Abstract
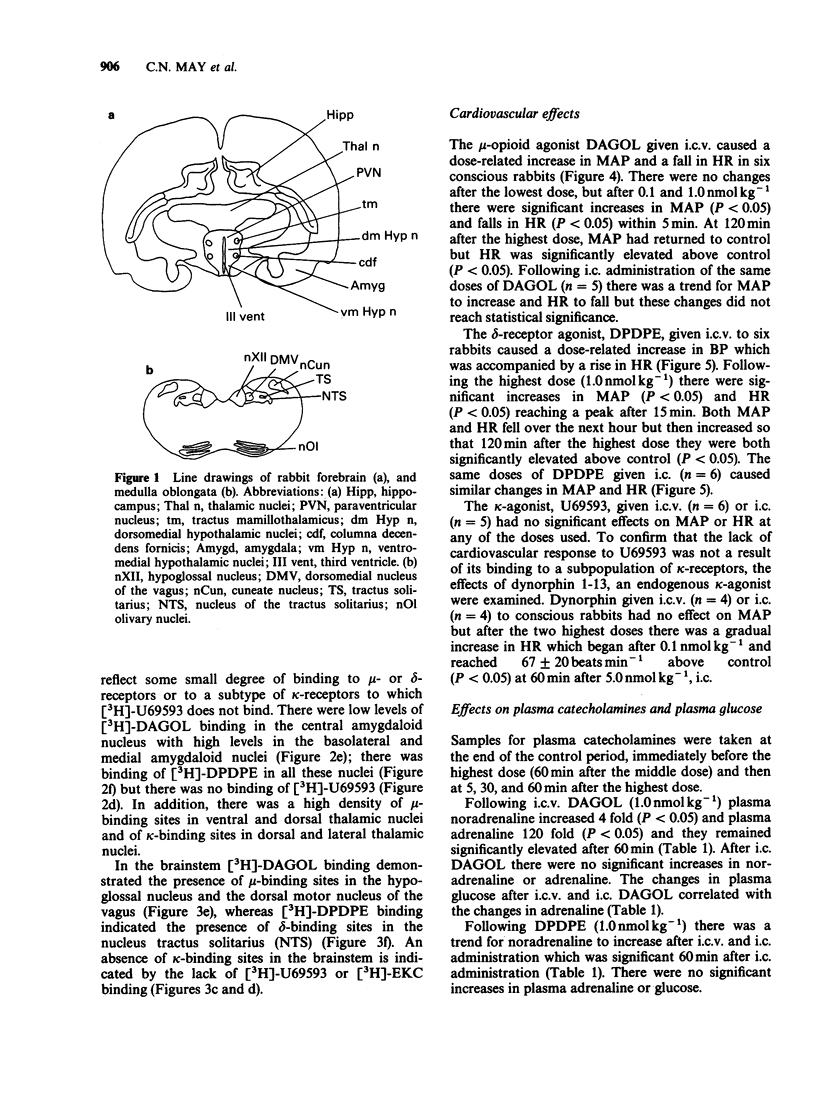
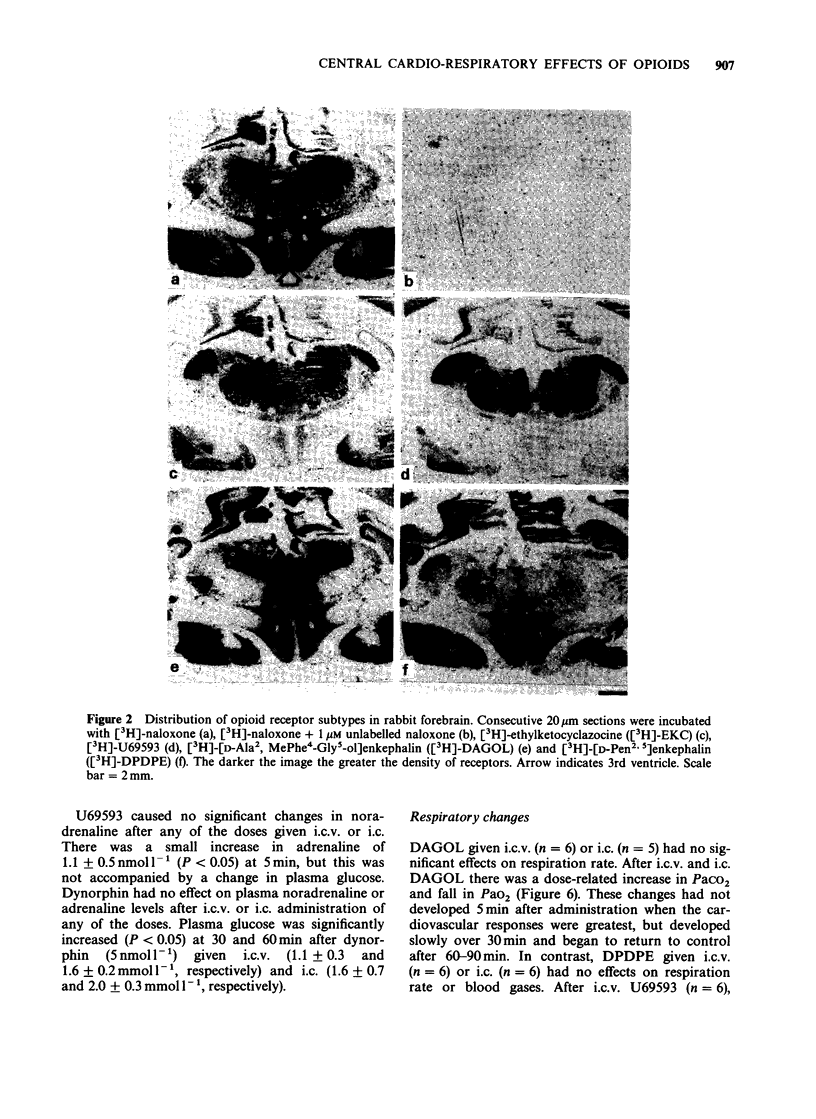
1. The effects of intracerebroventricular (i.c.v.) and intracisternal (i.c.) administration of a range of doses (0.01, 0.1 and 1.0 nmol kg-1) of specific mu- delta- and kappa-opioid agonists on cardiovascular and respiratory function and on plasma catecholamines have been studied in conscious rabbits. The distribution of mu- delta- and kappa-opioid receptors was localized in rabbit brain by in vitro autoradiography. 2. The mu-agonist [D-Ala2, MePhe4-Gly5-ol]enkephalin (DAGOL) given i.c.v. caused a large rise in plasma noradrenaline and adrenaline, hypertension accompanied by an initial bradycardia followed by tachycardia, respiratory depression and sedation. After i.c. administration there were similar changes in heart rate (HR) and respiration, but no significant changes in mean arterial pressure (MAP) or plasma catecholamines. 3. The delta-agonist [D-Pen2.5]enkephalin (DPDPE) increased MAP and HR after both i.c.v. and i.c. administration, caused a small increase in noradrenaline but had no effect on adrenaline and did not alter respiration rate or blood gases. After i.c.v. DPDPE the rabbits became more alert and active. 