Abstract
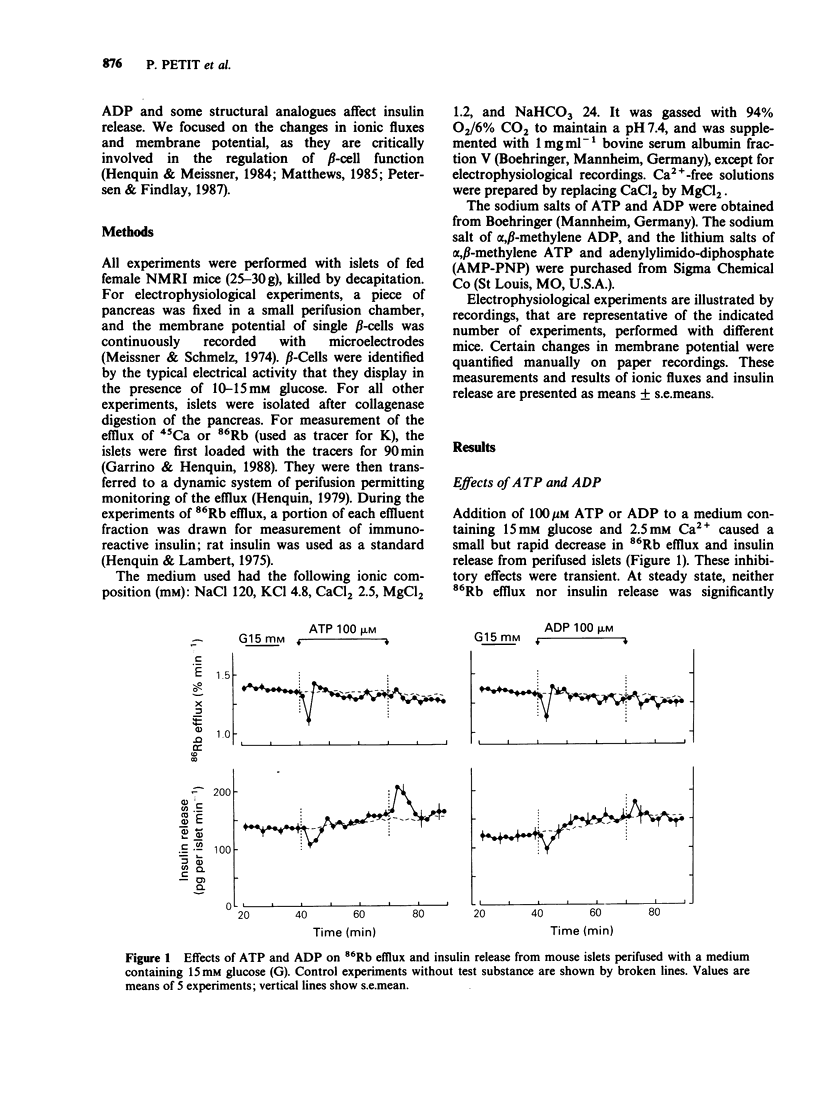
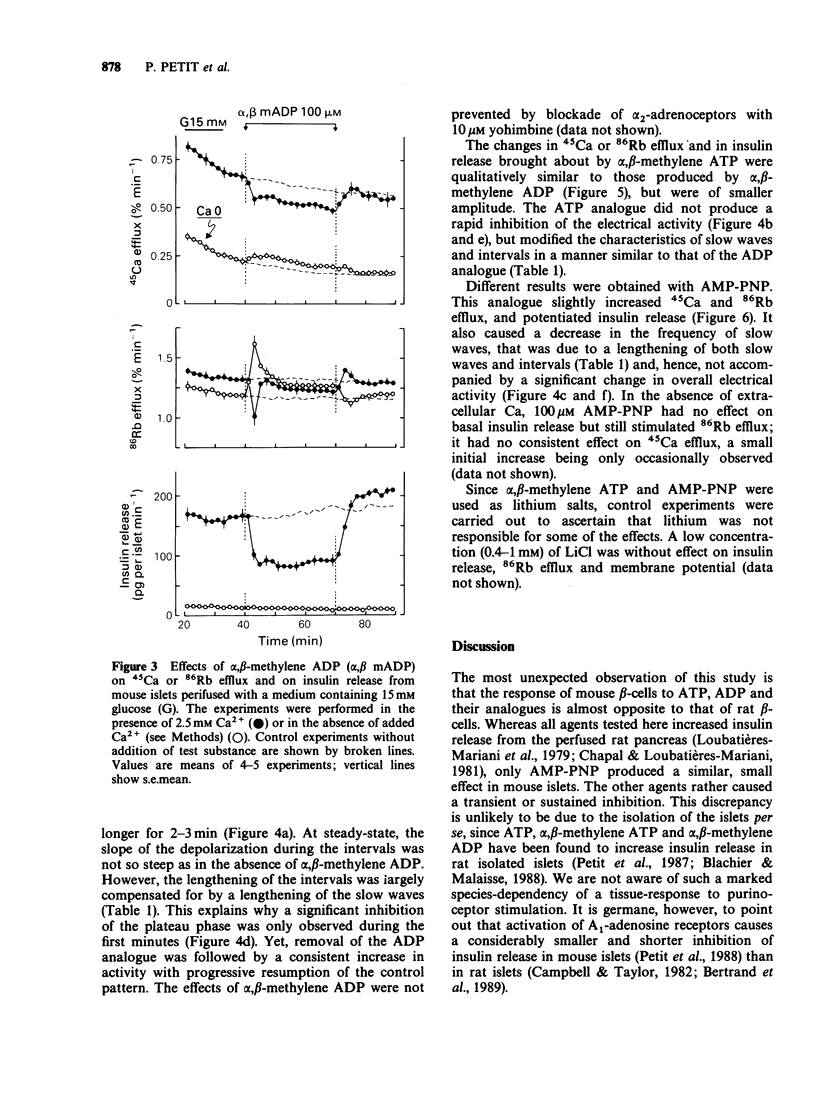
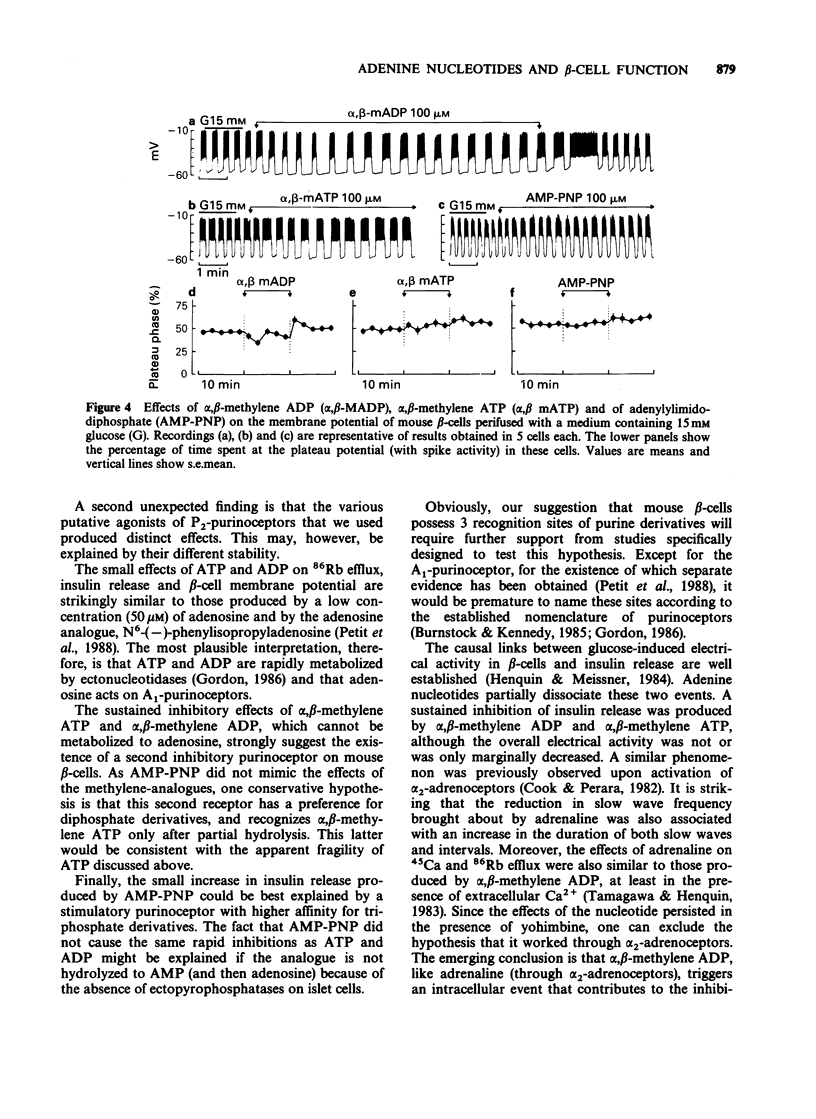
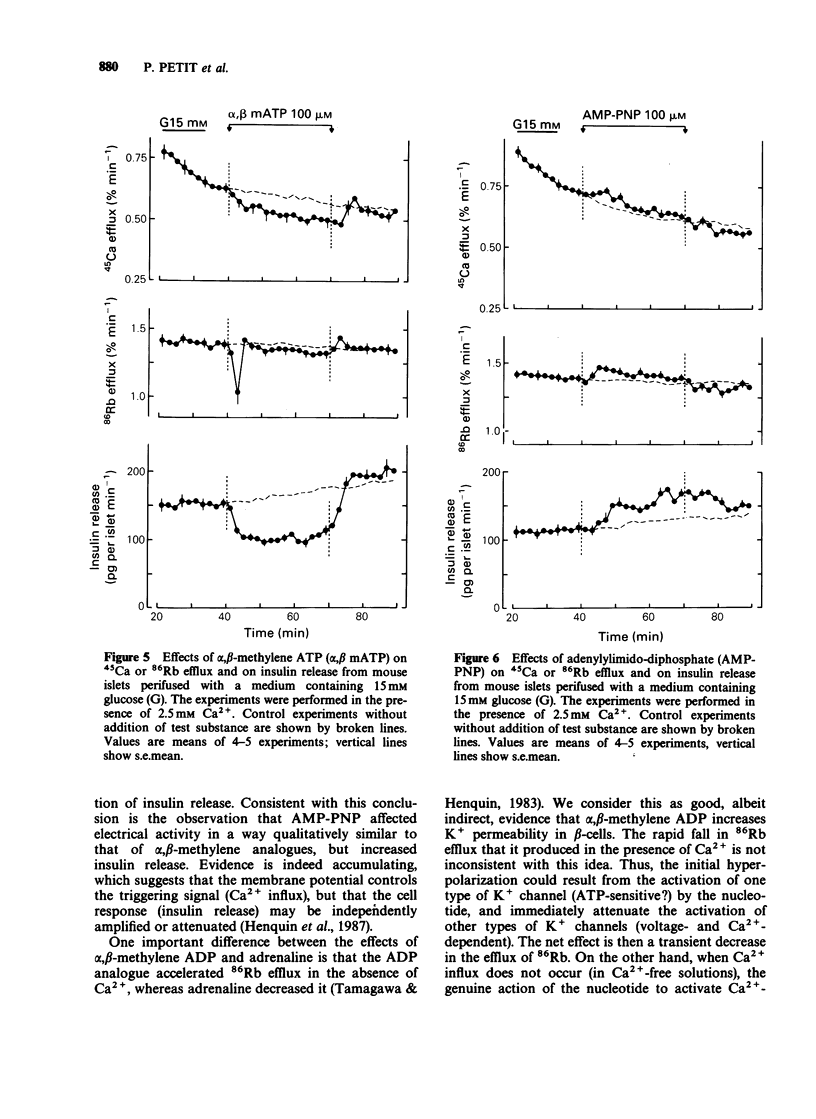
1. The mechanisms whereby extracellular adenine nucleotides modulate pancreatic beta-cell function were studied with mouse islets stimulated by 15 mM glucose. 2. Adenosine 5'-triphosphate (ATP) and adenosine 5'-diphosphate (ADP) (100 microM) inhibited insulin release, 45Ca efflux and 86Rb efflux from islet cells, and decreased electrical activity in beta-cells. These changes were rapid but small and transient. 3. alpha,beta-Methylene ADP caused a rapid and sustained inhibition of insulin release, 45Ca efflux and 86Rb efflux from islet cells. It also produced a slight hyperpolarization of the beta-cell membrane, with sustained modification of the pattern but only transient decrease of the intensity of the electrical activity. In the absence of extracellular Ca2+, alpha,beta-methylene ADP increased 45Ca and 86Rb efflux without changing insulin release. Most effects of alpha,beta-methylene ATP were qualitatively similar but quantitatively smaller than those of the ADP-analogue. 4. Adenylylimido-diphosphate (AMP-PNP) slightly increased 45Ca and 86Rb efflux and potentiated insulin release in the presence of extracellular Ca2+. However, its effects on electrical activity in beta-cells were qualitatively similar to those of the alpha,beta-methylene analogues. 