Abstract
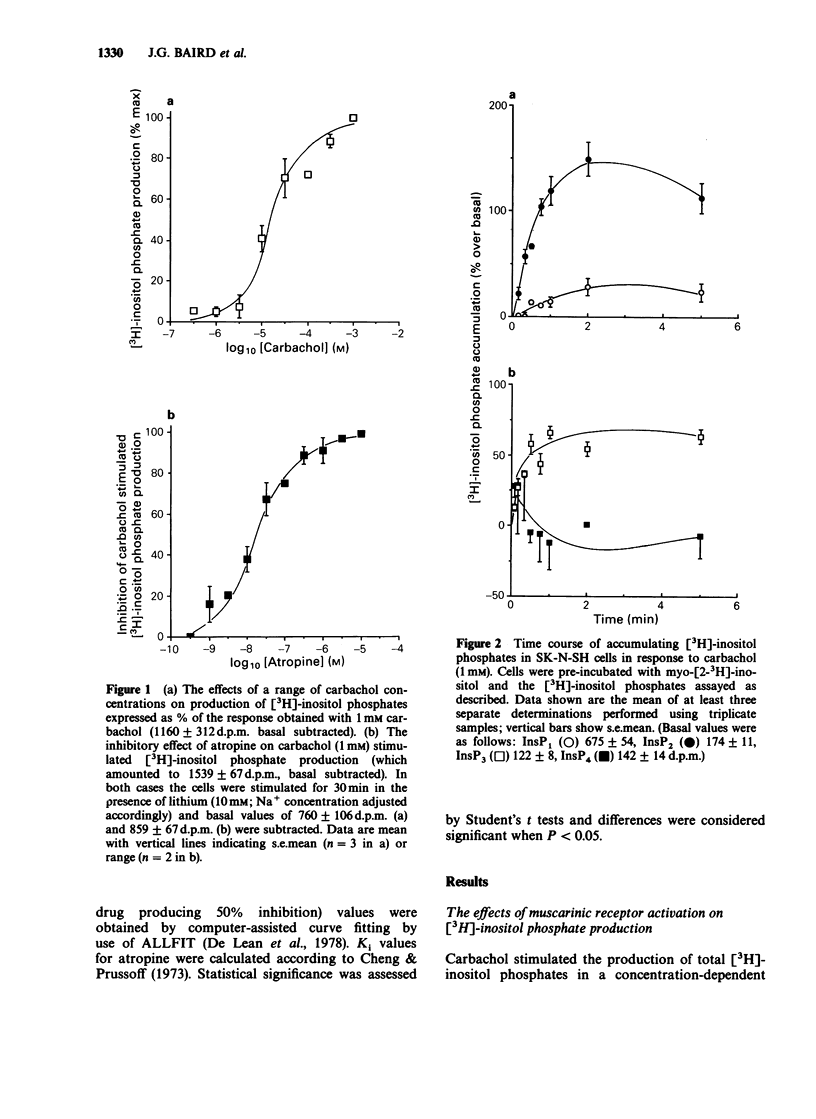
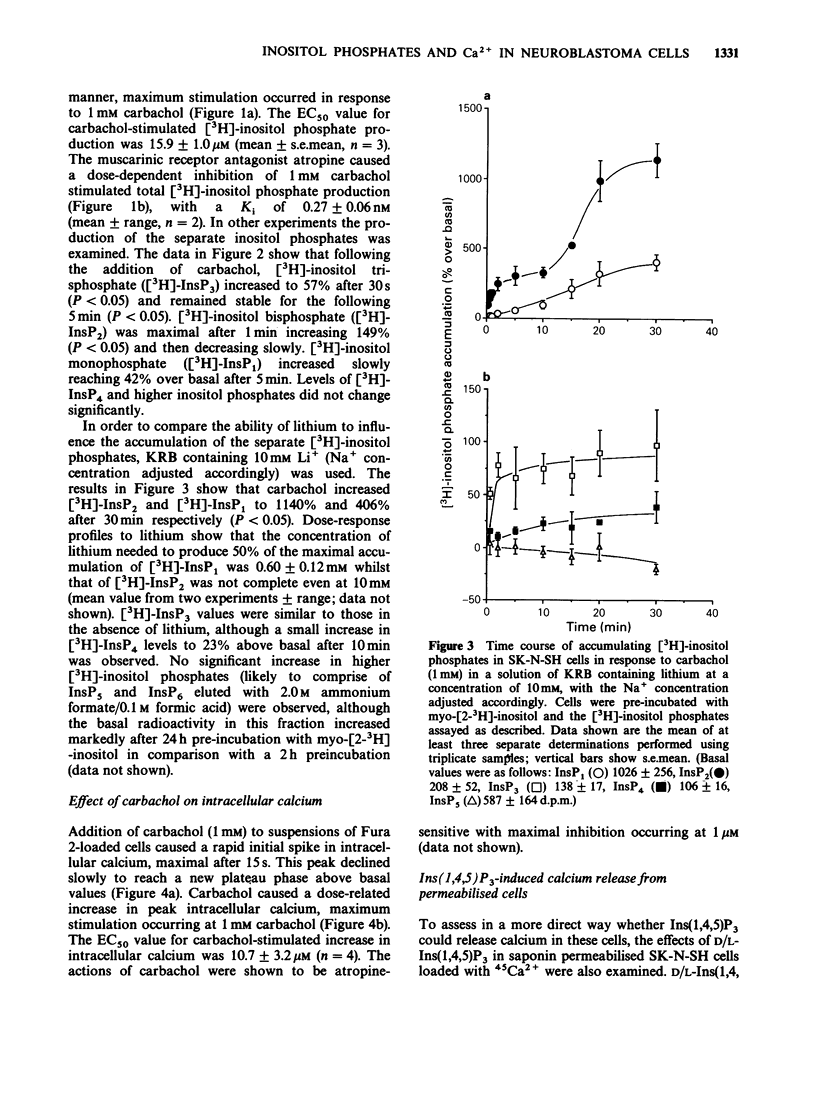
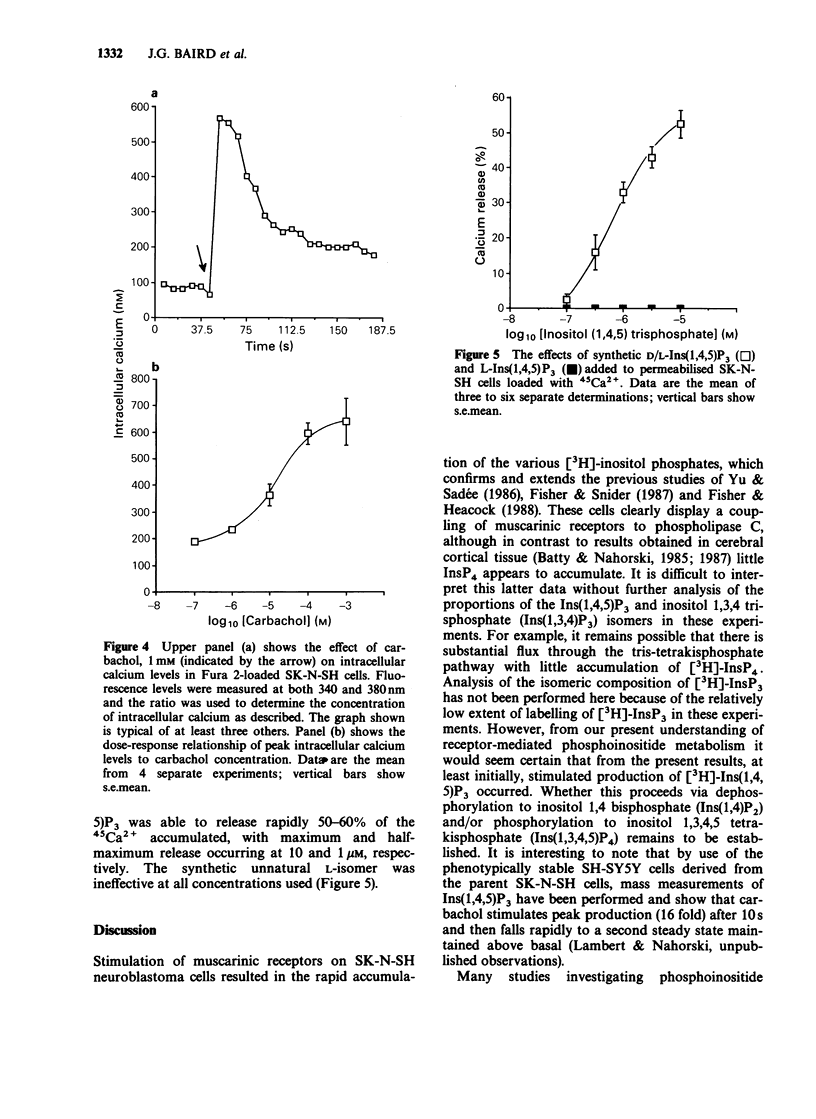
1. The effects of the muscarinic agonist carbachol on phosphoinositide metabolism and its relationship to alteration of intracellular calcium were examined in SK-N-SH human neuroblastoma cells. Muscarinic receptors on these cells are coupled to phospholipase C and the myo [2-3H]-inositol phosphates resulting from receptor activation of cells labelled with [3H]-inositol accumulate rapidly. The breakdown of both inositol monophosphate (InsP1) and inositol bisphosphate (InsP2) is sensitive to lithium with inhibition of the latter only observed at higher concentrations of this ion. 2. Use of the calcium indicator dye Fura 2 revealed that carbachol stimulates a biphasic increase in intracellular calcium. 3. Carbachol was able to stimulate both [3H]-inositol phosphate production and intracellular calcium levels with respective EC50 values of 15.9 +/- 1.0 microM and 10.7 +/- 3.2 microM, indicating that no amplification occurs between these steps in the signal transduction pathway. 4. Inositol 1,4,5 trisphosphate (Ins(1,4,5)P3) released 45Ca2+ in a stereospecific and dose-related manner from intracellular stores of permeabilised cells. 5. These results suggest that this cell line may represent a useful model system to investigate receptor-mediated phosphoinositide metabolism and calcium homeostasis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird J. G., Dobson P. R., Wojcikiewicz R. J., Brown B. L. Thyrotropin-releasing hormone stimulates inositol phosphate production in normal anterior pituitary cells and GH3 tumour cells in the presence of lithium. Biosci Rep. 1983 Dec;3(12):1091–1099. doi: 10.1007/BF01120201. [DOI] [PubMed] [Google Scholar]
