Abstract
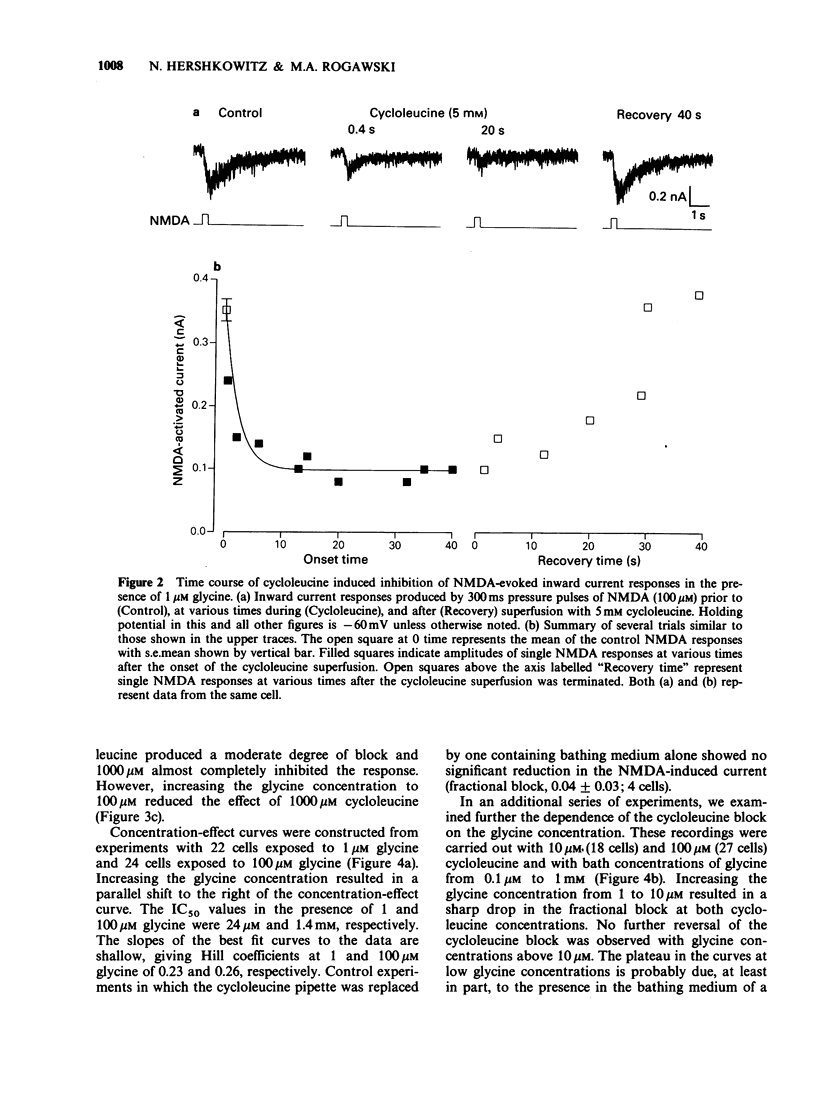
1. Radioligand binding studies have demonstrated that the neutral amino acid cycloleucine may act as a competitive antagonist at the glycine modulatory site on the N-methyl-D-aspartate (NMDA) receptor complex. In the present study, we examined the effects of cycloleucine on NMDA-evoked inward current responses in dissociated hippocampal neuronal cultures using the whole cell voltage-clamp technique. 2. In the presence of 1 microM glycine, cycloleucine caused a reversible, dose-dependent inhibition of NMDA responses with an IC50 of 24 microM. An increase in glycine to 100 microM resulted in a shift to the right of the cycloleucine concentration-effect curve (IC50, 1.4 mM). However, with cycloleucine concentrations less than or equal to 100 microM, a fraction of the block could not be overcome by glycine even at concentrations as high as 1 mM. 3. The cycloleucine block was unaffected by shifts in the holding potential (-60 to +60 mV), and there was no effect of cycloleucine on the reversal potential of the NMDA-evoked current. 4. Cycloleucine failed to effect kainic acid- and quisqualic acid-evoked currents at concentrations which inhibited NMDA responses. 5. We conclude that cycloleucine is a potent and selective antagonist of NMDA-receptor mediated responses. Although this effect occurs in part via competitive antagonism at the glycine modulatory site, the cycloleucine block cannot be completely reversed by glycine indicating an interaction with an additional site on the receptor-channel complex.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertolino M., Vicini S., Mazzetta J., Costa E. Phencyclidine and glycine modulate NMDA-activated high conductance cationic channels by acting at different sites. Neurosci Lett. 1988 Feb 3;84(3):351–355. doi: 10.1016/0304-3940(88)90534-4. [DOI] [PubMed] [Google Scholar]
- Birch P. J., Grossman C. J., Hayes A. G. 6,7-Dinitro-quinoxaline-2,3-dion and 6-nitro,7-cyano-quinoxaline-2,3-dion antagonise responses to NMDA in the rat spinal cord via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988 Oct 26;156(1):177–180. doi: 10.1016/0014-2999(88)90163-x. [DOI] [PubMed] [Google Scholar]
- Birch P. J., Grossman C. J., Hayes A. G. 6,7-Dinitro-quinoxaline-2,3-dion and 6-nitro,7-cyano-quinoxaline-2,3-dion antagonise responses to NMDA in the rat spinal cord via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988 Oct 26;156(1):177–180. doi: 10.1016/0014-2999(88)90163-x. [DOI] [PubMed] [Google Scholar]
- Birch P. J., Grossman C. J., Hayes A. G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988 Sep 1;154(1):85–87. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- Bonhaus D. W., Burge B. C., McNamara J. O. Biochemical evidence that glycine allosterically regulates an NMDA receptor-coupled ion channel. Eur J Pharmacol. 1987 Oct 27;142(3):489–490. doi: 10.1016/0014-2999(87)90096-3. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Drejer J., Sheardown M., Nielsen E. O., Honoré T. Glycine reverses the effect of HA-966 on NMDA responses in cultured rat cortical neurons and in chick retina. Neurosci Lett. 1989 Apr 10;98(3):333–338. doi: 10.1016/0304-3940(89)90424-2. [DOI] [PubMed] [Google Scholar]
