Abstract
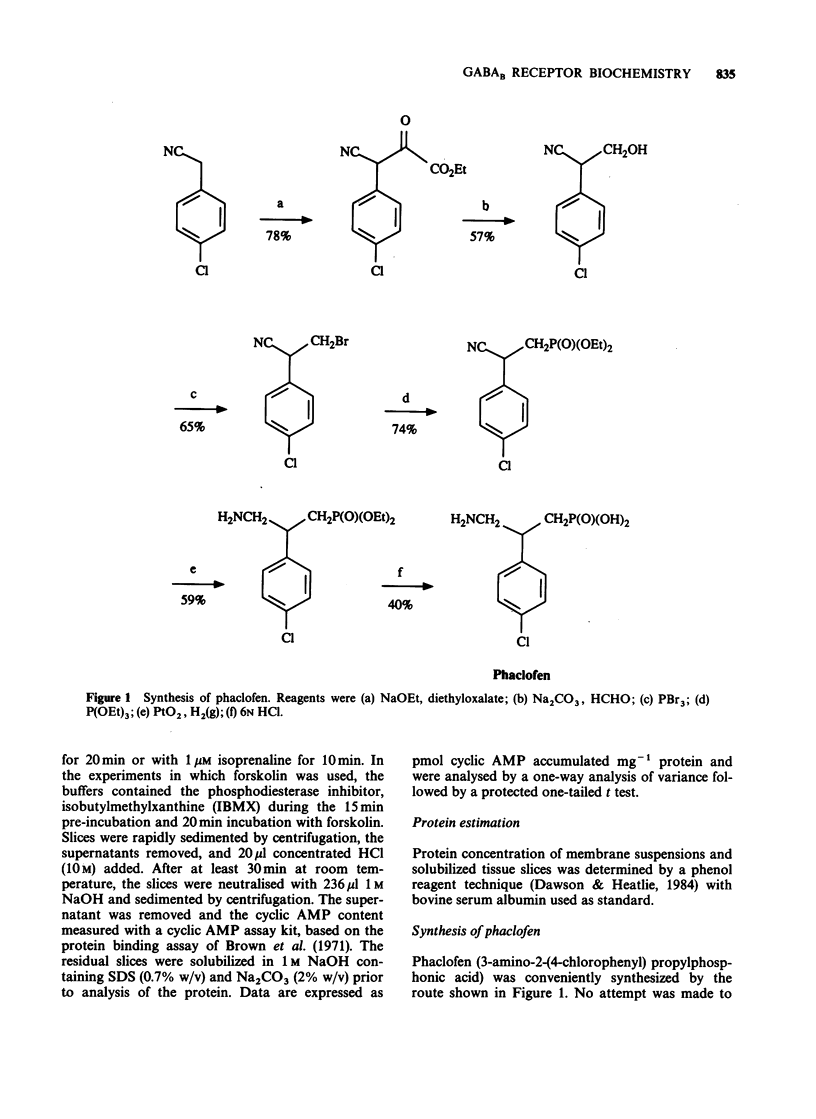
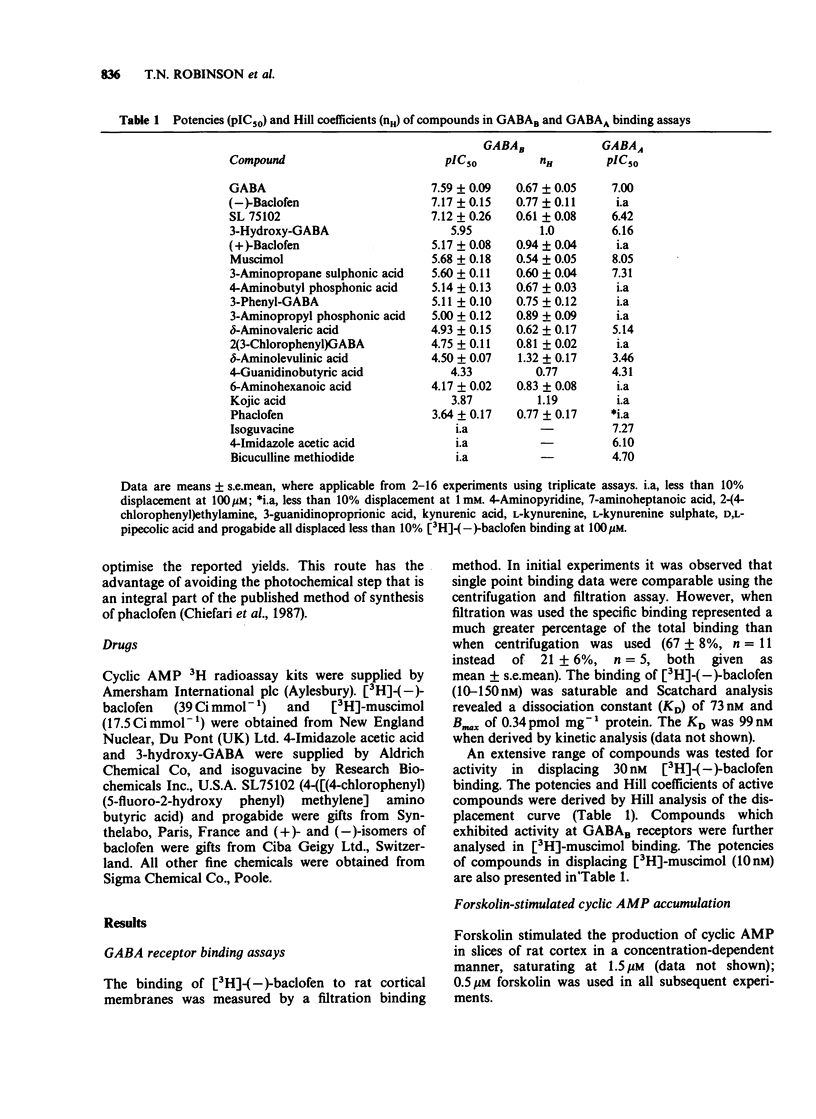
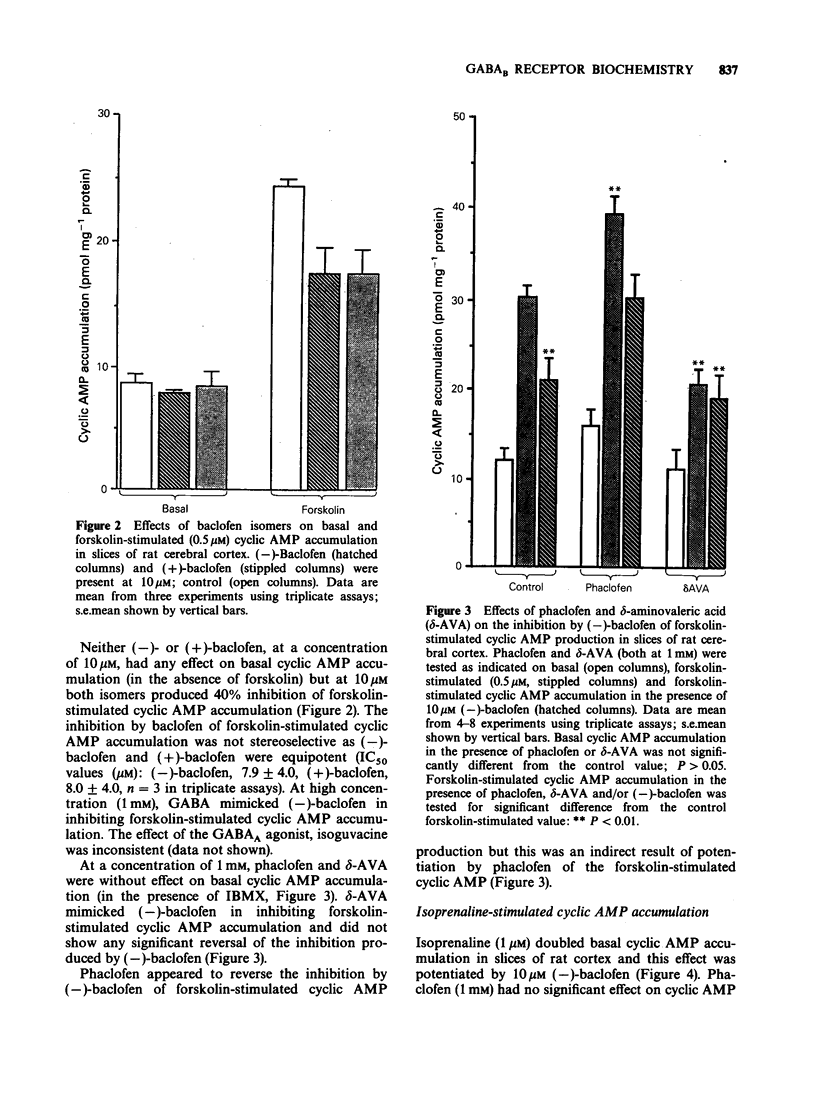
1. Phaclofen and delta-aminovaleric acid (delta-AVA) have been reported to be antagonists at gamma-aminobutyric acidB (GABAB) receptors. Phaclofen, delta-AVA and related compounds were examined for potency and specificity at GABAB and GABAA receptors in rat cortical membranes labelled with [3H]-(-)-baclofen and [3H]-muscimol, respectively. Additionally phaclofen and delta-AVA were examined in two functional tests of central GABAB activity in rat cortical slices, namely the inhibition of forskolin-stimulated cyclic AMP accumulation, and the potentiation of isoprenaline-stimulated cyclic AMP accumulation. 2. delta-AVA (IC50 = 11.7 microM) was 20 fold more potent than phaclofen (IC50 = 229 microM) on GABAB receptor binding. All compounds possessing a phosphonic acid group, including phaclofen, which were active at GABAB receptors were inactive at GABAA receptors, while delta-AVA was equally potent at both receptors. Several compounds exhibited Hill coefficients of less than unity in displacing [3H]-(-)-baclofen binding. 3. (-)-Baclofen inhibited forskolin-stimulated cyclic AMP accumulation (IC50 = 7.9 microM) but this effect was not stereospecific. Phaclofen (1 mM) was inactive against this inhibition but produced a potentiation of the forskolin effect. delta-AVA (1 mM) failed to antagonize the effect of baclofen; rather it mimicked baclofen. 