- Baird J. G., Nahorski S. R. Potassium depolarisation markedly enhances muscarinic receptor stimulated inositol tetrakisphosphate accumulation in rat cerebral cortical slices. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1130–1137. doi: 10.1016/s0006-291x(86)80161-9. [DOI] [PubMed] [Google Scholar]
- Batty I., Nahorski S. R. Differential effects of lithium on muscarinic receptor stimulation of inositol phosphates in rat cerebral cortex slices. J Neurochem. 1985 Nov;45(5):1514–1521. doi: 10.1111/j.1471-4159.1985.tb07221.x. [DOI] [PubMed] [Google Scholar]
- Batty I., Nahorski S. R. Lithium inhibits muscarinic-receptor-stimulated inositol tetrakisphosphate accumulation in rat cerebral cortex. Biochem J. 1987 Nov 1;247(3):797–800. doi: 10.1042/bj2470797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. K., Heacock A. M. A putative M3 muscarinic cholinergic receptor of high molecular weight couples to phosphoinositide hydrolysis in human SK-N-SH neuroblastoma cells. J Neurochem. 1988 Mar;50(3):984–987. doi: 10.1111/j.1471-4159.1988.tb03008.x. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Snider R. M. Differential receptor occupancy requirements for muscarinic cholinergic stimulation of inositol lipid hydrolysis in brain and in neuroblastomas. Mol Pharmacol. 1987 Jul;32(1):81–90. [PubMed] [Google Scholar]
- Gee N. S., Ragan C. I., Watling K. J., Aspley S., Jackson R. G., Reid G. G., Gani D., Shute J. K. The purification and properties of myo-inositol monophosphatase from bovine brain. Biochem J. 1988 Feb 1;249(3):883–889. doi: 10.1042/bj2490883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M., Pollock W. K., Smith P. M., Wreggett K. A. Inositol phosphates: proliferation, metabolism and function. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):281–298. doi: 10.1098/rstb.1988.0077. [DOI] [PubMed] [Google Scholar]
