Abstract
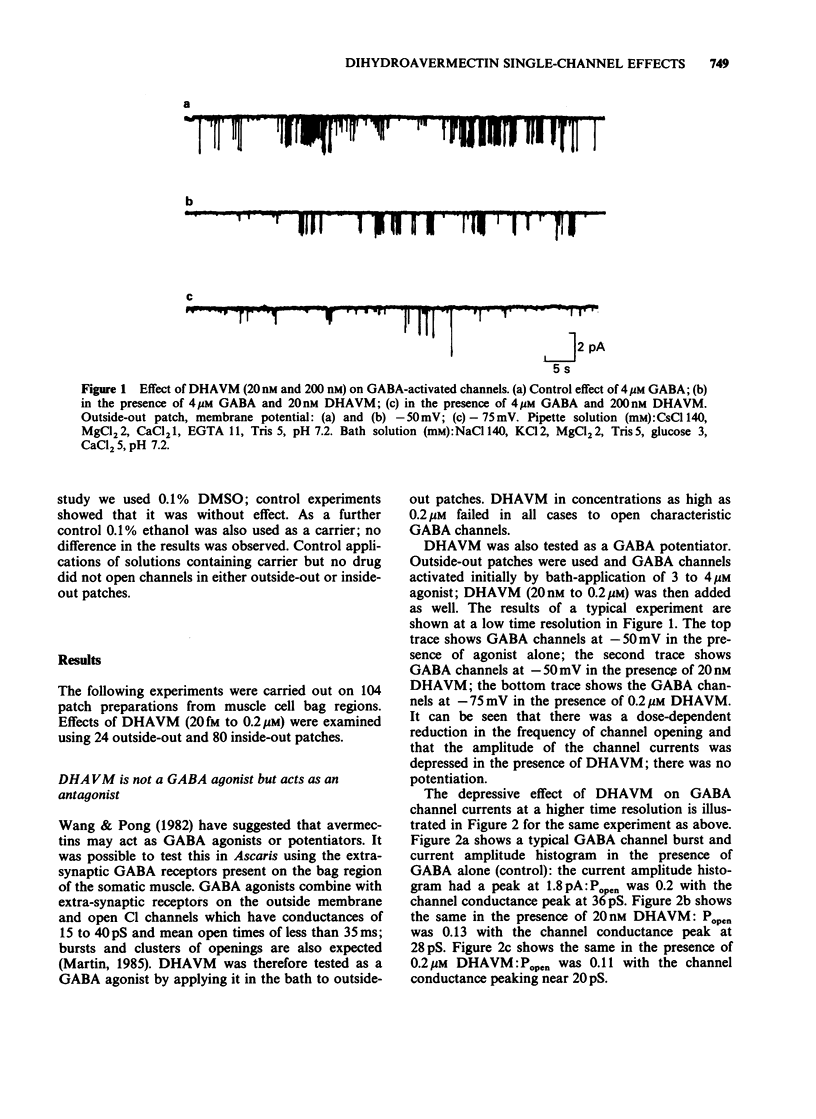
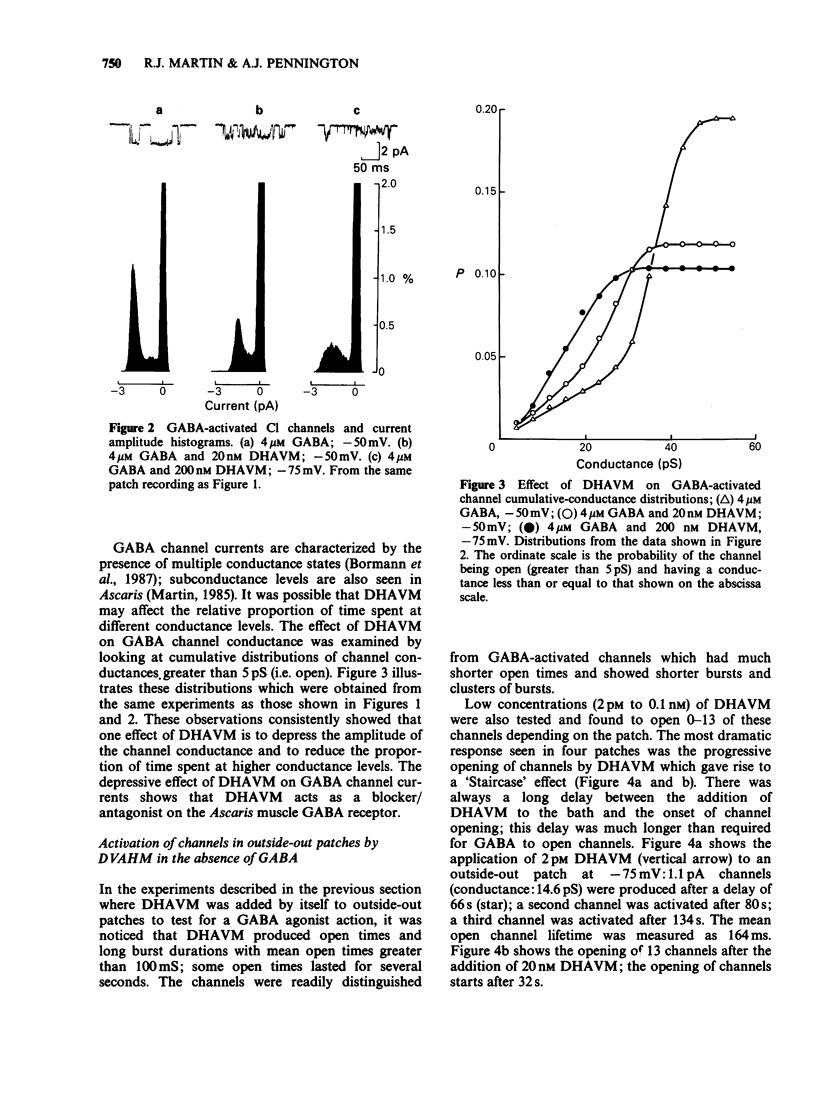
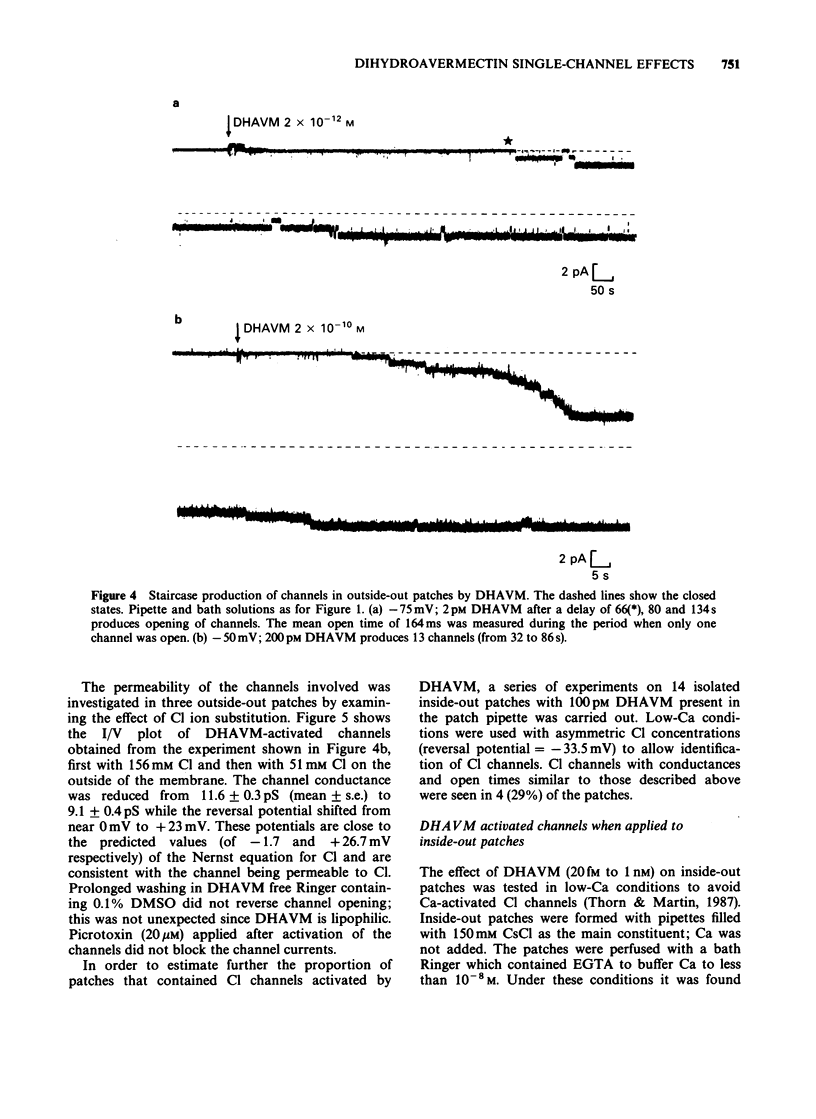
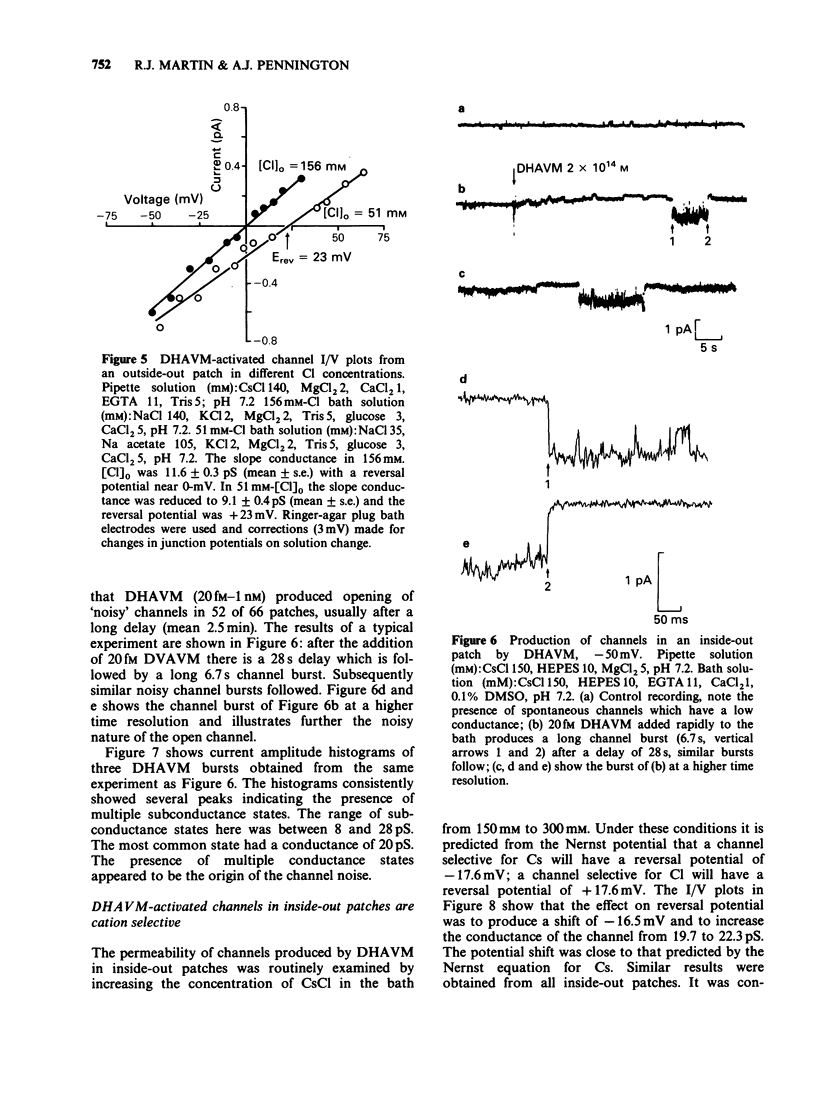
1. Effects of bath-application of the anti-parasitic agent 22,23-dihydroavermectin B1a (DHAVM 20 fM to 0.2 microM) on outside-out and inside-out patches from collagenase-treated muscle membranes of Ascaris suum were examined. 2. DHAVM was tested as a gamma-aminobutyric acid (GABA) agonist by application to outside-out patches. DHAVM failed to open characteristic GABA channels even when high concentrations (20 nM to 0.2 microM) were used. These high concentrations of DHAVM also failed to potentiate opening of channels activated by application of 3-4 microM GABA; instead they acted to depress GABA-activated channel currents by reducing mean conductances and P open. 3. In outside-out patches, low concentrations of DHAVM (1 pM to 100 pM) produced opening of 0-13 channels, the number depending on the patch. The progressive opening of channels gave rise to a 'Staircase' effect. The conductance of these channels was 9-15 pS and open times were long (greater than 100 mS). Ion substitution experiments showed these