4. (-)-Baclofen (10 microM) potentiated isoprenaline-stimulated cyclic AMP accumulation, an effect antagonized by phaclofen (1 mM). delta-AVA (1 mM) may be a weak antagonist but also potentiated basal cyclic AMP accumulation. 5. We conclude that neither delta-AVA nor phaclofen are potent specific GABAB receptor antagonists.
Full text
PDF

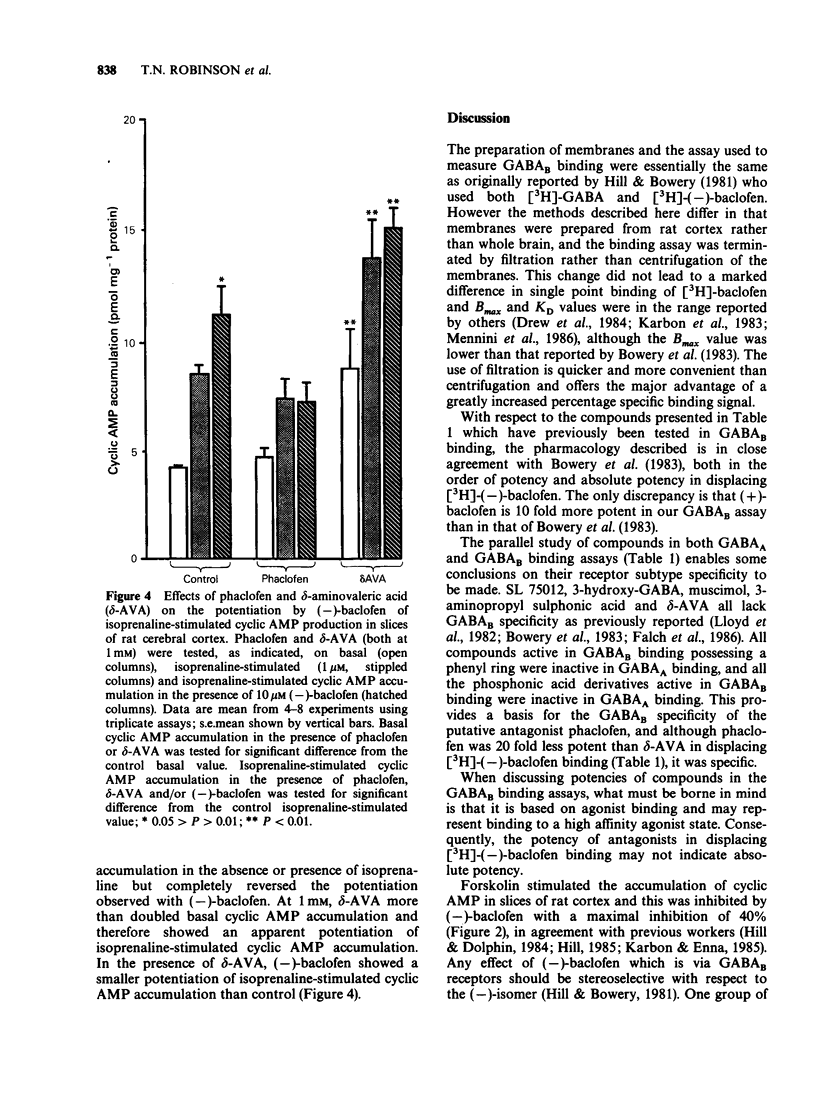


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R. D., Dickenson H. W. Evidence that antagonism by delta-aminovaleric acid of GABAB receptors in the guinea-pig ileum may be due to an interaction between GABAA and GABAB receptors. Eur J Pharmacol. 1986 Jan 14;120(1):119–122. doi: 10.1016/0014-2999(86)90650-3. [DOI] [PubMed] [Google Scholar]
- Arch J. R., Buckle D. R., Bumstead J., Clarke G. D., Taylor J. F., Taylor S. G. Evaluation of the potassium channel activator cromakalim (BRL 34915) as a bronchodilator in the guinea-pig: comparison with nifedipine. Br J Pharmacol. 1988 Nov;95(3):763–770. doi: 10.1111/j.1476-5381.1988.tb11702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983 Jan;78(1):191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. GABA, bicuculline and central inhibition. Nature. 1970 Jun 27;226(5252):1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- Drew C. A., Johnston G. A., Weatherby R. P. Bicuculline-insensitive GABA receptors: studies on the binding of (-)-baclofen to rat cerebellar membranes. Neurosci Lett. 1984 Dec 21;52(3):317–321. doi: 10.1016/0304-3940(84)90181-2. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Falch E., Hedegaard A., Nielsen L., Jensen B. R., Hjeds H., Krogsgaard-Larsen P. Comparative stereostructure-activity studies on GABAA and GABAB receptor sites and GABA uptake using rat brain membrane preparations. J Neurochem. 1986 Sep;47(3):898–903. doi: 10.1111/j.1471-4159.1986.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Hill D. R. GABAB receptor modulation of adenylate cyclase activity in rat brain slices. Br J Pharmacol. 1985 Jan;84(1):249–257. [PMC free article] [PubMed] [Google Scholar]
- Karbon E. W., Duman R., Enna S. J. Biochemical identification of multiple GABAB binding sites: association with noradrenergic terminals in rat forebrain. Brain Res. 1983 Sep 12;274(2):393–396. doi: 10.1016/0006-8993(83)90725-4. [DOI] [PubMed] [Google Scholar]
- Karbon E. W., Enna S. J. Characterization of the relationship between gamma-aminobutyric acid B agonists and transmitter-coupled cyclic nucleotide-generating systems in rat brain. Mol Pharmacol. 1985 Jan;27(1):53–59. [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Prager R. H., Gynther B. D., Curtis D. R. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987 Mar 3;405(1):150–154. doi: 10.1016/0006-8993(87)90999-1. [DOI] [PubMed] [Google Scholar]
- Lloyd K. G., Arbilla S., Beaumont K., Briley M., De Montis G., Scatton B., Langer S. Z., Bartholini G. gamma-Aminobutyric acid (GABA) receptor stimulation. II. Specificity of progabide (SL 76002) and SL 75102 for the GABA receptor. J Pharmacol Exp Ther. 1982 Mar;220(3):672–677. [PubMed] [Google Scholar]
