Abstract
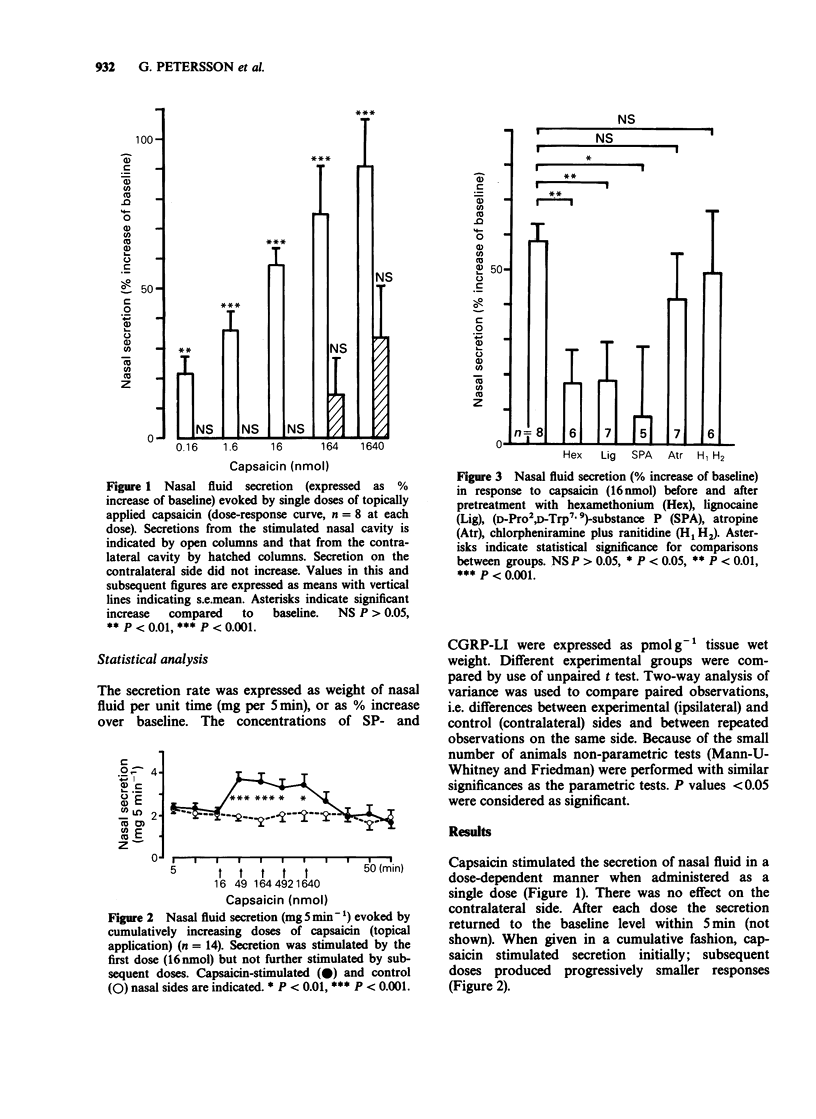
1. The secretion of nasal fluid was studied in anaesthetized rats after topical application of capsaicin, and of calcitonin gene-related peptide (CGRP) alone or CGRP in combination with substance P (SP). The flow of nasal fluid was stimulated and the secretions collected by a filter paper technique. The concentrations of SP and CGRP in nasal biopsies were determined after topical or systemic administration of capsaicin. 2. Capsaicin (single dose administration) stimulated nasal secretion in a dose-dependent manner. The effect was inhibited by hexamethonium, lignocaine, or by the tachykinin antagonist (D-Pro2, D-Trp7,9)-SP, but not by atropine, or by a combination of the histamine H1-receptor antagonist chlorpheniramine and the H2-receptor antagonist ranitidine. 3. When applied cumulatively, capsaicin rapidly produced desensitization. The concentrations of SP and CGRP in the nasal mucosa were reduced by capsaicin 6 days after topical or s.c. administration but not 15 min after topical application of desensitizing doses. 4. CGRP did not stimulate the secretion of nasal fluid and did not alter SP-evoked nasal secretion. 5. The inhibition by hexamethonium of the capsaicin-evoked nasal secretion suggests the involvement of ganglionic reflexes. In addition, the inhibition of the response to capsaicin by (D-Pro2,D-Trp7,9)-SP and lidocaine and the depletion of SP and CGRP after capsaicin indicate the involvement of tachykinin-mediated axon reflexes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beding-Barnekow B., Brodin E., Håkanson R. Substance P, neurokinin A and neurokinin B in the ocular response to injury in the rabbit. Br J Pharmacol. 1988 Sep;95(1):259–267. doi: 10.1111/j.1476-5381.1988.tb16572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br J Pharmacol. 1985 Dec;86(4):855–860. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J. Substance P regulates the vasodilator activity of calcitonin gene-related peptide. Nature. 1988 Sep 1;335(6185):73–75. doi: 10.1038/335073a0. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A., Henke H., Lundberg J. M., Petermann J. B., Hökfelt T., Fischer J. A. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987 Mar-Apr;8(2):399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
- Gamse R., Leeman S. E., Holzer P., Lembeck F. Differential effects of capsaicin on the content of somatostatin, substance P, and neurotensin in the nervous system of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1981 Sep;317(2):140–148. doi: 10.1007/BF00500070. [DOI] [PubMed] [Google Scholar]
- Gamse R., Molnar A., Lembeck F. Substance P release from spinal cord slices by capsaicin. Life Sci. 1979 Aug 13;25(7):629–636. doi: 10.1016/0024-3205(79)90558-7. [DOI] [PubMed] [Google Scholar]
