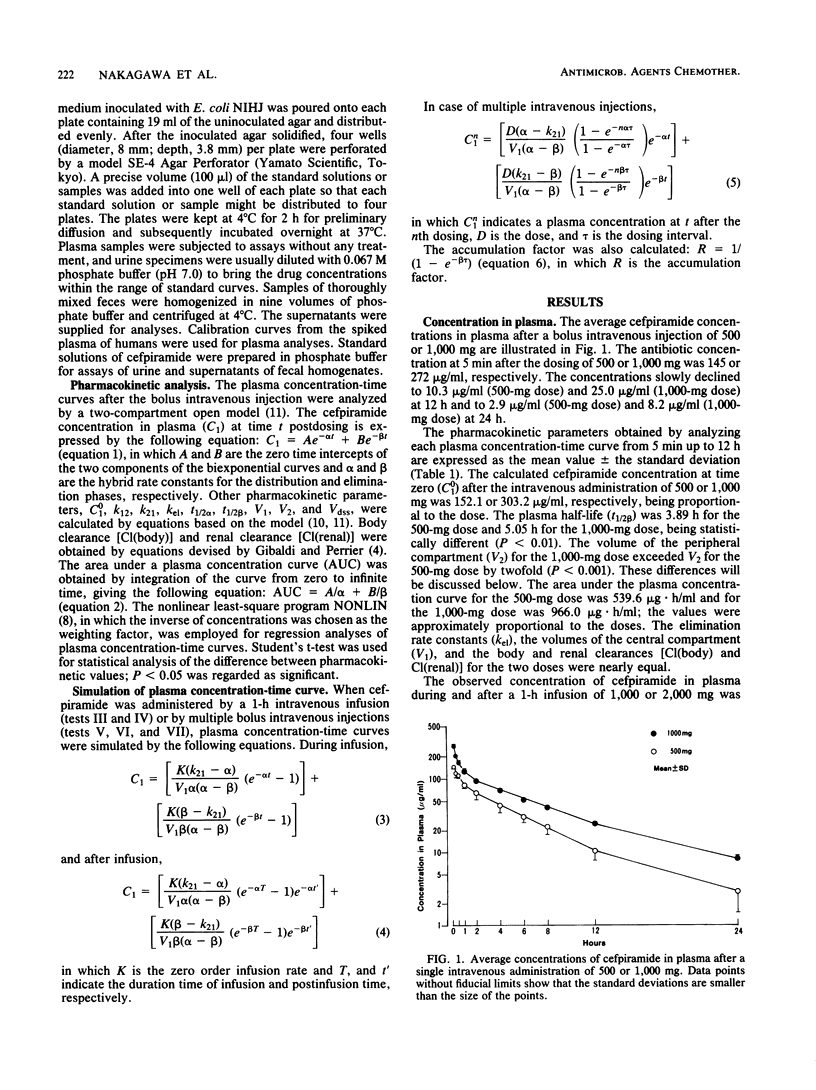
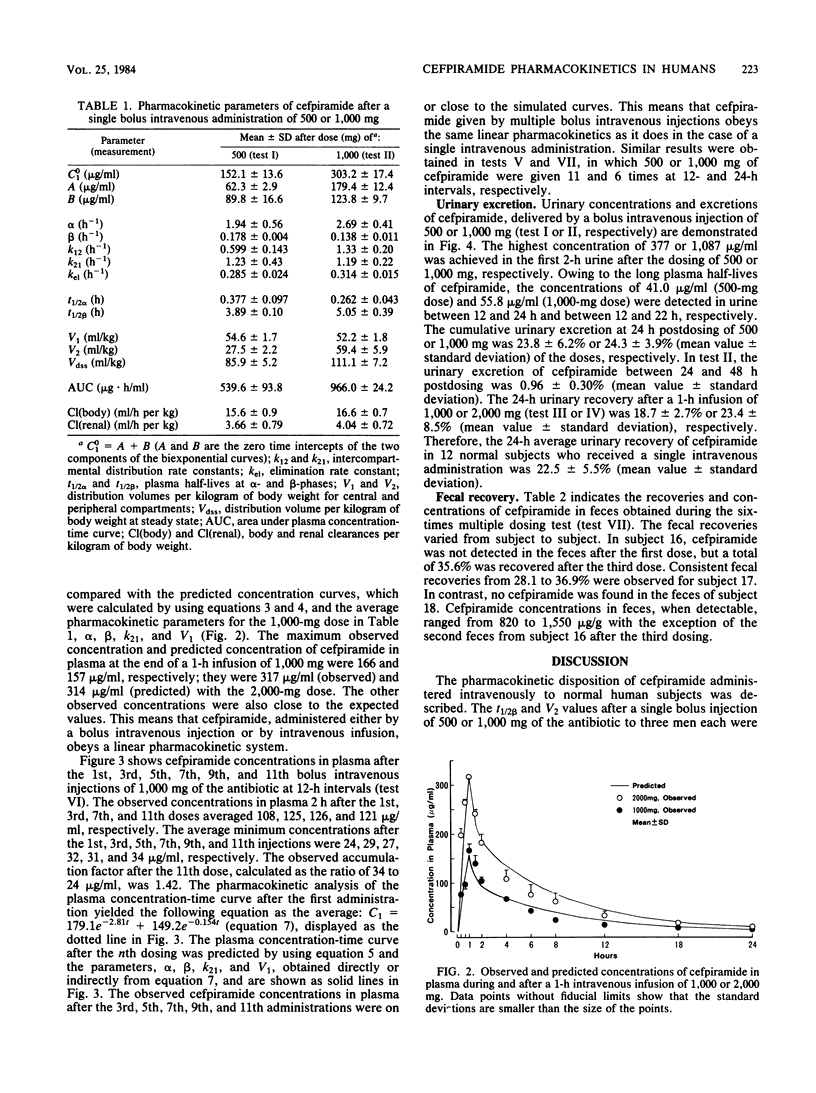
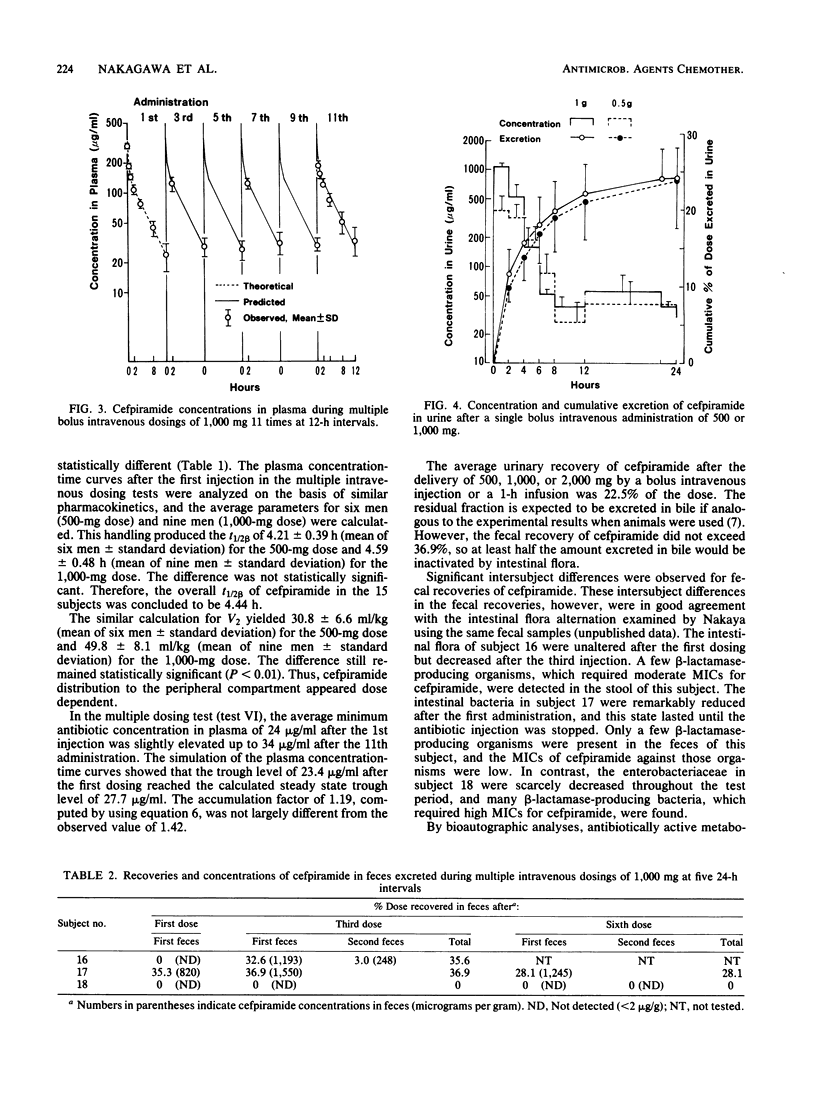
Abstract
The pharmacokinetics of cefpiramide (SM-1652) were studied after the intravenous administration of single or multiple doses to 21 healthy volunteers. The cefpiramide concentration in plasma at time zero after a bolus intravenous injection of 500 or 1,000 mg was 152 or 303 micrograms/ml, respectively. The maximum cefpiramide level in plasma at the end of a 1-h infusion of 1,000 or 2,000 mg was 166 or 317 micrograms/ml, respectively. The mean plasma half-life of cefpiramide in 15 subjects who received a single dose of 500 or 1,000 mg was 4.44 h. There was no evidence of drug accumulation in plasma when 500 or 1,000 mg of cefpiramide was administered 11 times at 12-h intervals. Urinary excretion of cefpiramide over a 24-h period was ca. 22.5%, regardless of the intravenous administration technique and the dosage. Fecal recoveries of cefpiramide varied from 0 to 36.9% in different subjects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. C., Wise R., Brown R. M., Hancox J. Comparison of ceftazidime and cefamandole pharmacokinetics and blister fluid concentrations. Antimicrob Agents Chemother. 