channels to be permeable to Cl. The channels were not blocked by 20 microM picrotoxin. There was a long delay (greater than 15 s) between DHAVM application and channel opening; this delay and lipophilic nature of DHAVM suggested a site of action in the lipid phase of the membrane. 4. The effect of bath-application of low concentrations of DHAVM on inside-out patches was investigated under low-Ca conditions (to avoid Ca-activated Cl channels). DHAVM (20 fM to 1 nM) did not produce opening of Cl channels but produced opening after a long delay (mean 2.5 minutes) of noisy cation-selective channels which had conductances of 5-30 pS. 5. The actions of DHAVM are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg R. W., Miller B. M., Baker E. E., Birnbaum J., Currie S. A., Hartman R., Kong Y. L., Monaghan R. L., Olson G., Putter I. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob Agents Chemother. 1979 Mar;15(3):361–367. doi: 10.1128/aac.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. C., Benz G. W. Ivermectin: a review of efficacy and safety. J Vet Pharmacol Ther. 1984 Mar;7(1):1–16. doi: 10.1111/j.1365-2885.1984.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Duce I. R., Scott R. H. Actions of dihydroavermectin B1a on insect muscle. Br J Pharmacol. 1985 Jun;85(2):395–401. doi: 10.1111/j.1476-5381.1985.tb08874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton J. R., Ostlind D. A., Blair L. S., Eary C. H., Suhayda D., Cifelli S., Riek R. F., Campbell W. C. Avermectins, new family of potent anthelmintic agents: efficacy of the B1a component. Antimicrob Agents Chemother. 1979 Mar;15(3):372–378. doi: 10.1128/aac.15.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz L. C., Wang C. C., Gorio A. Avermectin B1a irreversibly blocks postsynaptic potentials at the lobster neuromuscular junction by reducing muscle membrane resistance. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2062–2066. doi: 10.1073/pnas.76.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass I. S., Stretton A. O., Wang C. C. The effects of avermectin and drugs related to acetylcholine and 4-aminobutyric acid on neurotransmission in Ascaris suum. Mol Biochem Parasitol. 1984 Oct;13(2):213–225. doi: 10.1016/0166-6851(84)90114-2. [DOI] [PubMed] [Google Scholar]