4. The kappa-agonist U69593 given i.c.v. or i.c. had no effect on MAP or HR. After i.c.v. U69593, PaCO2 fell, but there were no other respiratory effects. The responses to dynorphin 1-13, an endogenous kappa-agonist, were similar to those of U69593. 5. The opioid antagonist naloxone (30 nmol kg-1) given intravenously (i.v.) blocked the effects of i.c.v. DAGOL (1 nmol kg-1). A 100 fold higher dose of i.v. naloxone (3 mumol kg-1) was required to abolish the effects of i.c.v. DPDPE (1 nmol kg-1). 6. Autoradiographic studies demonstrated a high density of mu- and delta-opioid receptors in hypothalamic sites. In the brainstem mu-receptors were demonstrated in the nucleus tractus solitarius (NTS) and delta-receptors in the dorsal motor nucleus of the vagus. kappa-Receptors were not detected in either the hypothalamus or brainstem. 7. These findings demonstrate that DAGOL increases sympatho-adrenal outflow, probably by stimulation of hypothalamic mu-receptors. The effects on HR are probably partly through a baroreflex and partly through an action of DAGOL on mu-receptors in the dorsal motor nucleus of the vagus. DPDPE probably acts on delta-receptors in the NTS to increase MAP and HR. Respiratory depression resulted from stimulation of mu-receptors in the brainstem with no evidence of delta- or kappa-receptors being involved.
