Abstract
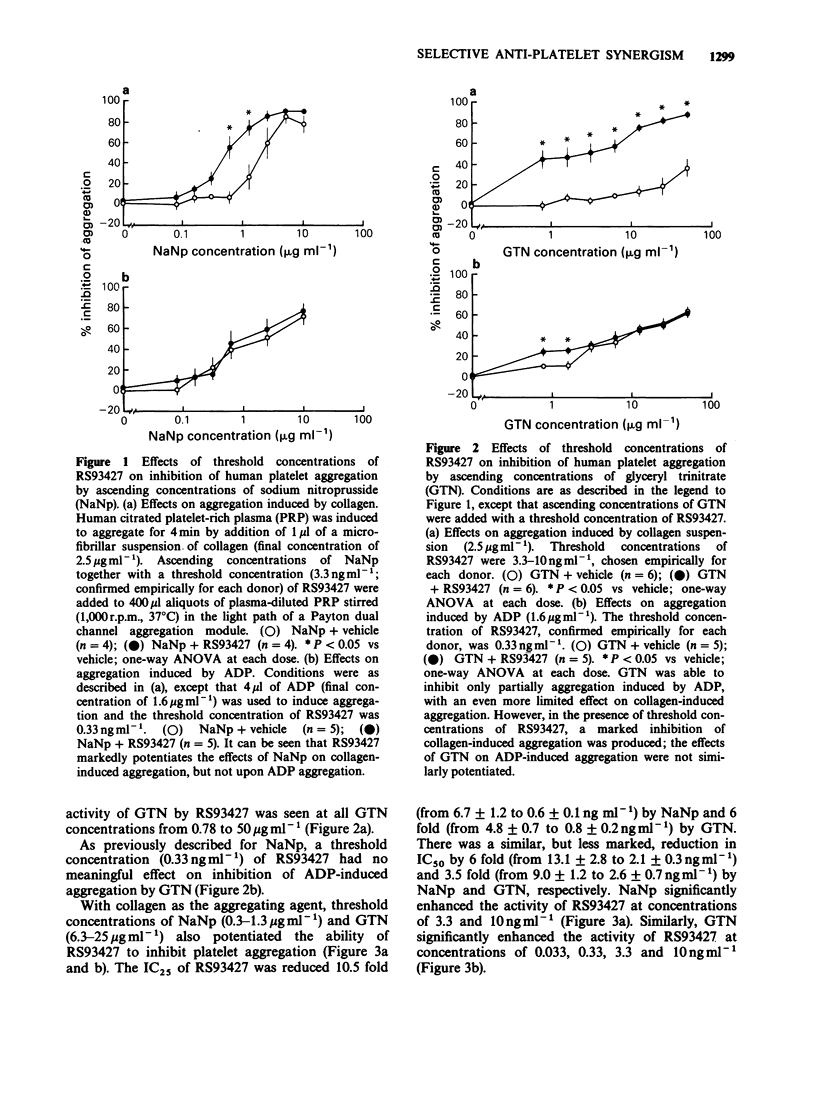
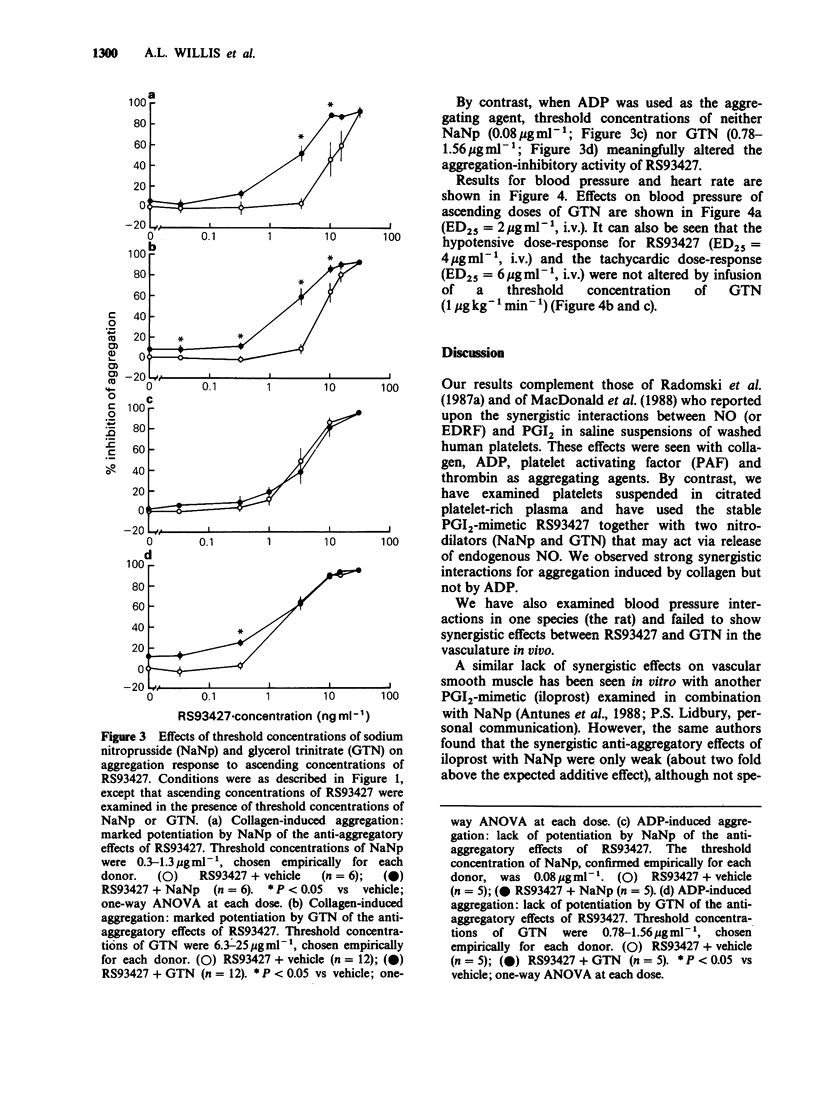
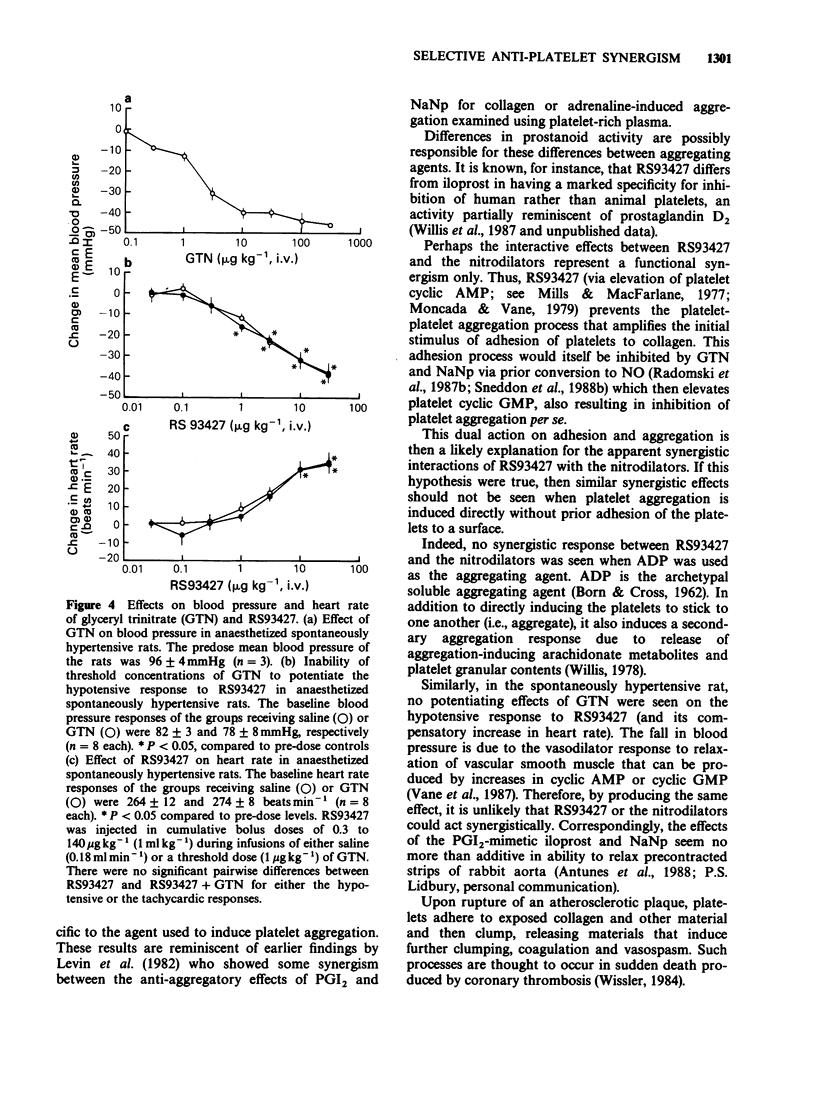
1. Citrated platelet-rich plasma from human donors was used to examine turbidometrically the platelet aggregation response to collagen (2.5 micrograms ml-1) and ADP (1.6 microgram ml-1). 2. With collagen as an aggregating agent, the limited (35% maximal inhibition) inhibitory effects of glyceryl trinitrate (GTN, 0.78-50 micrograms ml-1) were markedly potentiated by threshold (3.3-10 ng ml-1) concentrations of RS93427, an orally active prostacyclin-mimetic. Almost complete inhibition of aggregation could then be produced. 3. A threshold concentration of RS93427 (3.3 ng ml-1) similarly potentiated the ability of sodium nitroprusside (NaNp, 0.78-10 micrograms ml-1) to inhibit collagen-induced platelet aggregation. There was an 8 fold reduction in the IC25 concentration of NaNp. 4. Threshold concentrations of the nitrodilators were also able to potentiate