Abstract
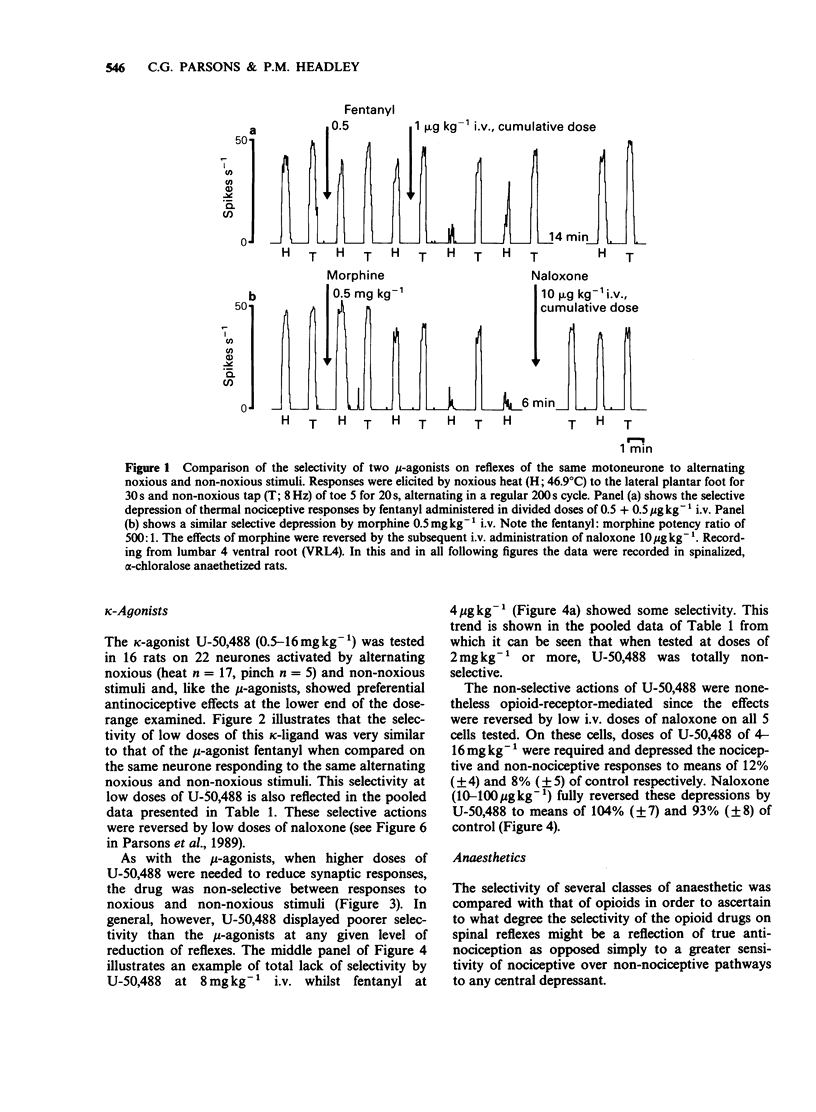
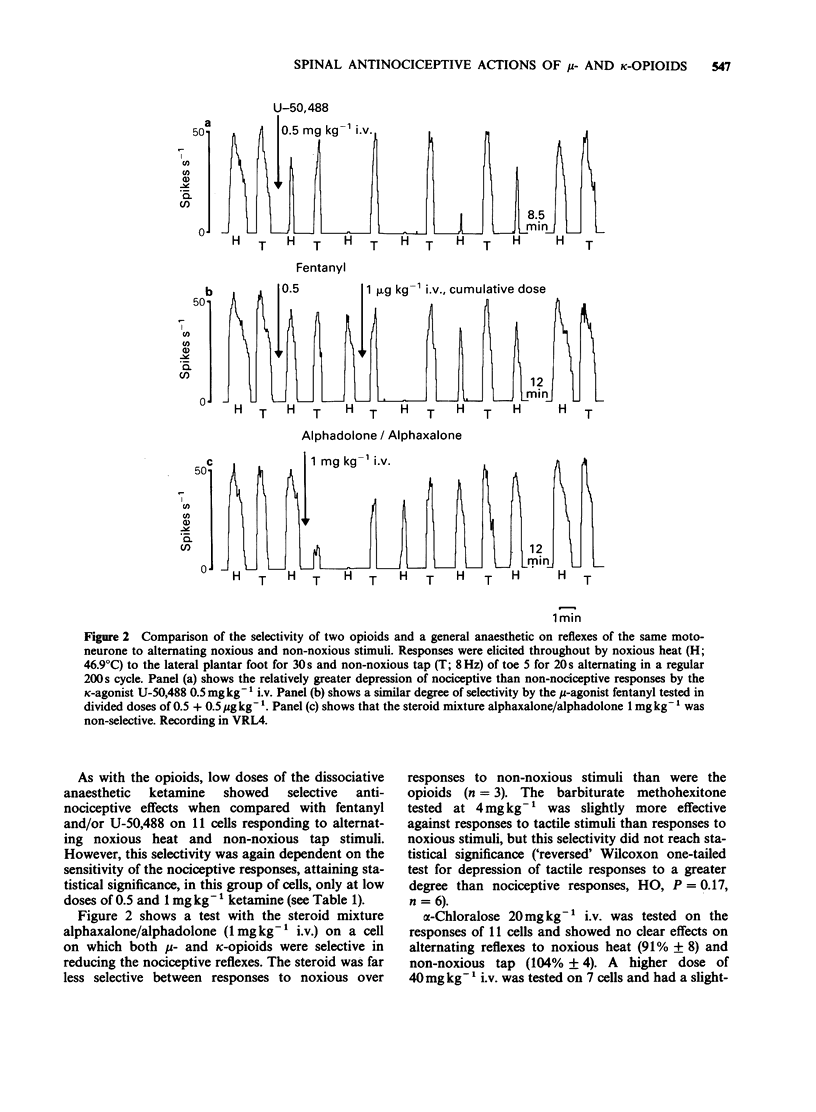
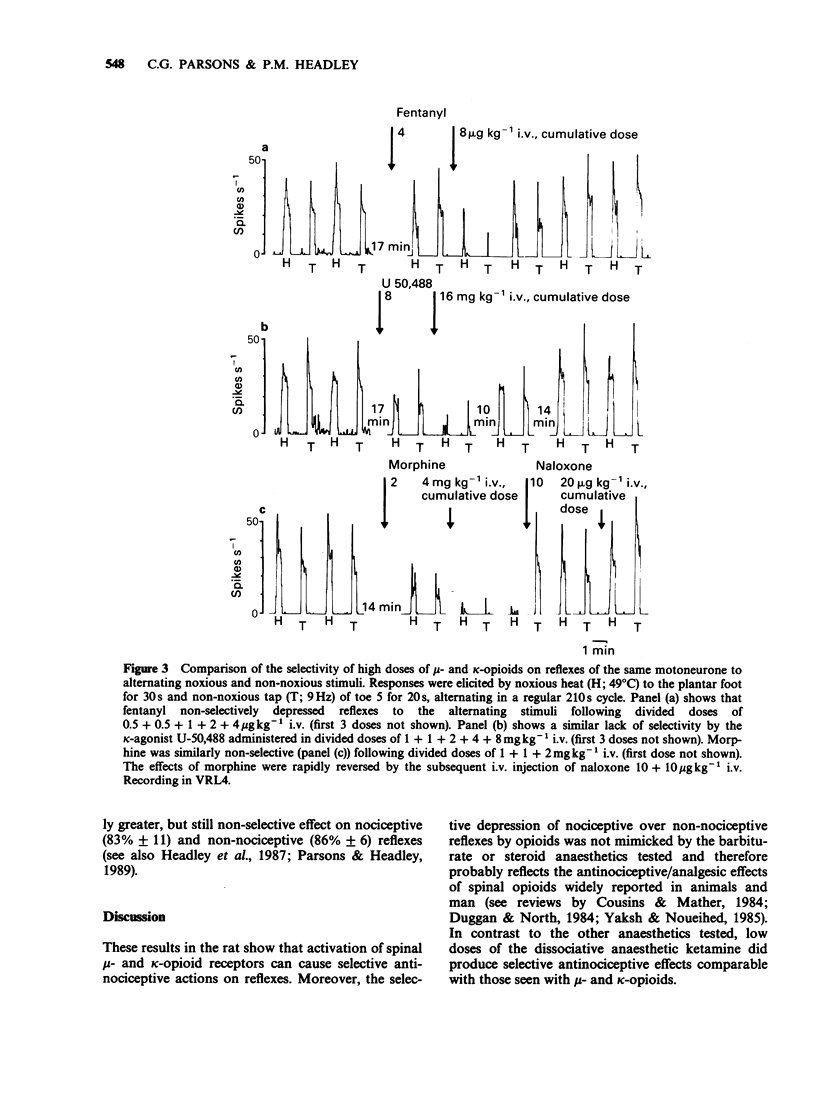
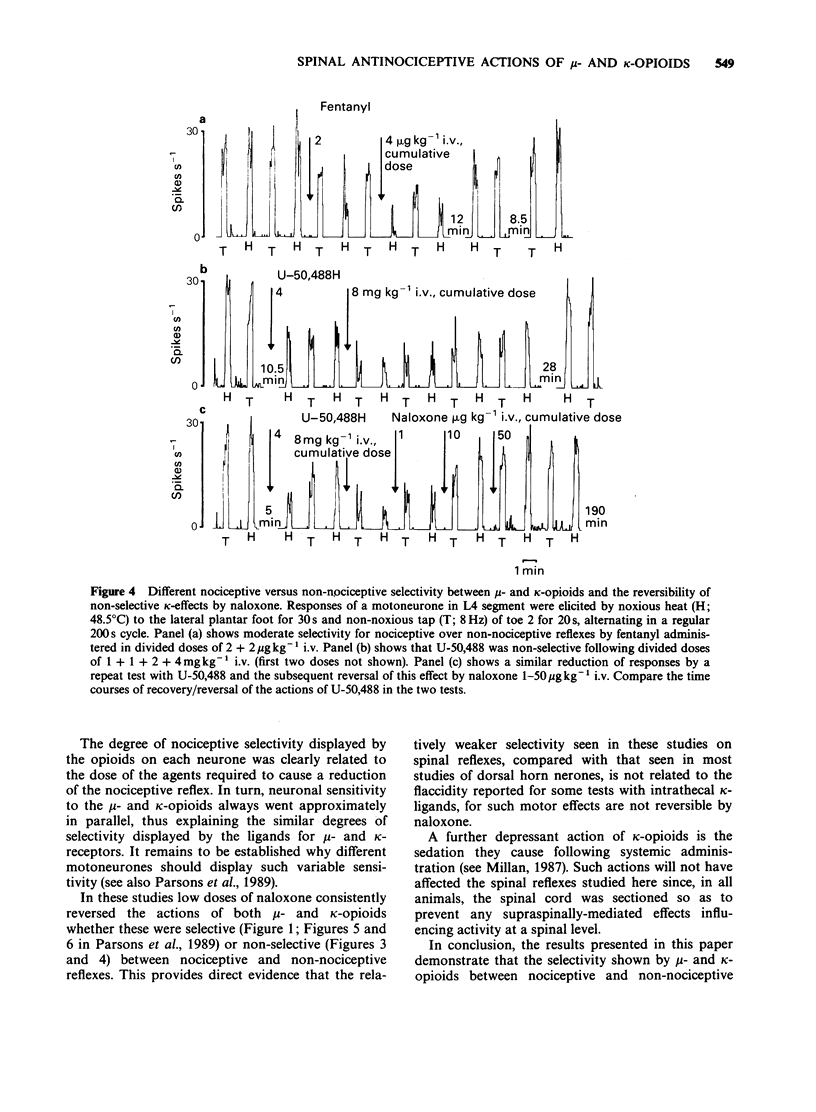
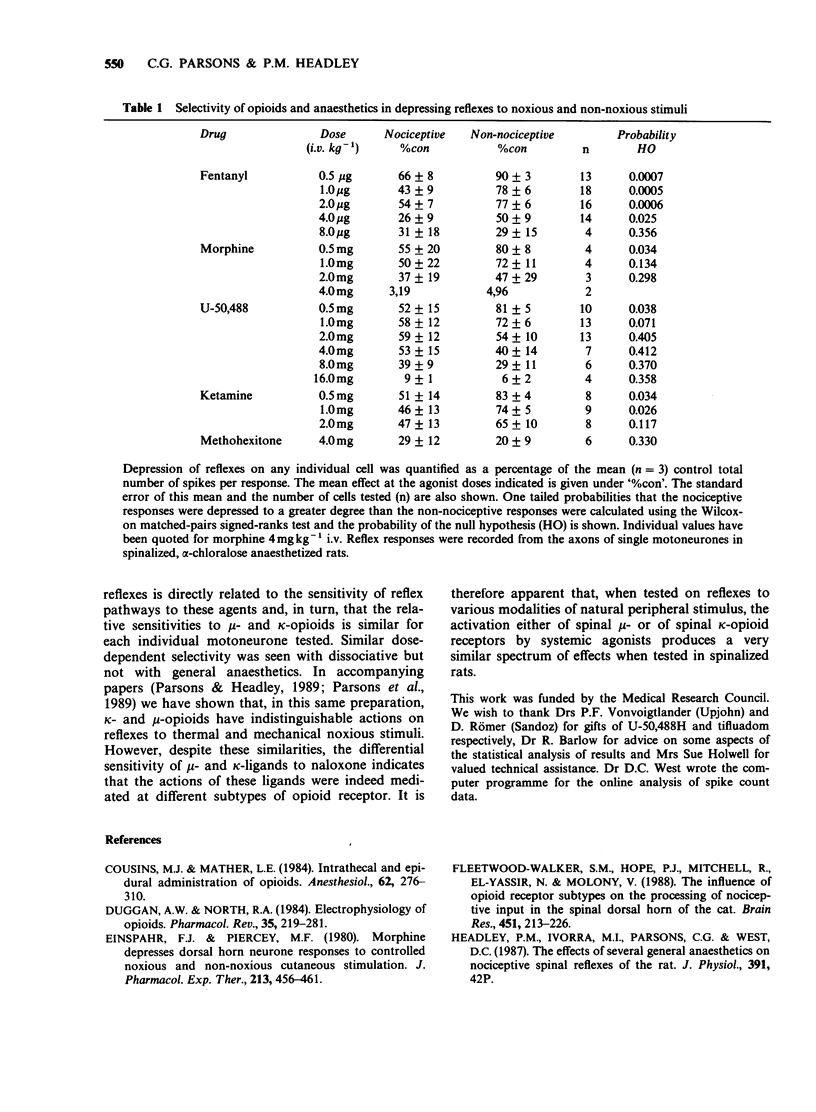
1. In electrophysiological experiments in spinalized, alpha-chloralose anaesthetized rats, opioids and anaesthetics were tested intravenously (i.v.) on the responses of individual motoneurones to alternating noxious (heat or pinch) and non-noxious (tap or vibration) stimuli. 2. On cells that were sensitive to low doses of mu-opioids, both fentanyl (0.5-4 micrograms kg-1 i.v.) and morphine (0.5 mg kg-1 i.v.) selectivity reduced reflexes to noxious stimuli to a greater degree than the higher doses required to reduce nociceptive reflexes (fentanyl 8 micrograms kg-1 i.v.; morphine 1-8 mg kg-1 i.v.) depressed non-nociceptive reflexes to a similar degree. 3. A similar spectrum of selectivity was seen with U-50,488 (0.5-16 mg kg-1 i.v.) although statistically significant selective depression of reflexes was only