Full text
PDF


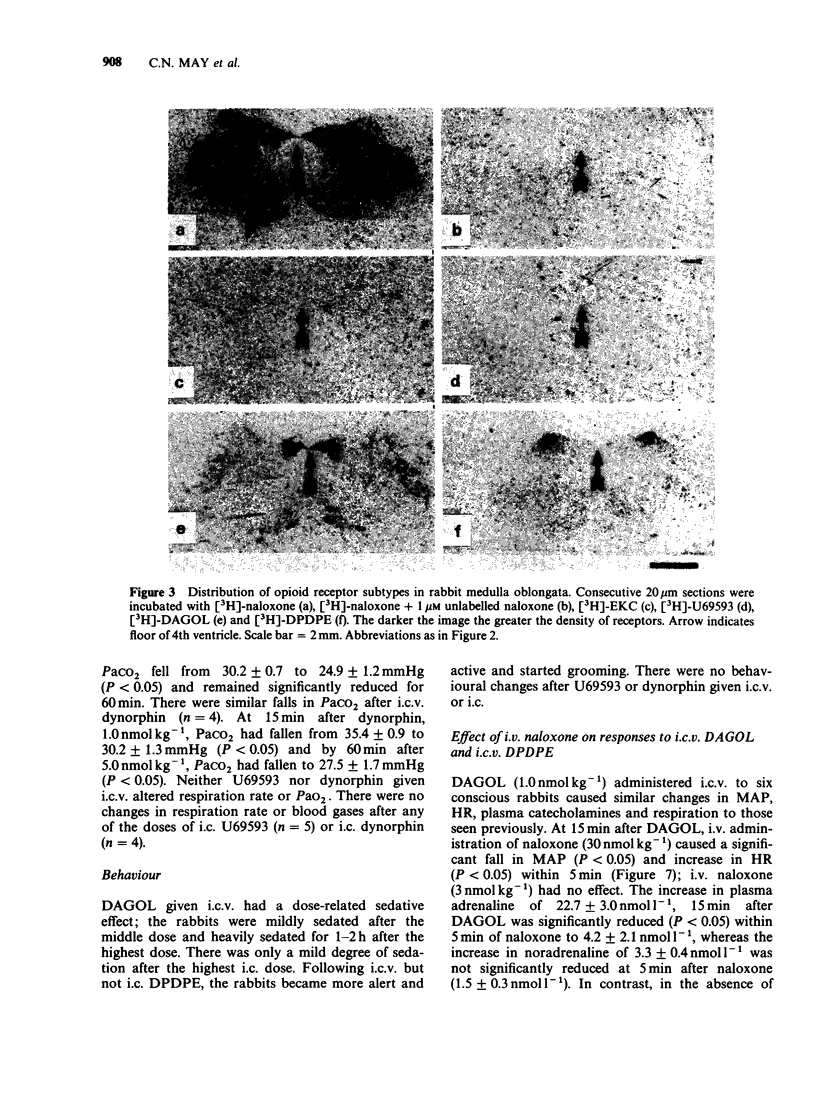

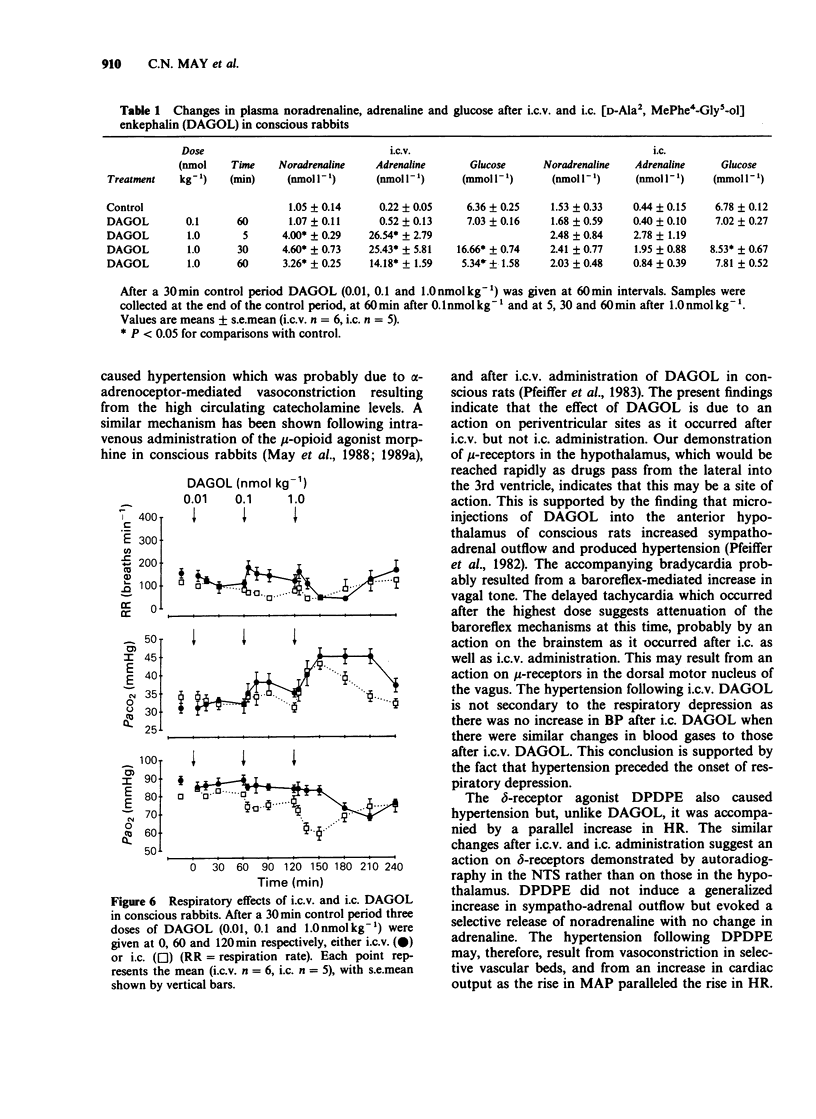
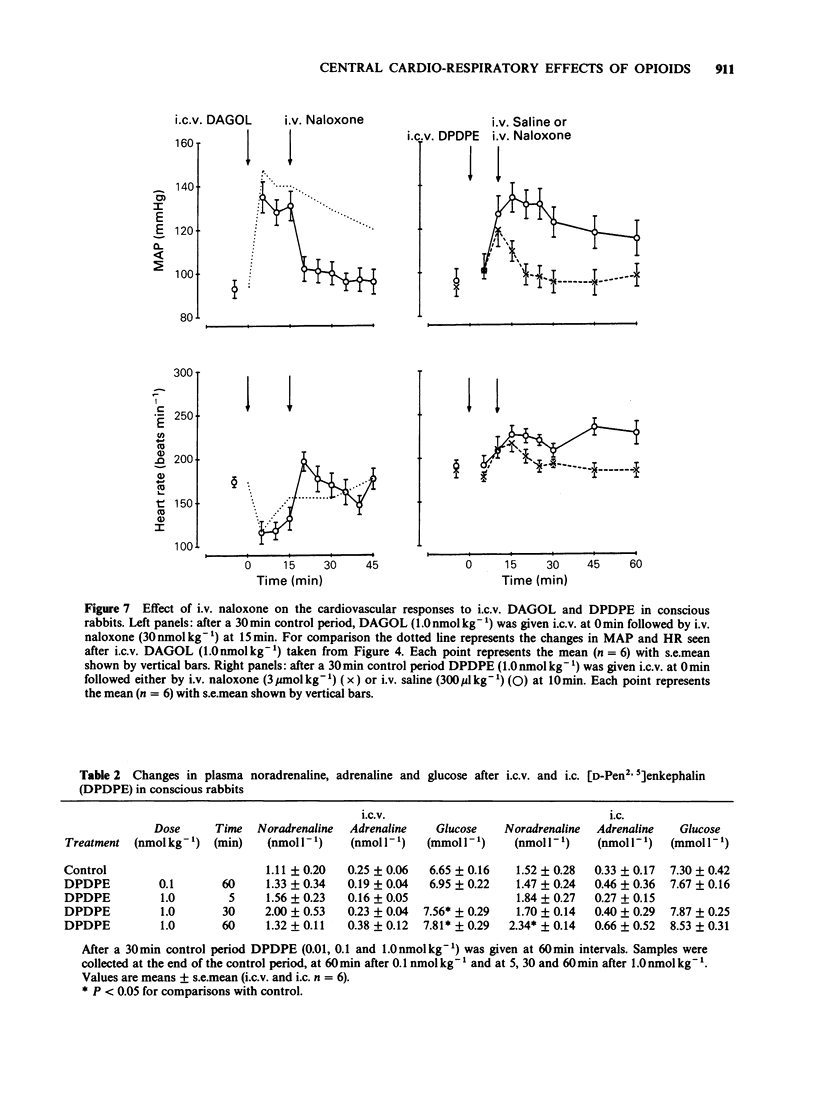


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dashwood M. R., Gilbey M. P., Spyer K. M. The localization of adrenoceptors and opiate receptors in regions of the cat central nervous system involved in cardiovascular control. Neuroscience. 1985 Jun;15(2):537–551. doi: 10.1016/0306-4522(85)90232-5. [DOI] [PubMed] [Google Scholar]
- Dashwood M. R., Muddle J. R., Spyer K. M. Opiate receptor subtypes in the nucleus tractus solitarii of the cat: the effect of vagal section. Eur J Pharmacol. 1988 Oct 11;155(1-2):85–92. doi: 10.1016/0014-2999(88)90405-0. [DOI] [PubMed] [Google Scholar]
- Dashwood M. R., Sykes R. M., Thompson C. S. Autoradiographic localization of opioid receptor types in the rat small intestine. NIDA Res Monogr. 1986;75:315–318. [PubMed] [Google Scholar]
- Gillan M. G., Kosterlitz H. W. Spectrum of the mu, delta- and kappa-binding sites in homogenates of rat brain. Br J Pharmacol. 1982 Nov;77(3):461–469. doi: 10.1111/j.1476-5381.1982.tb09319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H., Kuhar M. J., Young W. S., 3rd Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulya K., Gehlert D. R., Wamsley J. K., Mosberg H. I., Hruby V. J., Duckles S. P., Yamamura H. I. Autoradiographic localization of delta opioid receptors in the rat brain using a highly selective bis-penicillamine cyclic enkephalin analog. Eur J Pharmacol. 1985 May 8;111(2):285–286. doi: 10.1016/0014-2999(85)90770-8. [DOI] [PubMed] [Google Scholar]
