Abstract
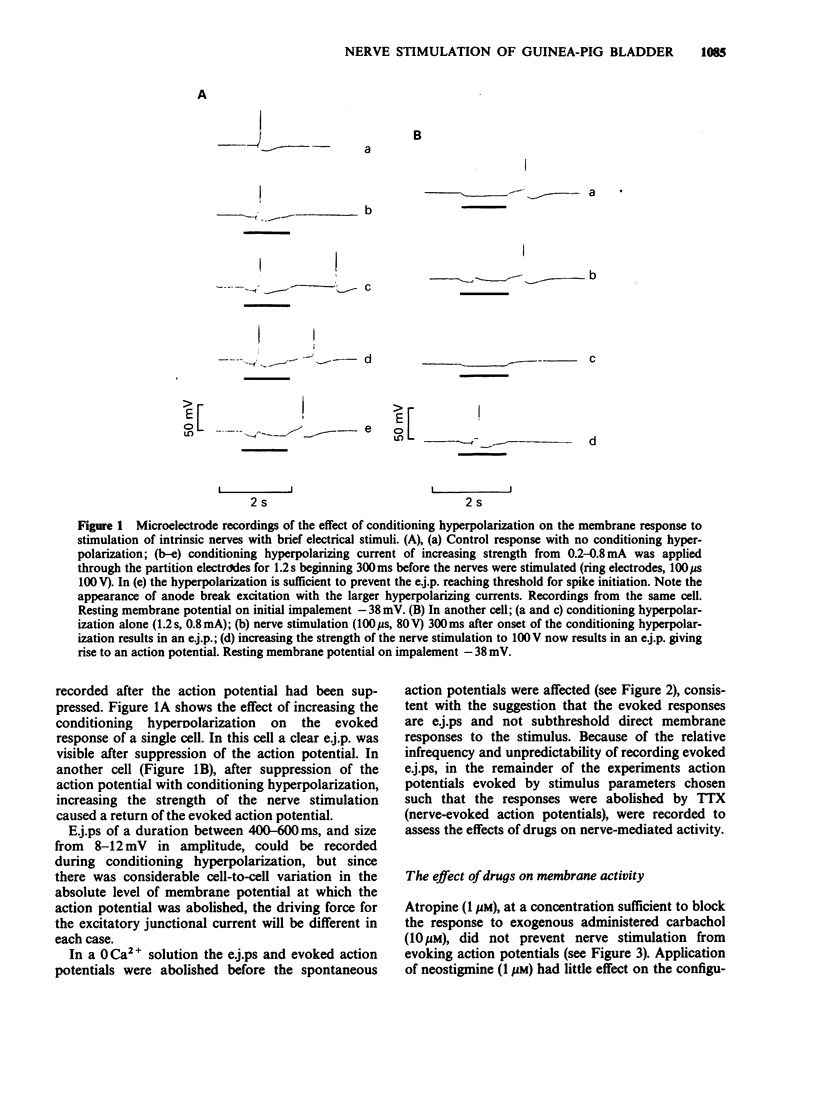
1 The electrical and mechanical responses to transmural stimulation of intrinsic nerves have been recorded from smooth muscle strips dissected from the dome of the guinea-pig bladder, by use of intracellular microelectrodes, and conventional tension recording techniques. 2 Stimulation of intrinsic nerves evoked action potentials in all cells studied. Hyperpolarization of the cells by extracellular current injection revealed subthreshold excitatory junction potentials (e.j.ps) in about a quarter of the cells studied. 3 Action potentials could still be evoked in the presence of atropine and neostigmine, but were abolished after desensitization of the cells to alpha, beta-methylene ATP, a stable analogue of ATP. 4 In the presence of neostigmine, the evoked action potential was followed by a slow depolarization of the membrane. The mechanical response increased in amplitude and duration. 5 The contractile response to transmural nerve stimulation was reduced but not abolished in the presence of either atropine or desensitizing doses of alpha, beta-methylene ATP. Atropine was more effective at high frequencies of stimulation (greater than or equal to 30 Hz), and alpha, beta-methylene ATP at low frequencies (less than or equal to 15 Hz). In combination the drugs abolished the response. 6 The results suggest that the mechanical response to excitatory nerve stimulation is biphasic. The early transient response is elicited by e.j.ps and evoked spikes, is resistant to atropine, but sensitive to desensitization of purinoceptors. The late response is mediated through muscarinic receptors, involves little membrane depolarization, and is unaffected by desensitization of purinoceptors. These responses are analogous to the responses seen in rabbit bladder, and in the sympathetically innervated rat tail artery and guinea-pig vas deferens.