5. The small effects of ATP and ADP could result from their degradation into adenosine. alpha,beta-Methylene ADP appears to increase K+ permeability of the beta-cell membrane and to produce a second, intracellular, effect which largely contributes to the inhibition of insulin release. Another recognition site, with higher affinity for triphosphate derivatives, could mediate the small stimulatory effects of AMP-PNP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand G., Chapal J., Loubatieres-Mariani M. M. Potentiating synergism between adenosine diphosphate or triphosphate and acetylcholine on insulin secretion. Am J Physiol. 1986 Oct;251(4 Pt 1):E416–E421. doi: 10.1152/ajpendo.1986.251.4.E416. [DOI] [PubMed] [Google Scholar]
- Bertrand G., Chapal J., Loubatières-Mariani M. M., Roye M. Evidence for two different P2-purinoceptors on beta cell and pancreatic vascular bed. Br J Pharmacol. 1987 Aug;91(4):783–787. doi: 10.1111/j.1476-5381.1987.tb11276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand G., Nenquin M., Henquin J. C. Comparison of the inhibition of insulin release by activation of adenosine and alpha 2-adrenergic receptors in rat beta-cells. Biochem J. 1989 Apr 1;259(1):223–228. doi: 10.1042/bj2590223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachier F., Malaisse W. J. Effect of exogenous ATP upon inositol phosphate production, cationic fluxes and insulin release in pancreatic islet cells. Biochim Biophys Acta. 1988 Jun 30;970(2):222–229. doi: 10.1016/0167-4889(88)90182-6. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Campbell I. L., Taylor K. W. Effects of adenosine, 2-deoxyadenosine and N6-phenylisopropyladenosine on rat islet function and metabolism. Biochem J. 1982 Jun 15;204(3):689–696. doi: 10.1042/bj2040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapal J., Loubatieres-Mariani M. M. Attempt to antagonized the stimulatory effect or ATP on insulin secretion. Eur J Pharmacol. 1981 Sep 11;74(2-3):127–134. doi: 10.1016/0014-2999(81)90522-7. [DOI] [PubMed] [Google Scholar]
