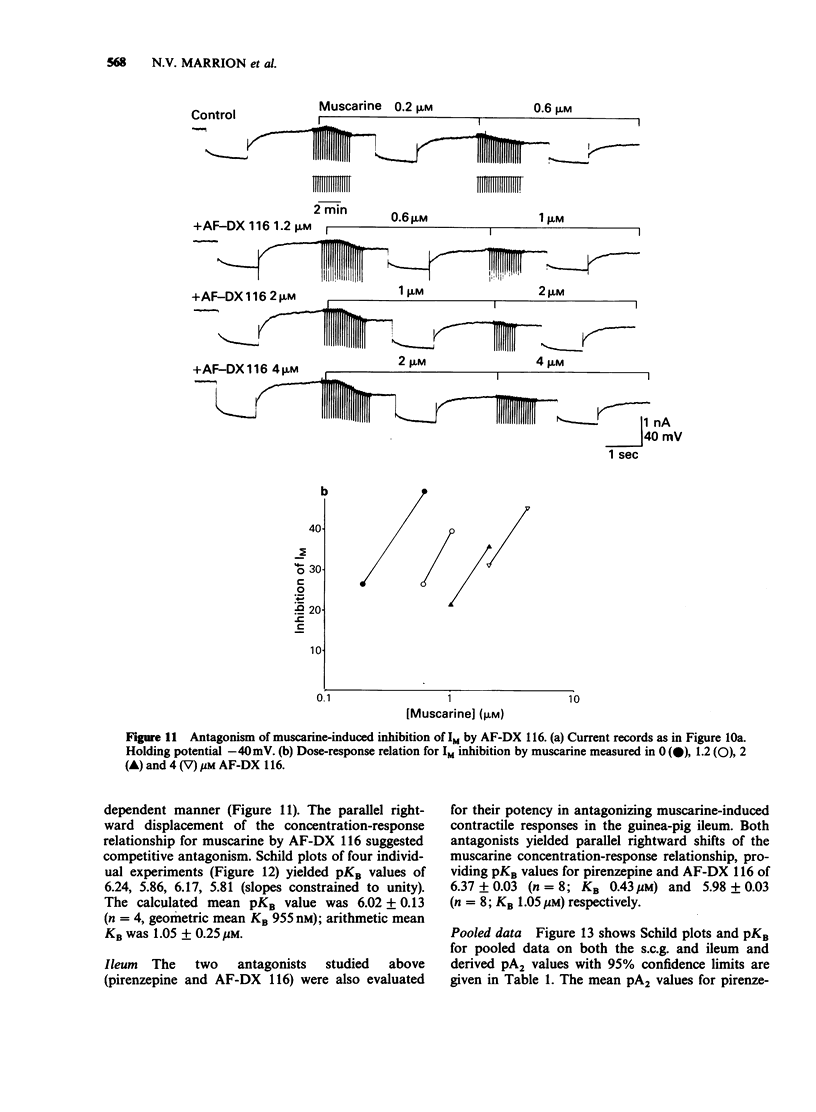
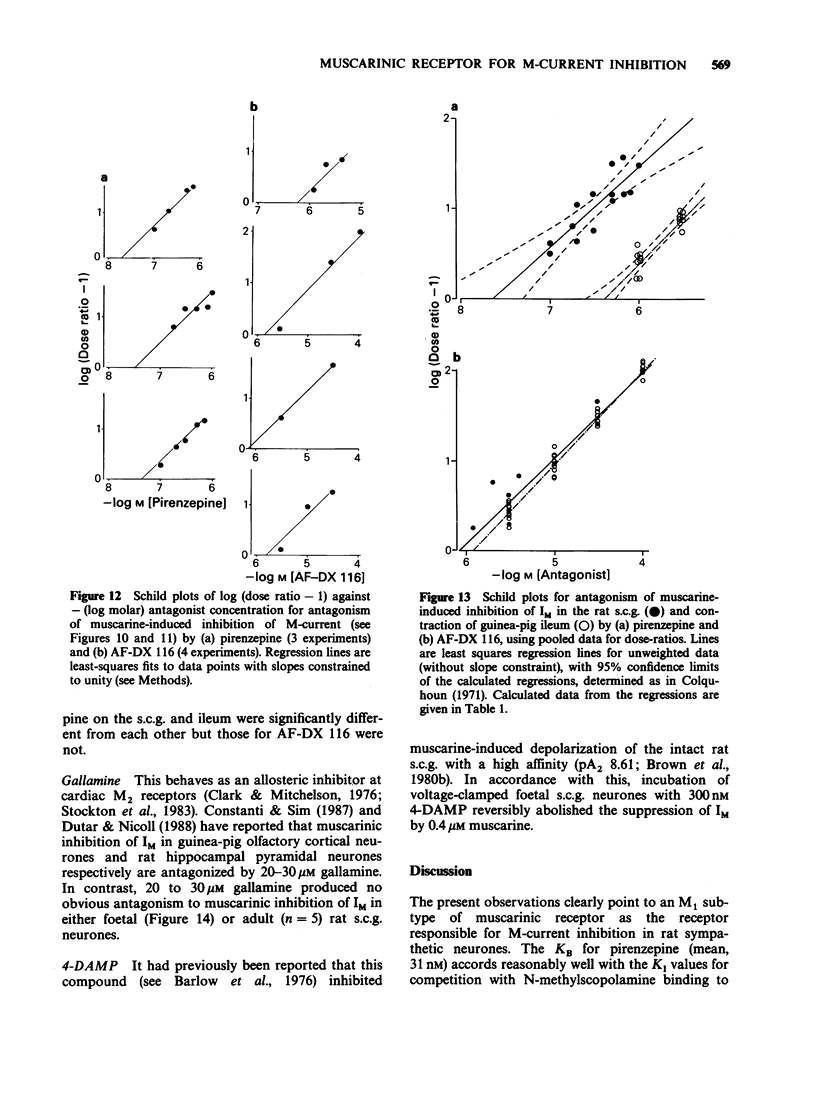
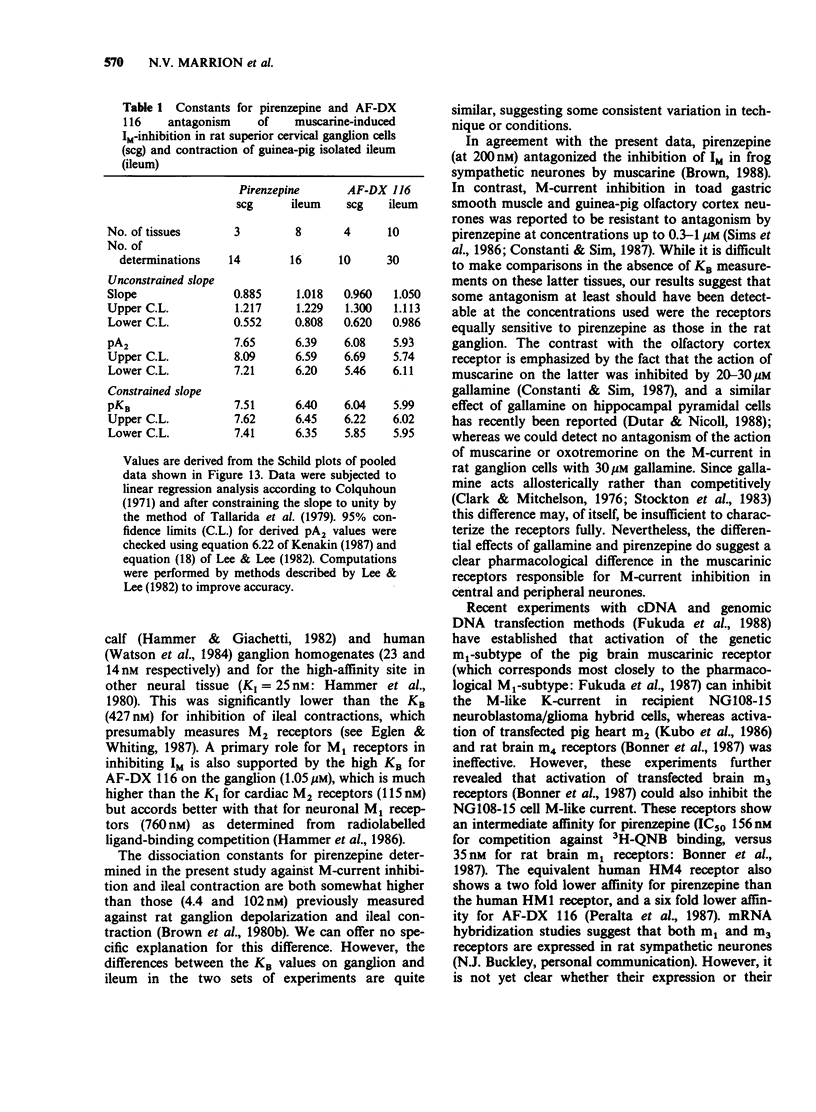
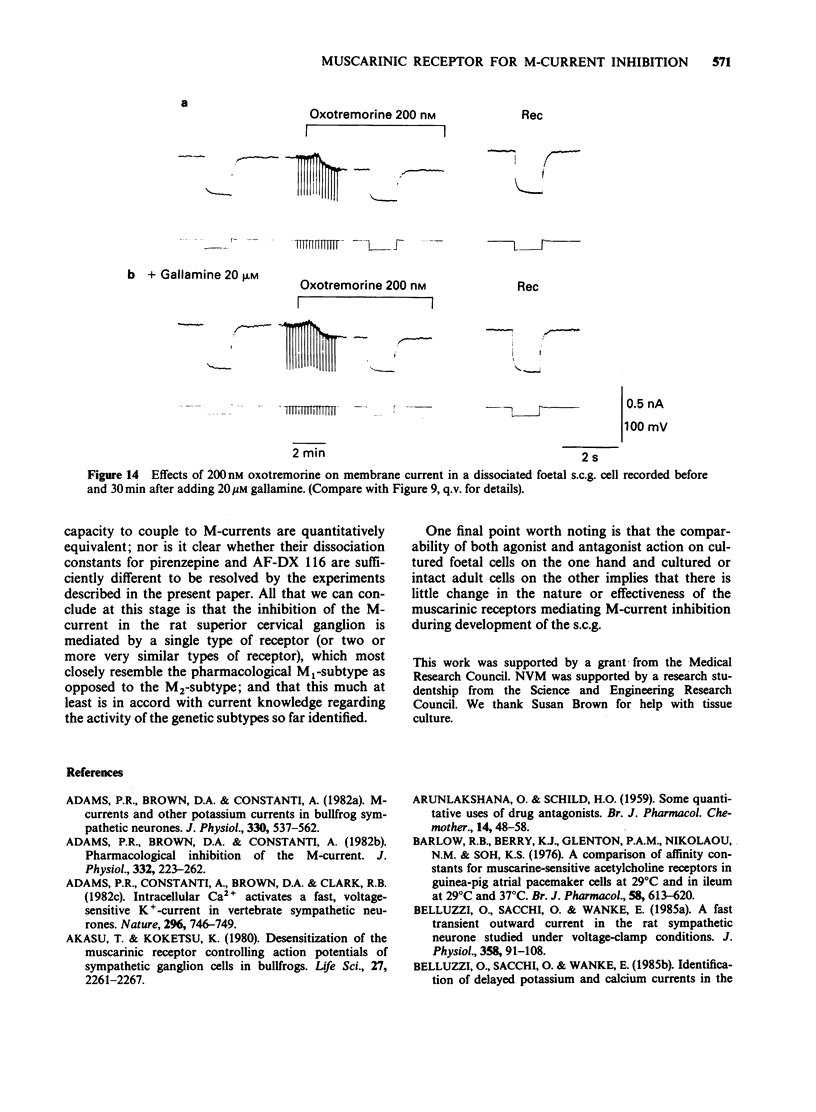
1. Under voltage-clamp dissociated adult and foetal rat superior cervical ganglion (s.c.g.) cells exhibited a non-inactivating voltage- and time-dependent component of K+ current termed the M-current (IM). IM was detected and measured from the current decay during hyperpolarizing voltage steps applied from potentials where IM was pre-activated. 2. Neither the resting membrane current nor the amplitude of these current decay relaxations were reduced by omitting Ca from the bathing fluid, showing that the M-current was not a 'Ca-activated' K-current dependent on a primary Ca-influx. Concentrations of (+)-tubocurarine sufficient to block the slow Ca-activated K-current IAHP did not inhibit IM or antagonize the effect of muscarinic agonists on IM, showing that IM was not contaminated by IAHP. Tetraethylammonium (1 mM), which blocks the fast Ca-activated K-current IC, produced a small inhibition of IM. This was not due to contamination of IM by IC since muscarinic agonists did not consistently block IC. 3. The muscarinic agonists muscarine, oxotremorine, McN-A-343 and methacholine reversibly suppressed IM, resulting in an inward (depolarizing) current. The rank order of potency was: oxotremorine greater than or equal to muscarine greater than McN-A-343 greater than methacholine. 4. The suppression of IM by muscarine was similar in cultured cells derived from adult and foetal tissue to that seen in the intact ganglia. 5. IM-suppression by muscarine was inhibited by pirenzepine (Pz) and AF-DX 116 with mean pKB values of 7.53 +/- 0.13 (n = 3) and 6.02 +/- 0.13 (n = 4) respectively. 6. The suppression of IM by muscarinic agonists was not affected by gallamine (10-30 microM). 4-Diphenylacetoxy-N-methylpiperidine methiodide inhibited the response at 300 nM. 7. Pirenzepine inhibited the contractions of the guinea-pig isolated ileum produced by muscarine with a mean pKB of 6.37 +/- 0.03 (n = 8). 