- Lambert D. G., Ghataorre A. S., Nahorski S. R. Muscarinic receptor binding characteristics of a human neuroblastoma SK-N-SH and its clones SH-SY5Y and SH-EP1. Eur J Pharmacol. 1989 Jun 8;165(1):71–77. doi: 10.1016/0014-2999(89)90771-1. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R. Inositol polyphosphates and neuronal calcium homeostasis. Trends Neurosci. 1988 Oct;11(10):444–448. doi: 10.1016/0166-2236(88)90196-8. [DOI] [PubMed] [Google Scholar]
- Ross R. A., Spengler B. A., Biedler J. L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983 Oct;71(4):741–747. [PubMed] [Google Scholar]
- Strupish J., Cooke A. M., Potter B. V., Gigg R., Nahorski S. R. Stereospecific mobilization of intracellular Ca2+ by inositol 1,4,5-triphosphate. Comparison with inositol 1,4,5-trisphosphorothioate and inositol 1,3,4-trisphosphate. Biochem J. 1988 Aug 1;253(3):901–905. doi: 10.1042/bj2530901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks A. L., Cooke A. M., Potter B. V., Nahorski S. R. Stereospecific recognition sites for [3H]inositol(1,4,5)-triphosphate in particulate preparations of rat cerebellum. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1071–1078. doi: 10.1016/0006-291x(87)90756-x. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Sadée W. Phosphatidylinositol turnover in neuroblastoma cells: regulation by bradykinin, acetylcholine, but not mu- and delta-opioid receptors. Neurosci Lett. 1986 Nov 11;71(2):219–223. doi: 10.1016/0304-3940(86)90562-8. [DOI] [PubMed] [Google Scholar]