- Chapal J., Loubatieres-Mariani M. M. Effects of phosphate-modified adenine nucleotide analogues on insulin secretion from perfused rat pancreas. Br J Pharmacol. 1981 May;73(1):105–110. doi: 10.1111/j.1476-5381.1981.tb16778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Cook D. L., Perara E. Islet electrical pacemaker response to alpha-adrenergic stimulation. Diabetes. 1982 Nov;31(11):985–990. doi: 10.2337/diacare.31.11.985. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R. Extracellular ATP activates polyphosphoinositide breakdown and Ca2+ mobilization in Ehrlich ascites tumor cells. Arch Biochem Biophys. 1986 Feb 15;245(1):84–95. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- Feldman J. M., Jackson T. B. Specificity of nucleotide-induced insulin secretion. Endocrinology. 1974 Feb;94(2):388–394. doi: 10.1210/endo-94-2-388. [DOI] [PubMed] [Google Scholar]
- Gallacher D. V. Are there purinergic receptors on parotid acinar cells? Nature. 1982 Mar 4;296(5852):83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- Garcia M. C., Hermans M. P., Henquin J. C. Glucose-, calcium- and concentration-dependence of acetylcholine stimulation of insulin release and ionic fluxes in mouse islets. Biochem J. 1988 Aug 15;254(1):211–218. doi: 10.1042/bj2540211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrino M. G., Henquin J. C. Highly potent and stereoselective effects of the benzoic acid derivative AZ-DF 265 on pancreatic beta-cells. Br J Pharmacol. 