8. These results suggest that the receptors mediating suppression of the M-current accord with those designated pharmacologically as M1 and that these receptors reach maturity at a very early stage in the development of the rat s.c.g.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Akasu T., Koketsu K. Desensitization of the muscarinic receptor controlling action potentials of sympathetic ganglion cells in bullfrogs. Life Sci. 1980 Dec 8;27(23):2261–2267. doi: 10.1016/0024-3205(80)90393-8. [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Berry K. J., Glenton P. A., Nilolaou N. M., Soh K. S. A comparison of affinity constants for muscarine-sensitive acetylcholine receptors in guinea-pig atrial pacemaker cells at 29 degrees C and in ileum at 29 degrees C and 37 degrees C. Br J Pharmacol. 1976 Dec;58(4):613–620. doi: 10.1111/j.1476-5381.1976.tb08631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. Identification of delayed potassium and calcium currents in the rat sympathetic neurone under voltage clamp. J Physiol. 1985 Jan;358:109–129. doi: 10.1113/jphysiol.1985.sp015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A., Adams P. R. Ca-activated potassium current in vertebrate sympathetic neurons. Cell Calcium. 1983 Dec;4(5-6):407–420. doi: 10.1016/0143-4160(83)90017-9. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A. Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol. 1980 Dec;70(4):593–608. doi: 10.1111/j.1476-5381.1980.tb09778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Fatherazi S., Garthwaite J., White R. D. Muscarinic receptors in rat sympathetic ganglia. Br J Pharmacol. 1980 Dec;70(4):577–592. doi: 10.1111/j.1476-5381.1980.tb09777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Forward A., Marsh S. Antagonist discrimination between ganglionic and ileal muscarinic receptors. Br J Pharmacol. 1980;71(2):362–364. doi: 10.1111/j.1476-5381.1980.tb10948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A. M currents. Ion Channels. 1988;1:55–94. doi: 10.1007/978-1-4615-7302-9_2. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Selyanko A. A. Two components of muscarine-sensitive membrane current in rat sympathetic neurones. J Physiol. 1985 Jan;358:335–363. doi: 10.1113/jphysiol.1985.sp015554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell J. F., McLachlan E. M. Muscarinic agonists block five different potassium conductances in guinea-pig sympathetic neurones. Br J Pharmacol. 1987 Jun;91(2):259–261. doi: 10.1111/j.1476-5381.1987.tb10279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. L., Mitchelson F. The inhibitory effect of gallamine on muscarinic receptors. Br J Pharmacol. 1976 Nov;58(3):323–331. doi: 10.1111/j.1476-5381.1976.tb07708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Brown D. A. M-Currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981 Jul 17;24(3):289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- Constanti A., Sim J. A. Muscarinic receptors mediating suppression of the M-current in guinea-pig olfactory cortex neurones may be of the M2-subtype. Br J Pharmacol. 1987 Jan;90(1):3–5. doi: 10.1111/j.1476-5381.1987.tb16818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci. 1988 Nov;8(11):4214–4224. doi: 10.1523/JNEUROSCI.08-11-04214.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Competitive and non-competitive antagonism exhibited by 'selective' antagonists at atrial and ileal muscarinic receptor subtypes. Br J Pharmacol. 1987 Apr;90(4):701–707. doi: 10.1111/j.1476-5381.1987.tb11223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi J. E. Membrane currents of cultured rat sympathetic neurons under voltage clamp. J Neurophysiol. 1983 Dec;50(6):1460–1478. doi: 10.1152/jn.1983.50.6.1460. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Higashida H., Kubo T., Maeda A., Akiba I., Bujo H., Mishina M., Numa S. Selective coupling with K+ currents of muscarinic acetylcholine receptor subtypes in NG108-15 cells. Nature. 1988 Sep 22;335(6188):355–358. doi: 10.1038/335355a0. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kubo T., Akiba I., Maeda A., Mishina M., Numa S. Molecular distinction between muscarinic acetylcholine receptor subtypes. Nature. 1987 Jun 18;327(6123):623–625. doi: 10.1038/327623a0. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., North R. A., Tokimasa T. Muscarinic agonists and potassium currents in guinea-pig myenteric neurones. Br J Pharmacol. 1989 Jan;96(1):193–203. doi: 10.1111/j.1476-5381.1989.tb11800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachetti A., Micheletti R., Montagna E. Cardioselective profile of AF-DX 116, a muscarine M2 receptor antagonist. Life Sci. 1986 May 5;38(18):1663–1672. doi: 10.1016/0024-3205(86)90410-8. [DOI] [PubMed] [Google Scholar]
- Giraldo E., Hammer R., Ladinsky H. Distribution of muscarinic receptor subtypes in rat brain as determined in binding studies with AF-DX 116 and pirenzepine. Life Sci. 1987 Mar 2;40(9):833–840. doi: 10.1016/0024-3205(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Goh J. W., Pennefather P. S. Pharmacological and physiological properties of the after-hyperpolarization current of bullfrog ganglion neurones. J Physiol. 1987 Dec;394:315–330. doi: 10.1113/jphysiol.1987.sp016872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. M., Tsien R. Y., Lester H. A. Activation of a potassium current by rapid photochemically generated step increases of intracellular calcium in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 May;84(10):3496–3500. doi: 10.1073/pnas.84.10.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982 Dec 27;31(26):2991–2998. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giraldo E., Schiavi G. B., Monferini E., Ladinsky H. Binding profile of a novel cardioselective muscarine receptor antagonist, AF-DX 116, to membranes of peripheral tissues and brain in the rat. Life Sci. 1986 May 5;38(18):1653–1662. doi: 10.1016/0024-3205(86)90409-1. [DOI] [PubMed] [Google Scholar]
- Hashiguchi T., Kobayashi H., Tosaka T., Libet B. Two muscarinic depolarizing mechanisms in mammalian sympathetic neurons. Brain Res. 1982 Jun 24;242(2):378–382. doi: 10.1016/0006-8993(82)90329-8. [DOI] [PubMed] [Google Scholar]