- Mennini T., Gobbi M., Romandini S. Localization of GABAA and GABAB receptor subtypes on serotonergic neurons. Brain Res. 1986 Apr 23;371(2):372–375. doi: 10.1016/0006-8993(86)90378-1. [DOI] [PubMed] [Google Scholar]
- Muhyaddin M., Roberts P. J., Woodruff G. N. Presynaptic gamma-aminobutyric acid receptors in the rat anococcygeus muscle and their antagonism by 5-aminovaleric acid. Br J Pharmacol. 1982 Sep;77(1):163–168. doi: 10.1111/j.1476-5381.1982.tb09282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahiro M., Saito K., Yamada I., Yoshida H. Antagonistic effect of delta-aminovaleric acid on bicuculline-insensitive gamma-aminobutyric acid B (GABA B) sites in the rat's brain. Neurosci Lett. 1985 Jun 24;57(3):263–266. doi: 10.1016/0304-3940(85)90502-6. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Steinmann M. W., Hall R. G., Brugger F., Pozza M. F. GABAA and GABAB receptors in locus coeruleus: effects of blockers. Eur J Pharmacol. 1988 Apr 27;149(1-2):183–185. doi: 10.1016/0014-2999(88)90061-1. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem. 1981 Jul;37(1):1–13. doi: 10.1111/j.1471-4159.1981.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Baclofen activates two distinct receptors in the rat spinal cord and guinea pig ileum. Neuropharmacology. 1986 Jul;25(7):795–798. doi: 10.1016/0028-3908(86)90098-5. [DOI] [PubMed] [Google Scholar]