evident at the lowest dose tested (0.5 mg kg-1 i.v.). All effects of U-50,488 were readily reversed by low doses of the opioid antagonist, naloxone (10-100 micrograms kg-1 i.v.). 4. The dissociative anaesthetic/PCP ligand ketamine (0.5-4 mg kg-1 i.v.) was similar in having selective actions at low doses on sensitive cells but non-selective actions when higher doses were required. In contrast, the general anaesthetics methohexitone (4 mg kg-1 i.v.) and alphadolone/alphaxalone (1 mg kg-1 i.v.) were consistently non-selective between reflexes to noxious and non-noxious stimuli. alpha-Chloralose (20-40 mg kg-1 i.v.) had very little effect on reflexes to any of the synaptic inputs tested.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cousins M. J., Mather L. E. Intrathecal and epidural administration of opioids. Anesthesiology. 1984 Sep;61(3):276–310. [PubMed] [Google Scholar]
- Duggan A. W., North R. A. Electrophysiology of opioids. Pharmacol Rev. 1983 Dec;35(4):219–281. [PubMed] [Google Scholar]
- Einspahr F. J., Piercey M. F. Morphine depresses dorsal horn neuron responses to controlled noxious and non-noxious cutaneous stimulation. J Pharmacol Exp Ther. 1980 Jun;213(3):456–461. [PubMed] [Google Scholar]
- Fleetwood-Walker S. M., Hope P. J., Mitchell R., el-Yassir N., Molony V. The influence of opioid receptor subtypes on the processing of nociceptive inputs in the spinal dorsal horn of the cat. Brain Res. 1988 Jun 7;451(1-2):213–226. doi: 10.1016/0006-8993(88)90766-4. [DOI] [PubMed] [Google Scholar]
- Herman B. H., Goldstein A. Antinociception and paralysis induced by intrathecal dynorphin A. J Pharmacol Exp Ther. 1985 Jan;232(1):27–32. [PubMed] [Google Scholar]
- Knox R. J., Dickenson A. H. Effects of selective and non-selective kappa-opioid receptor agonists on cutaneous C-fibre-evoked responses of rat dorsal horn neurones. Brain Res. 1987 Jul 7;415(1):21–29. doi: 10.1016/0006-8993(87)90265-4. [DOI] [PubMed] [Google Scholar]