Full text
PDF

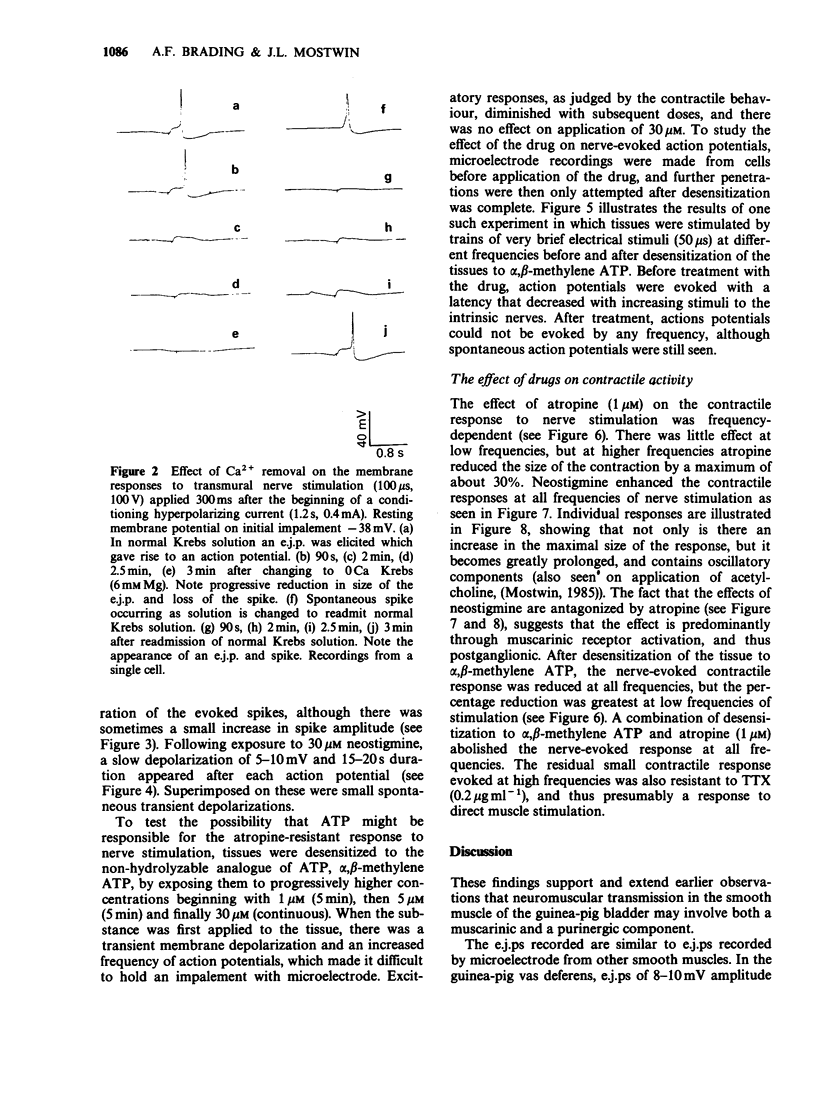
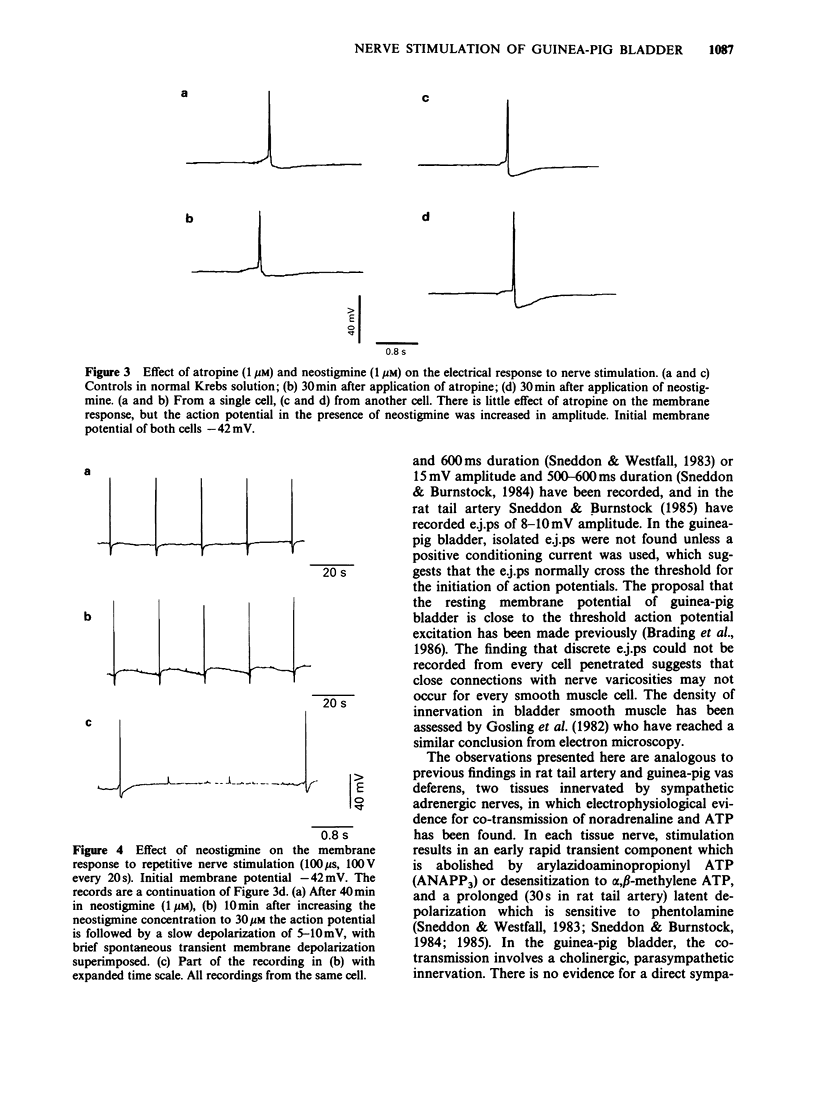

Selected References
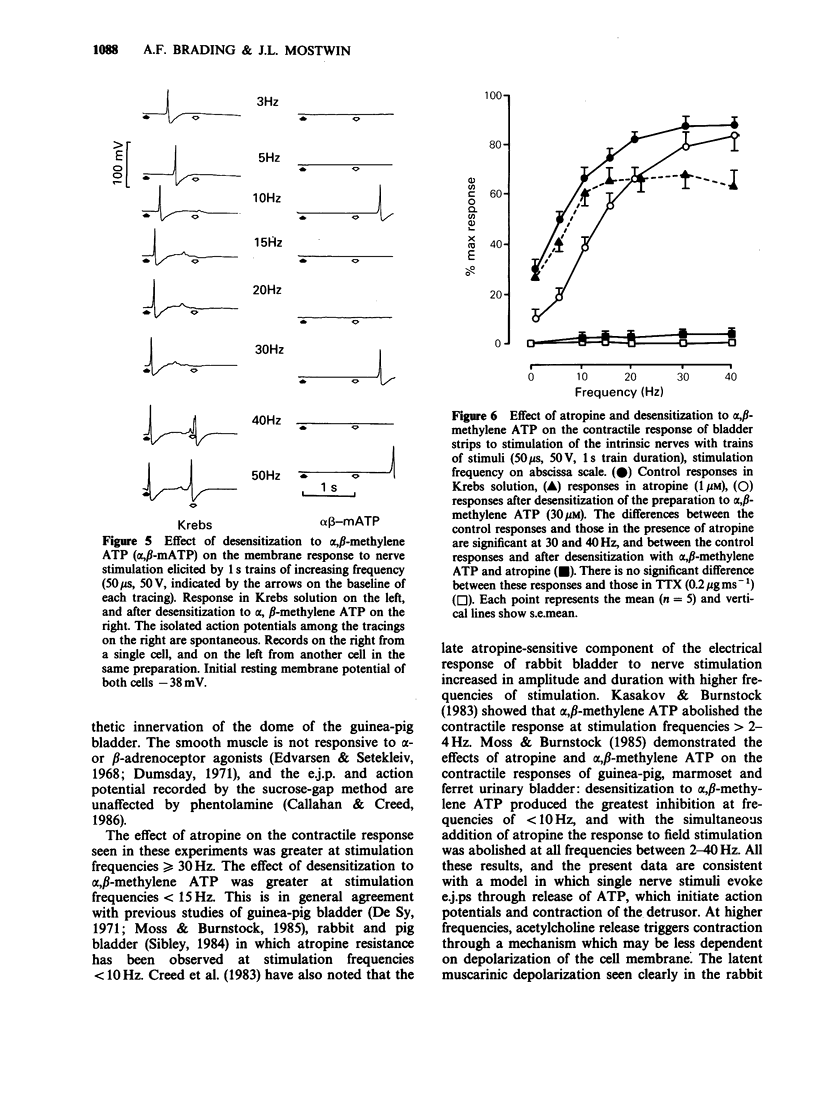
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Mostwin J. L., Sibley G. N., Speakman M. J. The role of smooth muscle and its possible involvement in diseases of the lower urinary tract. Clin Sci (Lond) 1986;70 (Suppl 14):7s–13s. doi: 10.1042/cs070s007. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R., Kasakov L. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978 May;63(1):125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Dumsday B., Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972 Mar;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan S. M., Creed K. E. Non-cholinergic neurotransmission and the effects of peptides on the urinary bladder of guinea-pigs and rabbits. J Physiol. 1986 May;374:103–115. doi: 10.1113/jphysiol.1986.sp016068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E., Ishikawa S., Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol. 1983 May;338:149–164. doi: 10.1113/jphysiol.1983.sp014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E. Membrane properties of the smooth muscle membrane of the guinea-pig urinary bladder. Pflugers Arch. 1971;326(2):115–126. doi: 10.1007/BF00586904. [DOI] [PubMed] [Google Scholar]