- Hassen A. H., Feuerstein G., Faden A. I. Kappa opioid receptors modulate cardiorespiratory function in hindbrain nuclei of rat. J Neurosci. 1984 Sep;4(9):2213–2221. doi: 10.1523/JNEUROSCI.04-09-02213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head G. A., Korner P. I., Lewis S. L., Badoer E. Contribution of noradrenergic and serotonergic neurons to the circulatory effects of centrally acting clonidine and alpha-methyldopa in rabbits. J Cardiovasc Pharmacol. 1983 Nov-Dec;5(6):945–953. doi: 10.1097/00005344-198311000-00006. [DOI] [PubMed] [Google Scholar]
- Holaday J. W. Cardiovascular effects of endogenous opiate systems. Annu Rev Pharmacol Toxicol. 1983;23:541–594. doi: 10.1146/annurev.pa.23.040183.002545. [DOI] [PubMed] [Google Scholar]
- Lahti R. A., Mickelson M. M., McCall J. M., Von Voigtlander P. F. [3H]U-69593 a highly selective ligand for the opioid kappa receptor. Eur J Pharmacol. 1985 Feb 26;109(2):281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- Ling G. S., Spiegel K., Nishimura S. L., Pasternak G. W. Dissociation of morphine's analgesic and respiratory depressant actions. Eur J Pharmacol. 1983 Jan 21;86(3-4):487–488. doi: 10.1016/0014-2999(83)90203-0. [DOI] [PubMed] [Google Scholar]
- May C. N., Ham I. W., Heslop K. E., Stone F. A., Mathias C. J. Intravenous morphine causes hypertension, hyperglycaemia and increases sympatho-adrenal outflow in conscious rabbits. Clin Sci (Lond) 1988 Jul;75(1):71–77. doi: 10.1042/cs0750071. [DOI] [PubMed] [Google Scholar]