- Leighton G. E., Rodriguez R. E., Hill R. G., Hughes J. kappa-Opioid agonists produce antinociception after i.v. and i.c.v. but not intrathecal administration in the rat. Br J Pharmacol. 1988 Mar;93(3):553–560. doi: 10.1111/j.1476-5381.1988.tb10310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M. J. Multiple opioid systems and pain. Pain. 1986 Dec;27(3):303–347. doi: 10.1016/0304-3959(86)90158-2. [DOI] [PubMed] [Google Scholar]
- Parsons C. G., Headley P. M. Spinal antinociceptive actions of mu- and kappa-opioids: the importance of stimulus intensity in determining 'selectivity' between reflexes to different modalities of noxious stimulus. Br J Pharmacol. 1989 Oct;98(2):523–532. doi: 10.1111/j.1476-5381.1989.tb12626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C. G., West D. C., Headley P. M. Spinal antinociceptive actions and naloxone reversibility of intravenous mu- and kappa-opioids in spinalized rats: potency mismatch with values reported for spinal administration. Br J Pharmacol. 1989 Oct;98(2):533–543. doi: 10.1111/j.1476-5381.1989.tb12627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewłocki R., Shearman G. T., Herz A. Mixed opioid/nonopioid effects of dynorphin and dynorphin related peptides after their intrathecal injection in rats. Neuropeptides. 1983 Jan;3(3):233–240. doi: 10.1016/0143-4179(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Schmauss C., Yaksh T. L. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984 Jan;228(1):1–12. [PubMed] [Google Scholar]
- Spampinato S., Candeletti S. Characterization of dynorphin A-induced antinociception at spinal level. Eur J Pharmacol. 1985 Mar 26;110(1):21–30. doi: 10.1016/0014-2999(85)90024-x. [DOI] [PubMed] [Google Scholar]
- Stevens C. W., Yaksh T. L. Dynorphin A and related peptides administered intrathecally in the rat: a search for putative kappa opiate receptor activity. J Pharmacol Exp Ther. 1986 Sep;238(3):833–838. [PubMed] [Google Scholar]
- Yaksh T. L., Noueihed R. The physiology and pharmacology of spinal opiates. Annu Rev Pharmacol Toxicol. 1985;25:433–462. doi: 10.1146/annurev.pa.25.040185.002245. [DOI] [PubMed] [Google Scholar]