- De Sy W. The reactivity of isolated urinary bladder strips of the guinea-pig towards electric stimulation. Arch Int Physiol Biochim. 1971 Aug;79(3):459–468. doi: 10.3109/13813457109085330. [DOI] [PubMed] [Google Scholar]
- Dumsday B. Atropine-resistance of the urinary bladder innervation. J Pharm Pharmacol. 1971 Mar;23(3):222–225. doi: 10.1111/j.2042-7158.1971.tb08649.x. [DOI] [PubMed] [Google Scholar]
- Edvardsen P., Setekleiv J. Distribution of adrenergic receptors in the urinary bladder of cats, rabbits, and guinea-pigs. Acta Pharmacol Toxicol (Copenh) 1968;26(5):437–445. doi: 10.1111/j.1600-0773.1968.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Fujii K. Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J Physiol. 1988 Oct;404:39–52. doi: 10.1113/jphysiol.1988.sp017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by alpha,beta-methylene ATP. Eur J Pharmacol. 1985 Aug 15;114(2):239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The Innervation of the Pelvic and adjoining Viscera: Part II. The Bladder. Part III. The External Generative Organs. Part IV. The Internal Generative Organs. Part V. Position of the Nerve Cells on the Course of the Efferent Nerve Fibres. J Physiol. 1895 Dec 30;19(1-2):71–139. doi: 10.1113/jphysiol.1895.sp000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie I., Burnstock G., Dolly J. O. The effects of purified botulinum neurotoxin type A on cholinergic, adrenergic and non-adrenergic, atropine-resistant autonomic neuromuscular transmission. Neuroscience. 1982 Apr;7(4):997–1006. doi: 10.1016/0306-4522(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Moss H. E., Burnstock G. A comparative study of electrical field stimulation of the guinea-pig, ferret and marmoset urinary bladder. Eur J Pharmacol. 1985 Aug 27;114(3):311–316. doi: 10.1016/0014-2999(85)90375-9. [DOI] [PubMed] [Google Scholar]
- Mostwin J. L. Receptor operated intracellular calcium stores in the smooth muscle of the guinea pig bladder. J Urol. 1985 May;133(5):900–905. doi: 10.1016/s0022-5347(17)49277-9. [DOI] [PubMed] [Google Scholar]
- Sibley G. N. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol. 1984 Sep;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald R. J., Jr Arylazido aminopropionyl atp (ANAPP3) antagonism of cat urinary bladder contractions. J Auton Pharmacol. 1982 Sep;2(3):175–179. doi: 10.1111/j.1474-8673.1982.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]