- Fletcher E. J., Lodge D. Glycine reverses antagonism of N-methyl-D-aspartate (NMDA) by 1-hydroxy-3-aminopyrrolidone-2 (HA-966) but not by D-2-amino-5-phosphonovalerate (D-AP5) on rat cortical slices. Eur J Pharmacol. 1988 Jun 22;151(1):161–162. doi: 10.1016/0014-2999(88)90711-x. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L., Mayer M. L. Modulation of excitatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J Neurosci. 1988 Oct;8(10):3733–3741. doi: 10.1523/JNEUROSCI.08-10-03733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C. R., Miljkovic Z., MacDonald J. F. Ketamine and phencyclidine cause a voltage-dependent block of responses to L-aspartic acid. Neurosci Lett. 1985 Oct 24;61(1-2):135–139. doi: 10.1016/0304-3940(85)90414-8. [DOI] [PubMed] [Google Scholar]
- Hood W. F., Sun E. T., Compton R. P., Monahan J. B. 1-Aminocyclobutane-1-carboxylate (ACBC): a specific antagonist of the N-methyl-D-aspartate receptor coupled glycine receptor. Eur J Pharmacol. 1989 Feb 28;161(2-3):281–282. doi: 10.1016/0014-2999(89)90861-3. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner J. E. Indole-2-carboxylic acid: a competitive antagonist of potentiation by glycine at the NMDA receptor. Science. 1989 Mar 24;243(4898):1611–1613. doi: 10.1126/science.2467381. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. The mobile receptor hypothesis and "cooperativity" of hormone binding. Application to insulin. Biochim Biophys Acta. 1976 May 21;433(3):482–495. doi: 10.1016/0005-2736(76)90275-3. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Johnson K. M., Sacaan A. I., Snell L. D. Equilibrium analysis of [3H]TCP binding: effects of glycine, magnesium and N-methyl-D-aspartate agonists. Eur J Pharmacol. 1988 Jul 26;152(1-2):141–146. doi: 10.1016/0014-2999(88)90845-x. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Foster A. C., Leeson P. D., Priestley T., Tridgett R., Iversen L. L., Woodruff G. N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Larson A. A., Beitz A. J. Glycine potentiates strychnine-induced convulsions: role of NMDA receptors. J Neurosci. 1988 Oct;8(10):3822–3826. doi: 10.1523/JNEUROSCI.08-10-03822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Miljkovic Z., Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987 Aug;58(2):251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Vyklický L., Jr Sites of antagonist action on N-methyl-D-aspartic acid receptors studied using fluctuation analysis and a rapid perfusion technique. J Neurophysiol. 1988 Aug;60(2):645–663. doi: 10.1152/jn.1988.60.2.645. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ransom R. W., Deschenes N. L. Glycine modulation of NMDA-evoked release of [3H]acetylcholine and [3H]dopamine from rat striatal slices. Neurosci Lett. 1989 Jan 30;96(3):323–328. doi: 10.1016/0304-3940(89)90399-6. [DOI] [PubMed] [Google Scholar]