- Gamse R., Saria A. Potentiation of tachykinin-induced plasma protein extravasation by calcitonin gene-related peptide. Eur J Pharmacol. 1985 Aug 7;114(1):61–66. doi: 10.1016/0014-2999(85)90520-5. [DOI] [PubMed] [Google Scholar]
- Geppetti P., Fusco B. M., Marabini S., Maggi C. A., Fanciullacci M., Sicuteri F. Secretion, pain and sneezing induced by the application of capsaicin to the nasal mucosa in man. Br J Pharmacol. 1988 Mar;93(3):509–514. doi: 10.1111/j.1476-5381.1988.tb10305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunditz T., Ekman R., Håkanson R., Rerup C., Sundler F., Uddman R. Calcitonin gene-related peptide in thyroid nerve fibers and C cells: effects on thyroid hormone secretion and response to hypercalcemia. Endocrinology. 1986 Nov;119(5):2313–2324. doi: 10.1210/endo-119-5-2313. [DOI] [PubMed] [Google Scholar]
- Håkanson R., Beding B., Ekman R., Heilig M., Wahlestedt C., Sundler F. Multiple tachykinin pools in sensory nerve fibres in the rabbit iris. Neuroscience. 1987 Jun;21(3):943–950. doi: 10.1016/0306-4522(87)90049-2. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L., Cuello A. C. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978 Aug 18;152(1):183–188. doi: 10.1016/0006-8993(78)90146-4. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Release of histamine from mast cells by vasoactive peptides. Proc Soc Exp Biol Med. 1973 Apr;142(4):1252–1256. doi: 10.3181/00379727-142-37219. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J. Time course of capsaicin-induced functional impairments in comparison with changes in neuronal substance P content. Naunyn Schmiedebergs Arch Pharmacol. 1981 Jun;316(3):240–243. doi: 10.1007/BF00505656. [DOI] [PubMed] [Google Scholar]
- Lindberg S., Dolata J., Mercke U. Effects of neurokinin A and calcitonin gene-related peptide on mucociliary activity in rabbit maxillary sinus. Regul Pept. 1986 Dec 1;16(1):15–25. doi: 10.1016/0167-0115(86)90191-6. [DOI] [PubMed] [Google Scholar]
- Lindberg S., Mercke U. Capsaicin stimulates mucociliary activity by releasing substance P and acetylcholine. Eur J Respir Dis. 1986 Feb;68(2):96–106. [PubMed] [Google Scholar]
- Lundberg J. M., Brodin E., Saria A. Effects and distribution of vagal capsaicin-sensitive substance P neurons with special reference to the trachea and lungs. Acta Physiol Scand. 1983 Nov;119(3):243–252. doi: 10.1111/j.1748-1716.1983.tb07334.x. [DOI] [PubMed] [Google Scholar]
- Lundblad L., Brodin E., Lundberg J. M., Anggård A. Effects of nasal capsaicin pretreatment and cryosurgery on sneezing reflexes, neurogenic plasma extravasation, sensory and sympathetic neurons. Acta Otolaryngol. 1985 Jul-Aug;100(1-2):117–127. doi: 10.3109/00016488509108596. [DOI] [PubMed] [Google Scholar]
- Lundblad L., Lundberg J. M., Anggård A. Local and systemic capsaicin pretreatment inhibits sneezing and the increase in nasal vascular permeability induced by certain chemical irritants. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jun;326(3):254–261. doi: 10.1007/BF00505327. [DOI] [PubMed] [Google Scholar]