1988 Jan;93(1):61–68. doi: 10.1111/j.1476-5381.1988.tb11405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Endothelium-dependent relaxation of the pig aorta: relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br J Pharmacol. 1983 Jun;79(2):531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylfe E., Hellman B. External ATP mimics carbachol in initiating calcium mobilization from pancreatic beta-cells conditioned by previous exposure to glucose. Br J Pharmacol. 1987 Oct;92(2):281–289. doi: 10.1111/j.1476-5381.1987.tb11322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Bozem M., Schmeer W., Nenquin M. Distinct mechanisms for two amplification systems of insulin release. Biochem J. 1987 Sep 1;246(2):393–399. doi: 10.1042/bj2460393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Lambert A. E. Cobalt inhibition of insulin secretion and calcium uptake by isolated rat islets. Am J Physiol. 1975 Jun;228(6):1669–1677. doi: 10.1152/ajplegacy.1975.228.6.1669. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Opposite effects of intracellular Ca2+ and glucose on K+ permeability of pancreatic islet cells. Nature. 1979 Jul 5;280(5717):66–68. doi: 10.1038/280066a0. [DOI] [PubMed] [Google Scholar]
- Leitner J. W., Sussman K. E., Vatter A. E., Schneider F. H. Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology. 1975 Mar;96(3):662–677. doi: 10.1210/endo-96-3-662. [DOI] [PubMed] [Google Scholar]
- Loubatieres-Mariani M. M., Chapal J., Lignon F., Valette G. Structural specificity of nucleotides for insulin secretory action from the isolated perfused rat pancreas. Eur J Pharmacol. 1979 Nov 16;59(3-4):277–286. doi: 10.1016/0014-2999(79)90291-7. [DOI] [PubMed] [Google Scholar]