- Lees G., Beadle D. J. Dihydroavermectin B1: actions on cultured neurones from the insect central nervous system. Brain Res. 1986 Feb 26;366(1-2):369–372. doi: 10.1016/0006-8993(86)91321-1. [DOI] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J. The effect of gamma-aminobutyric acid on the input conductance and membrane potential of Ascaris muscle. Br J Pharmacol. 1980;71(1):99–106. doi: 10.1111/j.1476-5381.1980.tb10914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J. gamma-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Br J Pharmacol. 1985 Feb;84(2):445–461. doi: 10.1111/j.1476-5381.1985.tb12929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P., Martin R. J. A high-conductance calcium-dependent chloride channel in Ascaris suum muscle. Q J Exp Physiol. 1987 Jan;72(1):31–49. doi: 10.1113/expphysiol.1987.sp003053. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Pong S. S. Actions of avermectin B1a on GABA nerves. Prog Clin Biol Res. 1982;97:373–395. [PubMed] [Google Scholar]
- Williams M., Yarbrough G. G. Enhancement of in vitro binding and some of the pharmacological properties of diazepam by a novel anthelmintic agent, Avermectin B1a. Eur J Pharmacol. 1979 Jun 15;56(3):273–276. doi: 10.1016/0014-2999(79)90183-3. [DOI] [PubMed] [Google Scholar]