- Lundblad L., Lundberg J. M., Brodin E., Anggård A. Origin and distribution of capsaicin-sensitive substance P-immunoreactive nerves in the nasal mucosa. Acta Otolaryngol. 1983 Nov-Dec;96(5-6):485–493. doi: 10.3109/00016488309132735. [DOI] [PubMed] [Google Scholar]
- Martling C. R., Anggård A., Lundberg J. M. Non-cholinergic vasodilation in the tracheobronchial tree of the cat induced by vagal nerve stimulation. Acta Physiol Scand. 1985 Oct;125(2):343–346. doi: 10.1111/j.1748-1716.1985.tb07726.x. [DOI] [PubMed] [Google Scholar]
- Martling C. R., Saria A., Fischer J. A., Hökfelt T., Lundberg J. M. Calcitonin gene-related peptide and the lung: neuronal coexistence with substance P, release by capsaicin and vasodilatory effect. Regul Pept. 1988 Feb;20(2):125–139. doi: 10.1016/0167-0115(88)90046-8. [DOI] [PubMed] [Google Scholar]
- Petersson G., Malm L., Rosengren E., Håkanson R. Hyperosmolarity but not histamine evokes secretion of nasal fluid in the rat. Eur J Pharmacol. 1989 Feb 14;161(1):37–43. doi: 10.1016/0014-2999(89)90177-5. [DOI] [PubMed] [Google Scholar]
- Piotrowski W., Foreman J. C. Some effects of calcitonin gene-related peptide in human skin and on histamine release. Br J Dermatol. 1986 Jan;114(1):37–46. doi: 10.1111/j.1365-2133.1986.tb02777.x. [DOI] [PubMed] [Google Scholar]
- Stjärne P., Lundblad L., Lundberg J. M., Anggård A. Capsaicin and nicotine-sensitive afferent neurones and nasal secretion in healthy human volunteers and in patients with vasomotor rhinitis. Br J Pharmacol. 1989 Mar;96(3):693–701. doi: 10.1111/j.1476-5381.1989.tb11870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddman R., Malm L., Sundler F. Substance-P-containing nerve fibers in the nasal mucosa. Arch Otorhinolaryngol. 1983;238(1):9–16. doi: 10.1007/BF00453736. [DOI] [PubMed] [Google Scholar]
- Uddman R., Malm L., Sundler F. The origin of vasoactive intestinal polypeptide (VIP) nerves in the feline nasal mucosa. Acta Otolaryngol. 1980 Jan-Feb;89(1-2):152–156. doi: 10.3109/00016488009127121. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C., Beding B., Ekman R., Oksala O., Stjernschantz J., Håkanson R. Calcitonin gene-related peptide in the eye: release by sensory nerve stimulation and effects associated with neurogenic inflammation. Regul Pept. 1986 Dec 22;16(2):107–115. doi: 10.1016/0167-0115(86)90054-6. [DOI] [PubMed] [Google Scholar]
- Wells U., Widdicombe J. G. Lateral nasal gland secretion in the anaesthetized dog. J Physiol. 1986 May;374:359–374. doi: 10.1113/jphysiol.1986.sp016084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner-Menzel L., Schulz B., Vakilzadeh F., Czarnetzki B. M. Electron microscopical evidence for a direct contact between nerve fibres and mast cells. Acta Derm Venereol. 1981;61(6):465–469. doi: 10.2340/0001555561465469. [DOI] [PubMed] [Google Scholar]