- Schwarz M., Klockgether T., Wüllner U., Turski L., Sontag K. H. Delta-aminovaleric acid antagonizes the pharmacological actions of baclofen in the central nervous system. Exp Brain Res. 1988;70(3):618–626. doi: 10.1007/BF00247610. [DOI] [PubMed] [Google Scholar]
- Stirling J. M., Cross A. J., Robinson T. N., Green A. R. The effects of GABAB receptor agonists and antagonists on potassium-stimulated [Ca2+]i in rat brain synaptosomes. Neuropharmacology. 1989 Jul;28(7):699–704. doi: 10.1016/0028-3908(89)90153-6. [DOI] [PubMed] [Google Scholar]
- Watling K. J., Bristow D. R. GABAB receptor-mediated enhancement of vasoactive intestinal peptide-stimulated cyclic AMP production in slices of rat cerebral cortex. J Neurochem. 1986 Jun;46(6):1755–1762. doi: 10.1111/j.1471-4159.1986.tb08493.x. [DOI] [PubMed] [Google Scholar]
- Williams M., Risley E. A. Characterization of the binding of [3H]muscimol, a potent gamma-aminobutyric acid agonist, to rat brain synaptosomal membranes using a filtration assay. J Neurochem. 1979 Mar;32(3):713–718. doi: 10.1111/j.1471-4159.1979.tb04553.x. [DOI] [PubMed] [Google Scholar]
- Wojcik W. J., Neff N. H. gamma-aminobutyric acid B receptors are negatively coupled to adenylate cyclase in brain, and in the cerebellum these receptors may be associated with granule cells. Mol Pharmacol. 1984 Jan;25(1):24–28. [PubMed] [Google Scholar]
- Xu J., Wojcik W. J. Gamma aminobutyric acid B receptor-mediated inhibition of adenylate cyclase in cultured cerebellar granule cells: blockade by islet-activating protein. J Pharmacol Exp Ther. 1986 Nov;239(2):568–573. [PubMed] [Google Scholar]