- May C. N., Whitehead C. J., Dashwood M. R., Mathias C. J. Investigation of the central sites at which morphine acts to cause hypertension in conscious rabbits. Br J Pharmacol. 1989 Jul;97(3):873–881. doi: 10.1111/j.1476-5381.1989.tb12027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C. N., Whitehead C. J., Heslop K. E., Mathias C. J. Evidence that intravenous morphine stimulates central opiate receptors to increase sympatho-adrenal outflow and cause hypertension in conscious rabbits. Clin Sci (Lond) 1989 Apr;76(4):431–437. doi: 10.1042/cs0760431. [DOI] [PubMed] [Google Scholar]
- Mosberg H. I., Hurst R., Hruby V. J., Gee K., Yamamura H. I., Galligan J. J., Burks T. F. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo J. R., Urdín M. C., Borrell J., Fuentes J. A. Evidence for a central but not adrenal, opioid mediation in hypertension induced by brief isolation in the rat. Life Sci. 1986 May 26;38(21):1923–1930. doi: 10.1016/0024-3205(86)90221-3. [DOI] [PubMed] [Google Scholar]
- Pazos A., Flórez J. A comparative study in rats of the respiratory depression and analgesia induced by mu- and delta-opioid agonists. Eur J Pharmacol. 1984 Mar 16;99(1):15–21. doi: 10.1016/0014-2999(84)90427-8. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Feuerstein G., Faden A., Kopin I. J. Evidence for an involvement of mu-, but not delta- or kappa-opiate receptors in sympathetically and parasympathetically mediated cardiovascular responses to opiates upon anterior hypothalamic injection. Life Sci. 1982 Sep 20;31(12-13):1279–1282. doi: 10.1016/0024-3205(82)90361-7. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Feuerstein G., Zerbe R. L., Faden A. I., Kopin I. J. Mu-receptors mediate opioid cardiovascular effects at anterior hypothalamic sites through sympatho-adrenomedullary and parasympathetic pathways. Endocrinology. 1983 Sep;113(3):929–938. doi: 10.1210/endo-113-3-929. [DOI] [PubMed] [Google Scholar]
- Schaz K., Stock G., Simon W., Schlör K. H., Unger T., Rockhold R., Ganten D. Enkephalin effects on blood pressure, heart rate, and baroreceptor reflex. Hypertension. 1980 Jul-Aug;2(4):395–407. doi: 10.1161/01.hyp.2.4.395. [DOI] [PubMed] [Google Scholar]
- Szilagyi J. E., Chelly J., Doursout M. F. Suppression of renin release by antagonism of endogenous opiates in the dog. Am J Physiol. 1986 Apr;250(4 Pt 2):R633–R637. doi: 10.1152/ajpregu.1986.250.4.R633. [DOI] [PubMed] [Google Scholar]
- Van Loon G. R., Appel N. M., Ho D. beta-Endorphin-induced stimulation of central sympathetic outflow: beta-endorphin increases plasma concentrations of epinephrine, norepinephrine, and dopamine in rats. Endocrinology. 1981 Jul;109(1):46–53. doi: 10.1210/endo-109-1-46. [DOI] [PubMed] [Google Scholar]
- Wexler B. C. Naloxone ameliorates the pathophysiologic changes which lead to and attend an acute stroke in stroke-prone/SHR. Stroke. 1984 Jul-Aug;15(4):630–634. doi: 10.1161/01.str.15.4.630. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Kuhar M. J. A new method for receptor autoradiography: [3H]opioid receptors in rat brain. Brain Res. 1979 Dec 28;179(2):255–270. doi: 10.1016/0006-8993(79)90442-6. [DOI] [PubMed] [Google Scholar]