1981 Sep;20(3):356–358. doi: 10.1128/aac.20.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton W. K., Scheld W. M., Spyker D. A., Sande M. A. Pharmacokinetics of cefoperazone in normal volunteers and subjects with renal insufficiency. Antimicrob Agents Chemother. 1981 May;19(5):821–825. doi: 10.1128/aac.19.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa M., Noguchi H., Okuda T., Komatsu T., Yano K. In vitro antibacterial activity of SM-1652, a new broad-spectrum cephalosporin with antipseudomonal activity. Antimicrob Agents Chemother. 1983 Feb;23(2):195–200. doi: 10.1128/aac.23.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Inoue M., Mitsuhashi S. Antibacterial activities of SM-1652 compared with those of other broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1982 Nov;22(5):721–727. doi: 10.1128/aac.22.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. H., Pfeffer M., Van Harken D. R., Smyth R. D., Hottendorf G. H. Comparative pharmacokinetics of ceforanide (BL-S786R) and cefazolin in laboratory animals and humans. Antimicrob Agents Chemother. 1980 Feb;17(2):188–192. doi: 10.1128/aac.17.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Yano K., Okuda T. Pharmacokinetics of the cephalosporin SM-1652 in mice, rats, rabbits, dogs, and rhesus monkeys. Antimicrob Agents Chemother. 1982 Aug;22(2):213–217. doi: 10.1128/aac.22.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K., Koyama M., Tachibana A., Komiya M., Kikuchi Y., Yano K. Pharmacokinetics of cefotetan (YM09330) in humans. Antimicrob Agents Chemother. 1982 Dec;22(6):935–941. doi: 10.1128/aac.22.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegelman S., Loo J. C., Rowland M. Shortcomings in pharmacokinetic analysis by conceiving the body to exhibit properties of a single compartment. J Pharm Sci. 1968 Jan;57(1):117–123. doi: 10.1002/jps.2600570123. [DOI] [PubMed] [Google Scholar]
- Riegelman S., Loo J., Rowland M. Concept of a volume of distribution and possible errors in evaluation of this parameter. J Pharm Sci. 1968 Jan;57(1):128–133. doi: 10.1002/jps.2600570125. [DOI] [PubMed] [Google Scholar]
- Seddon M., Wise R., Gillett A. P., Livingston R. Pharmacokinetics of Ro 13-9904, a broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1980 Aug;18(2):240–242. doi: 10.1128/aac.18.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]



