Abstract
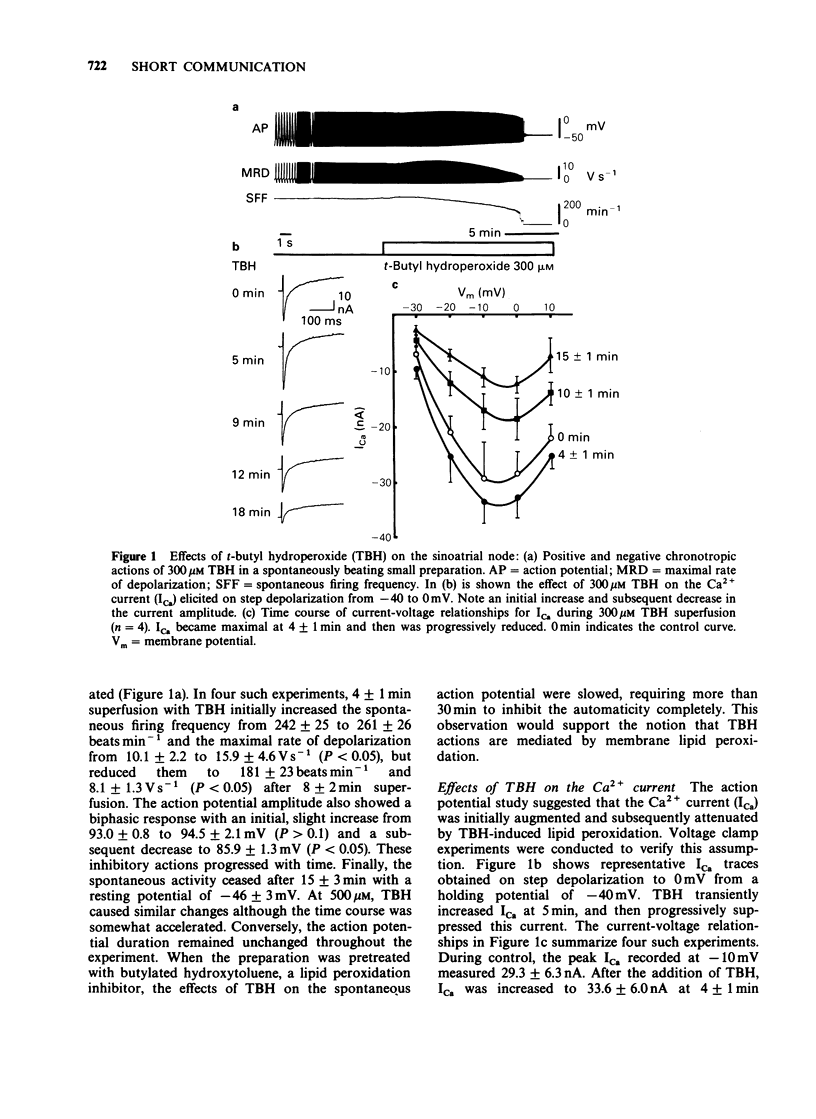
Cellular electrophysiological effects of membrane lipid peroxidation by t-butyl hydroperoxide (TBH) were studied in the rabbit sinoatrial (SA) node. Superfusion for 1-5 min with 300 microM TBH caused an initial increase and subsequent decrease in the spontaneous firing frequency of the SA node. Voltage clamp experiments revealed that TBH initially enhanced but later blocked the Ca2+ current. Thus, membrane lipid peroxidation appears to accelerate and then suppress physiological automaticity by causing biphasic changes in the Ca2+ current.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrington P. L., Meier C. F., Jr, Weglicki W. B. Abnormal electrical activity induced by free radical generating systems in isolated cardiocytes. J Mol Cell Cardiol. 1988 Dec;20(12):1163–1178. doi: 10.1016/0022-2828(88)90596-2. [DOI] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L., Brehm P. Calcium-mediated control of Ca and K currents. Fed Proc. 1981 Jun;40(8):2226–2232. [PubMed] [Google Scholar]
- Hess M. L., Manson N. H. Molecular oxygen: friend and foe. The role of the oxygen free radical system in the calcium paradox, the oxygen paradox and ischemia/reperfusion injury. J Mol Cell Cardiol. 1984 Nov;16(11):969–985. doi: 10.1016/s0022-2828(84)80011-5. [DOI] [PubMed] [Google Scholar]
- Hess M. L., Okabe E., Kontos H. A. Proton and free oxygen radical interaction with the calcium transport system of cardiac sarcoplasmic reticulum. J Mol Cell Cardiol. 1981 Aug;13(8):767–772. doi: 10.1016/0022-2828(81)90258-3. [DOI] [PubMed] [Google Scholar]
- Kramer J. H., Mak I. T., Weglicki W. B. Differential sensitivity of canine cardiac sarcolemmal and microsomal enzymes to inhibition by free radical-induced lipid peroxidation. Circ Res. 1984 Jul;55(1):120–124. doi: 10.1161/01.res.55.1.120. [DOI] [PubMed] [Google Scholar]
- Lebedev A. V., Levitsky D. O., Loginov V. A., Smirnov V. N. The effect of primary products of lipid peroxidation on the transmembrane transport of calcium ions. J Mol Cell Cardiol. 1982 Sep;14 (Suppl 3):99–103. doi: 10.1016/0022-2828(82)90136-5. [DOI] [PubMed] [Google Scholar]
- Marban E., Tsien R. W. Enhancement of calcium current during digitalis inotropy in mammalian heart: positive feed-back regulation by intracellular calcium? J Physiol. 1982 Aug;329:589–614. doi: 10.1113/jphysiol.1982.sp014321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerson F. Z., Kagan V. E., Kozlov YuP, Belkina L. M., Arkhipenko YuV The role of lipid peroxidation in pathogenesis of ischemic damage and the antioxidant protection of the heart. Basic Res Cardiol. 1982 Sep-Oct;77(5):465–485. doi: 10.1007/BF01907940. [DOI] [PubMed] [Google Scholar]
- Nakaya H., Tohse N., Kanno M. Electrophysiological derangements induced by lipid peroxidation in cardiac tissue. Am J Physiol. 1987 Nov;253(5 Pt 2):H1089–H1097. doi: 10.1152/ajpheart.1987.253.5.H1089. [DOI] [PubMed] [Google Scholar]
- Noma A., Kotake H., Irisawa H. Slow inward current and its role mediating the chronotropic effect of epinephrine in the rabbit sinoatrial node. Pflugers Arch. 1980 Oct;388(1):1–9. doi: 10.1007/BF00582621. [DOI] [PubMed] [Google Scholar]