- Loubatieres-Mariani M. M., Chapal J. Purinergic receptors involved in the stimulation of insulin and glucagon secretion. Diabete Metab. 1988 Mar-Apr;14(2):119–126. [PubMed] [Google Scholar]
- Loubatiéres A. L., Loubatiéres-Mariani M. M., Chapal J. Adénosine triphosphate (ATP), adénosine 3'5' monophosphate syclique (3'5' AMP c) et sécrétion d'insuline. C R Seances Soc Biol Fil. 1972;166(12):1742–1746. [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I. Electrophysiology of the pancreas. Physiol Rev. 1987 Jul;67(3):1054–1116. doi: 10.1152/physrev.1987.67.3.1054. [DOI] [PubMed] [Google Scholar]
- Petit P., Manteghetti M., Puech R., Loubatieres-Mariani M. M. ATP and phosphate-modified adenine nucleotide analogues. Effects on insulin secretion and calcium uptake. Biochem Pharmacol. 1987 Feb 1;36(3):377–380. doi: 10.1016/0006-2952(87)90297-8. [DOI] [PubMed] [Google Scholar]
- RODRIGUE-CANDELA J. L., MARTIN-HERNANDEZ D., CASTILLA-CORTAZAR T. Stimulation of insulin secretion in vitro by adenosine triphosphate. Nature. 1963 Mar 30;197:1304–1304. doi: 10.1038/1971304a0. [DOI] [PubMed] [Google Scholar]
- Richardson P. J., Brown S. J., Bailyes E. M., Luzio J. P. Ectoenzymes control adenosine modulation of immunoisolated cholinergic synapses. Nature. 1987 May 21;327(6119):232–234. doi: 10.1038/327232a0. [DOI] [PubMed] [Google Scholar]
- Tamagawa T., Henquin J. C. Epinephrine modifications of insulin release and of 86Rb+ or 45Ca2+ fluxes in rat islets. Am J Physiol. 1983 Mar;244(3):E245–E252. doi: 10.1152/ajpendo.1983.244.3.E245. [DOI] [PubMed] [Google Scholar]
- White T. D. Role of adenine compounds in autonomic neurotransmission. Pharmacol Ther. 1988;38(2):129–168. doi: 10.1016/0163-7258(88)90095-2. [DOI] [PubMed] [Google Scholar]


