Abstract
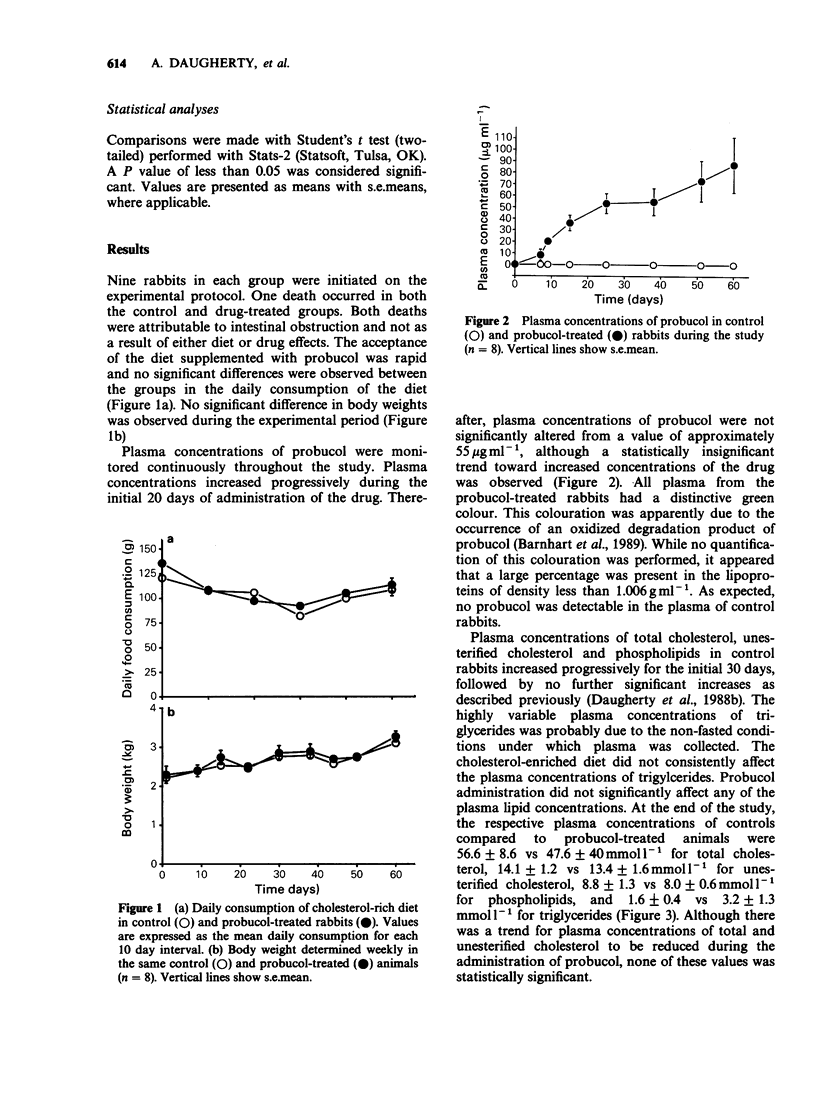
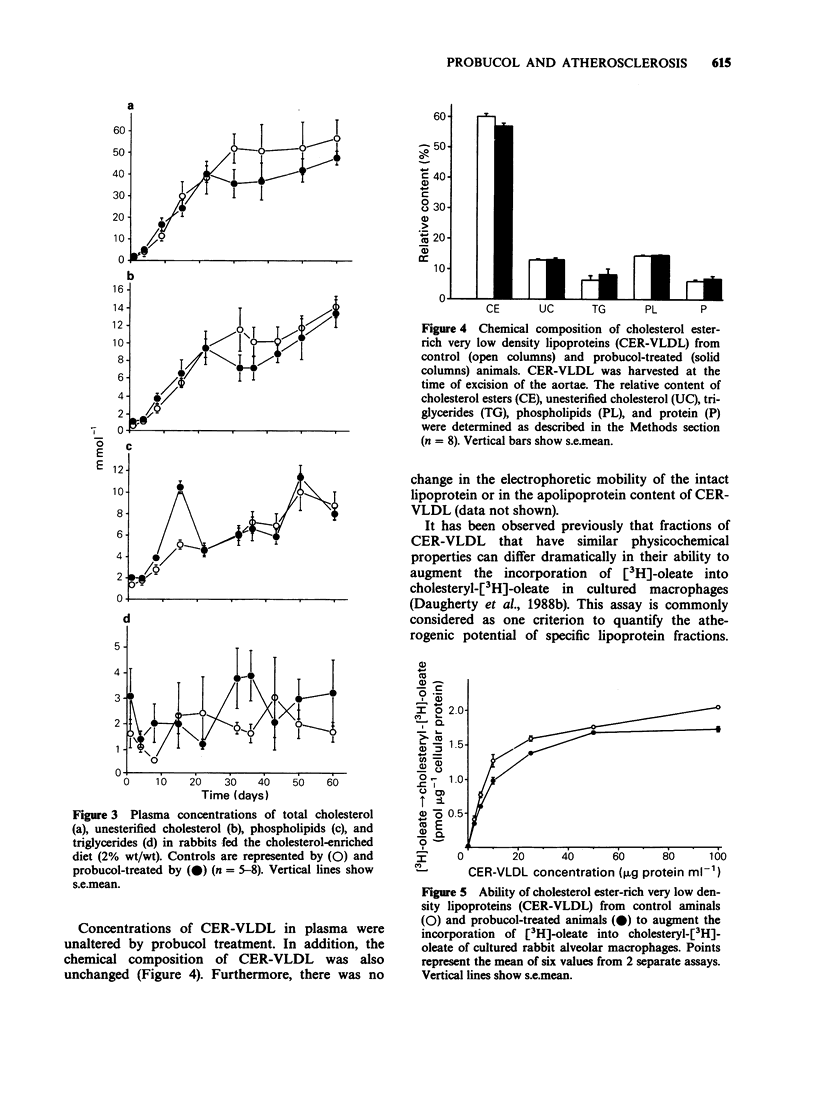
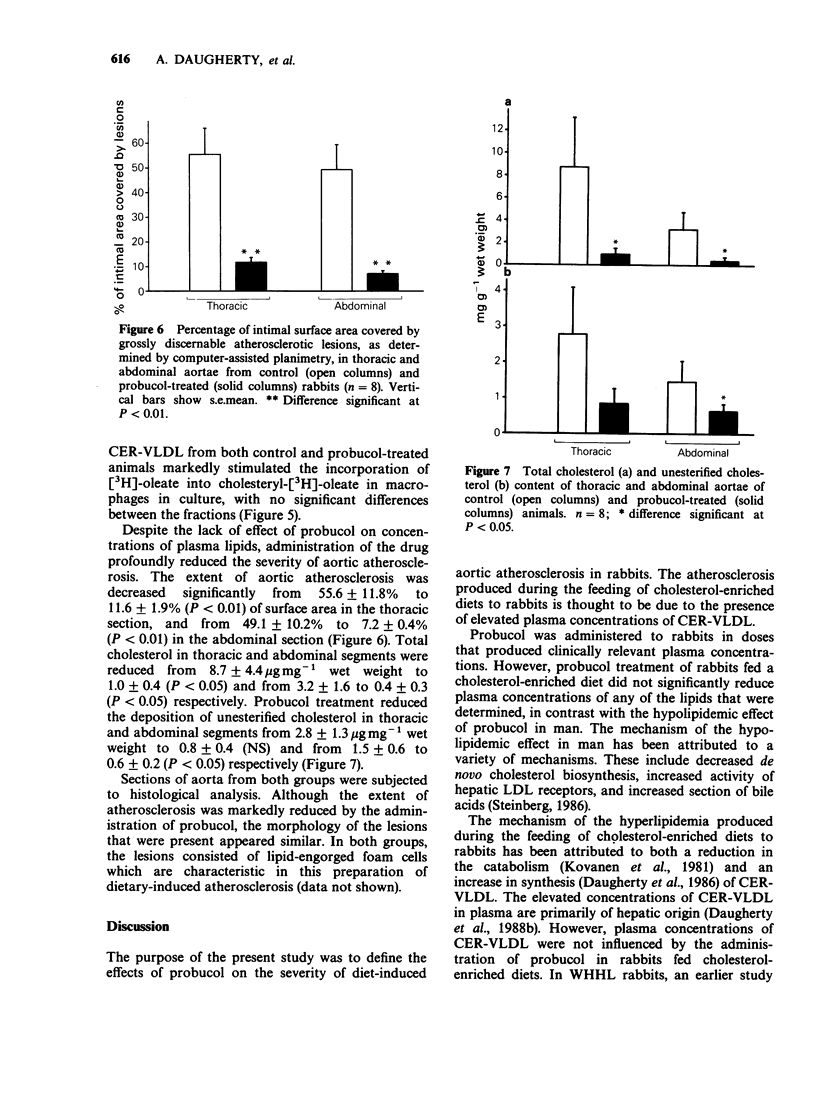
1. Probucol was administered to rabbits fed a cholesterol-enriched (2% wt/wt) diet to determine potential anti-atherogenic effects in a preparation in which the disease process is due to elevated plasma concentrations of cholesterol ester-rich very low density lipoproteins (CER-VLDL). 2. Probucol was supplemented to the diet at 1% wt/wt which resulted in plasma concentrations rising steadily to 53 +/- 8 micrograms ml-1 after 14 days, with no significant changes during continued administration. Dietary consumption and body weight gains were comparable in the drug-treated and control groups during the observation period. 3. Probucol treatment did not significantly affect plasma concentrations of total cholesterol, unesterified cholesterol, triglycerides or phospholipids. 4. The concentration of CER-VLDL in plasma and its physicochemical characteristics were not significantly changed during administration of probucol. CER-VLDL from both control and probucol-treated animals was a potent stimulant of the augmentation of the intracellular incorporation of [3H]-oleate into cholesteryl-[3H]-oleate in cultured macrophages. 5. Despite the lack of effect of probucol on concentrations of plasma lipids and the cell interaction characteristics of CER-VLDL, administration of the drug markedly decreased the extent of intimal aortic surface area covered by grossly discernible atherosclerotic lesions from 55.6 +/- 11.8% to 11.6 +/- 1.9% in thoracic sections, and from 49.1 +/- 10.2% to 7.2 +/- 0.4% in abdominal sections. Furthermore, probucol treatment significantly reduced the deposition of total cholesterol in vascular tissue. 6. Probucol reduced the extent of aortic atherosclerosis produced by diet-induced hypercholesterolemia in rabbits. This reduction occurred in the absence of any significant change in the characteristics of plasma lipoproteins that were determined.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daugherty A., Lange L. G., Sobel B. E., Schonfeld G. Aortic accumulation and plasma clearance of beta-VLDL and HDL: effects of diet-induced hypercholesterolemia in rabbits. J Lipid Res. 1985 Aug;26(8):955–963. [PubMed] [Google Scholar]