- Jones S. W. Muscarinic and peptidergic excitation of bull-frog sympathetic neurones. J Physiol. 1985 Sep;366:63–87. doi: 10.1113/jphysiol.1985.sp015785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Analysis of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Jpn J Physiol. 1976;26(6):651–669. doi: 10.2170/jjphysiol.26.651. [DOI] [PubMed] [Google Scholar]
- Kubo T., Maeda A., Sugimoto K., Akiba I., Mikami A., Takahashi H., Haga T., Haga K., Ichiyama A., Kangawa K. Primary structure of porcine cardiac muscarinic acetylcholine receptor deduced from the cDNA sequence. FEBS Lett. 1986 Dec 15;209(2):367–372. doi: 10.1016/0014-5793(86)81144-9. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Weight F. F. Action potential repolarization may involve a transient, Ca2+-sensitive outward current in a vertebrate neurone. Nature. 1982 Nov 11;300(5888):185–188. doi: 10.1038/300185a0. [DOI] [PubMed] [Google Scholar]
- Marrion N. V., Smart T. G., Brown D. A. Membrane currents in adult rat superior cervical ganglia in dissociated tissue culture. Neurosci Lett. 1987 Jun 1;77(1):55–60. doi: 10.1016/0304-3940(87)90606-9. [DOI] [PubMed] [Google Scholar]
- Mochida S., Kobayashi H. Multiple muscarinic responses directly evoked in isolated neurones dissociated from rabbit sympathetic ganglia. J Auton Nerv Syst. 1986 Dec;17(4):289–301. doi: 10.1016/0165-1838(86)90095-0. [DOI] [PubMed] [Google Scholar]
- Nohmi M., Kuba K. (+)-Tubocurarine blocks the Ca2+-dependent K+-channel of the bullfrog sympathetic ganglion cell. Brain Res. 1984 May 28;301(1):146–148. doi: 10.1016/0006-8993(84)90412-8. [DOI] [PubMed] [Google Scholar]
- North R. A., Slack B. E., Surprenant A. Muscarinic M1 and M2 receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous system. J Physiol. 1985 Nov;368:435–452. doi: 10.1113/jphysiol.1985.sp015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Depression of calcium-dependent potassium conductance of guinea-pig myenteric neurones by muscarinic agonists. J Physiol. 