the anti-aggregatory effects of RS93427 (0.03-30 ng ml-1) on collagen-induced platelet aggregation. With threshold concentrations of either GTN (6.3-25 micrograms ml-1) or NaNp (0.3-1.3 microgram ml-1), the mean IC50 concentration of RS93427 was reduced 4 or 6 fold, respectively, while the IC25 concentration was reduced 6 or 10 fold, respectively. 5. No similar synergistic interactions were seen between RS93427 and the nitrodilators when ADP was used as an aggregating agent. 6. In spontaneously hypertensive rats, the dose-response for the hypotensive response to bolus doses of RS93427 was not altered by concomitant steady state infusion of a threshold dose (1 micrograms kg-1 min-1) of GTN. 7. Possible therapeutic implications of these findings are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. Effect of adenosine diphosphate on the concentration of platelets in circulating blood. Nature. 1963 Mar 9;197:974–976. doi: 10.1038/197974a0. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Levin R. I., Weksler B. B., Jaffe E. A. The interaction of sodium nitroprusside with human endothelial cells and platelets: nitroprusside and prostacyclin synergistically inhibit platelet function. Circulation. 1982 Dec;66(6):1299–1307. doi: 10.1161/01.cir.66.6.1299. [DOI] [PubMed] [Google Scholar]
- Macdonald P. S., Read M. A., Dusting G. J. Synergistic inhibition of platelet aggregation by endothelium-derived relaxing factor and prostacyclin. Thromb Res. 1988 Mar 1;49(5):437–449. doi: 10.1016/s0049-3848(98)90001-9. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978 Sep;30(3):293–331. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Willis A. L., Smith D. L., Vigo C., Kluge A., O'Yang C., Kertesz D., Wu H. Orally active prostacyclin-mimetic RS-93427: therapeutic potential in vascular occlusive disease associated with atherosclerosis. Adv Prostaglandin Thromboxane Leukot Res. 1987;17A:254–265. [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]