- Daugherty A., Oida K., Sobel B. E., Schonfeld G. Dependence of metabolic and structural heterogeneity of cholesterol ester-rich very low density lipoproteins on the duration of cholesterol feeding in rabbits. J Clin Invest. 1988 Aug;82(2):562–570. doi: 10.1172/JCI113633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Rateri D. L., Schonfeld G., Sobel B. E. Inhibition of cholesteryl ester deposition in macrophages by calcium entry blockers: an effect dissociable from calcium entry blockade. Br J Pharmacol. 1987 May;91(1):113–118. doi: 10.1111/j.1476-5381.1987.tb08989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Schonfeld G., Sobel B. E., Lange L. G. Metabolism of very low density lipoproteins after cessation of cholesterol feeding in rabbits. A factor potentially contributing to the slow regression of atheromatous plaques. J Clin Invest. 1986 Apr;77(4):1108–1115. doi: 10.1172/JCI112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Zweifel B. S., Sobel B. E., Schonfeld G. Isolation of low density lipoprotein from atherosclerotic vascular tissue of Watanabe heritable hyperlipidemic rabbits. Arteriosclerosis. 1988 Nov-Dec;8(6):768–777. doi: 10.1161/01.atv.8.6.768. [DOI] [PubMed] [Google Scholar]
- Fowler S., Shio H., Haley N. J. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. Lab Invest. 1979 Oct;41(4):372–378. [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Ishikawa T. T., MacGee J., Morrison J. A., Glueck C. J. Quantitative analysis of cholesterol in 5 to 20 microliter of plasma. J Lipid Res. 1974 May;15(3):286–291. [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Basu S. K., Bilheimer D. W., Goldstein J. L. Saturation and suppression of hepatic lipoprotein receptors: a mechanism for the hypercholesterolemia of cholesterol-fed rabbits. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1396–1400. doi: 10.1073/pnas.78.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W. Atherogenic hyperlipoproteinemia. The cellular and molecular biology of plasma lipoproteins altered by dietary fat and cholesterol. Med Clin North Am. 1982 Mar;66(2):375–402. doi: 10.1016/s0025-7125(16)31426-2. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Brown M. S., Ho Y. K., Goldstein J. L. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980 Nov;21(8):970–980. [PubMed] [Google Scholar]
- McLean L. R., Hagaman K. A. Effect of probucol on the physical properties of low-density lipoproteins oxidized by copper. Biochemistry. 1989 Jan 10;28(1):321–327. doi: 10.1021/bi00427a043. [DOI] [PubMed] [Google Scholar]
- Naruszewicz M., Carew T. E., Pittman R. C., Witztum J. L., Steinberg D. A novel mechanism by which probucol lowers low density lipoprotein levels demonstrated in the LDL receptor-deficient rabbit. J Lipid Res. 1984 Nov;25(11):1206–1213. [PubMed] [Google Scholar]
- Parthasarathy S., Young S. G., Witztum J. L., Pittman R. C., Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986 Feb;77(2):641–644. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satonin D. K., Coutant J. E. Comparison of gas chromatography and high-performance liquid chromatography for the analysis of probucol in plasma. J Chromatogr. 1986 Aug 2;380(2):401–406. doi: 10.1016/s0378-4347(00)83670-1. [DOI] [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Steinberg D. Studies on the mechanism of action of probucol. Am J Cardiol. 1986 Jun 27;57(16):16H–21H. doi: 10.1016/0002-9149(86)90430-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Takaichi S., Hara H., Nishikawa O., Yokoyama S., Yamamura T., Yamaguchi T. Probucol prevents lipid storage in macrophages. Atherosclerosis. 1986 Dec;62(3):209–217. doi: 10.1016/0021-9150(86)90095-x. [DOI] [PubMed] [Google Scholar]