- Ransom R. W., Deschenes N. L. NMDA-induced hippocampal [3H]norepinephrine release is modulated by glycine. Eur J Pharmacol. 1988 Oct 26;156(1):149–155. doi: 10.1016/0014-2999(88)90157-4. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Harris K. M., Miller R. J. NMDA receptor antagonists that bind to the strychnine-insensitive glycine site and inhibit NMDA-induced Ca2+ fluxes and [3H]GABA release. Eur J Pharmacol. 1989 Mar 7;172(1):9–17. doi: 10.1016/0922-4106(89)90040-0. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Murphy S. N., Miller R. J. 3H-labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is enhanced by glycine. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7744–7748. doi: 10.1073/pnas.84.21.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman S. Noncompetitive N-methyl-D-aspartate antagonists affect multiple ionic currents. J Pharmacol Exp Ther. 1988 Jul;246(1):137–142. [PubMed] [Google Scholar]
- Segal M. Rat hippocampal neurons in culture: responses to electrical and chemical stimuli. J Neurophysiol. 1983 Dec;50(6):1249–1264. doi: 10.1152/jn.1983.50.6.1249. [DOI] [PubMed] [Google Scholar]
- Snell L. D., Johnson K. M. Cycloleucine competitively antagonizes the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988 Jun 22;151(1):165–166. doi: 10.1016/0014-2999(88)90713-3. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Kleckner N. W., Dingledine R. N-methyl-D-aspartate/glycine and quisqualate/kainate receptors expressed in Xenopus oocytes: antagonist pharmacology. Mol Pharmacol. 1989 Mar;35(3):360–368. [PubMed] [Google Scholar]
- Verdoorn T. A., Kleckner N. W., Dingledine R. Rat brain N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Science. 1987 Nov 20;238(4830):1114–1116. doi: 10.1126/science.2825347. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Knight A. R., Ransom R. Glycine modulates [3H]MK-801 binding to the NMDA receptor in rat brain. Eur J Pharmacol. 1987 Oct 27;142(3):487–488. doi: 10.1016/0014-2999(87)90095-1. [DOI] [PubMed] [Google Scholar]
- de Haën C. The non-stoichiometric floating receptor model for hormone sensitive adenylyl cyclase. J Theor Biol. 1976 May 21;58(2):383–400. doi: 10.1016/s0022-5193(76)80126-9. [DOI] [PubMed] [Google Scholar]
- ffrench-Mullen J. M., Rogawski M. A., Barker J. L. Phencyclidine at low concentrations selectively blocks the sustained but not the transient voltage-dependent potassium current in cultured hippocampal neurons. Neurosci Lett. 1988 Jun 7;88(3):325–330. doi: 10.1016/0304-3940(88)90232-7. [DOI] [PubMed] [Google Scholar]