1983 Sep;342:253–266. doi: 10.1113/jphysiol.1983.sp014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Persistent calcium-sensitive potassium current and the resting properties of guinea-pig myenteric neurones. J Physiol. 1987 May;386:333–353. doi: 10.1113/jphysiol.1987.sp016537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Leibowitz M. D., Subers E. M., Nathanson N. M., Almers W., Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988 Aug;1(6):477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Smart T. G. Single calcium-activated potassium channels recorded from cultured rat sympathetic neurones. J Physiol. 1987 Aug;389:337–360. doi: 10.1113/jphysiol.1987.sp016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton J. M., Birdsall N. J., Burgen A. S., Hulme E. C. Modification of the binding properties of muscarinic receptors by gallamine. Mol Pharmacol. 1983 May;23(3):551–557. [PubMed] [Google Scholar]
- Tallarida R. J., Cowan A., Adler M. W. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979 Aug 20;25(8):637–654. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]
- Tokimasa T. Intracellular Ca2+-ions inactivate K+-current in bullfrog sympathetic neurons. Brain Res. 1985 Jul 1;337(2):386–391. doi: 10.1016/0006-8993(85)90081-2. [DOI] [PubMed] [Google Scholar]
- Tokimasa T. Muscarinic agonists depress calcium-dependent gK in bullfrog sympathetic neurons. J Auton Nerv Syst. 1984 Apr;10(2):107–116. doi: 10.1016/0165-1838(84)90049-3. [DOI] [PubMed] [Google Scholar]
- Wanke E., Ferroni A., Malgaroli A., Ambrosini A., Pozzan T., Meldolesi J. Activation of a muscarinic receptor selectively inhibits a rapidly inactivated Ca2+ current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4313–4317. doi: 10.1073/pnas.84.12.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M., Roeske W. R., Johnson P. C., Yamamura H. I. [3H]Pirenzepine identifies putative M1 muscarinic receptors in human stellate ganglia. Brain Res. 1984 Jan 2;290(1):179–182. doi: 10.1016/0006-8993(84)90751-0. [DOI] [PubMed] [Google Scholar]


