Abstract
Cytoskeleton disorganization is an early step in the activation process of matrix metalloproteinase 2 (MMP-2) by membrane type 1 MMP (MT1-MMP) but is also associated with endoplasmic reticulum (ER) dysfunction and subsequent cell death. Given evidence that the ER-embedded glucose-6-phosphate transporter (G6PT) regulates glioblastoma cell survival and that MT1-MMP is a key enzyme in the cancer cell invasive phenotype, we explored the molecular link between G6PT and MT1-MMP. Cytoskeleton-disrupting agents such as concanavalin A (ConA) and cytochalasin D triggered proMMP-2 activation and cell death in U87 glioma cells. ConA decreased G6PT gene expression, an event that was also observed in cells overexpressing the fulllength recombinant MT1-MMP protein. Overexpression of a membrane-bound catalytically active but cytoplasmic domain-deleted MT1-MMP was unable to downregulate G6PT gene expression or to trigger necrosis. Gene silencing of MT1-MMP with small interfering RNA prevented proMMP-2 activation and induced G6PT gene expression. ConA inhibited Akt phosphorylation, whereas overexpression of recombinant G6PT rescued the cells from ConA-induced proMMP-2 activation and increased Akt phosphorylation. Altogether, new functions of MT1-MMP in cell death signaling may be linked to those of G6PT. Our study indicates a molecular signaling axis regulating the invasive phenotype of brain tumor cells and highlights a new “bioswitch” function for G6PT in cell survival.
Keywords: Glioma, cytoskeleton, glucose-6-phosphate transporter, MT1-MMP, necrosis
Introduction
The endoplasmic reticulum (ER) is a membrane-bound organelle present in all eukaryotic cells. Recently, ER stress signaling has been linked to disease states involving insulin resistance, disordered lipid metabolism, and hypoxia tolerance in tumor progression [1,2]. In addition, the ER is a multifunctional metabolic compartment that controls entry and release of calcium, sterol biosynthesis, apoptosis, and the release of arachidonic acid [3,4]. Despite its complex organization, the ER is a continuous membrane compartment whose architecture depends on microtubule dynamics [5]. The ER is primarily known as the site of synthesis and folding of secreted, membrane-bound, and some organelle-targeted proteins. Recent evidence suggests that the microtubulin cytoskeleton and the centrosomes (the microtubulin cytoskeleton-organizing centers) are essential for the trafficking and internalization of ‘the membrane-bound matrix metalloproteinase MT1-MMP [6], involved in brain tumor cell invasion, extracellular matrix (ECM) degradation and cell-ECM interaction [7]. Interestingly, altered expression, maturation and trafficking of MT1-MMP to the plasma membrane were observed in diabetic states [8,9], a condition known to upregulate the expression of an ER-embedded protein, the glucose-6-phosphate transporter (G6PT) [10]. G6PT expression was shown to be downregulated by MT1-MMP in bone marrow-derived stromal cells, where it was suggested to provide a molecular link between proMMP-2 activation and chemotaxis processes in cell mobilization [11].
Several factors are required for optimum protein folding, including ATP, Ca2+, and an oxidizing environment that will allow disulfide-bond formation [12]. Because of this specialized environment requirement, the stresses that perturb cellular energy levels, redox state, or Ca2+ concentration can often result in the intracellular accumulation of unfolded protein, which is called ER stress response. Recently, we have provided evidence that G6PTG6PT regulated U87 glioma cell chemotaxis [13] and survival [14]. Tumor cells often show evidence of constitutive ER stress, possibly due to hypoxia and glucose depletion [15]. In fact, the ATP-depleting agents and ER stress inducers 2-deoxyglucose and 5-thioglucose have been shown to inhibit MMP-2 secretion from U87 glioma cells [13], a process known to contribute to tumor development [16]. G6PT is thought to have a role in sequestering intracellular Ca2+ within the ER through an ATP-mediated process [17]. Because manipulating the ER stress response of tumor cells is a promising therapeutic strategy [15] and because various anticancer drugs have been shown to induce ER stress and to affect the invasive or metabolic control of cancer cells [18,19], we explored the potential molecular link between MT1-MMP and G6PT functions within the ER that could potentially regulate the brain tumor cell invasive phenotype.
Results
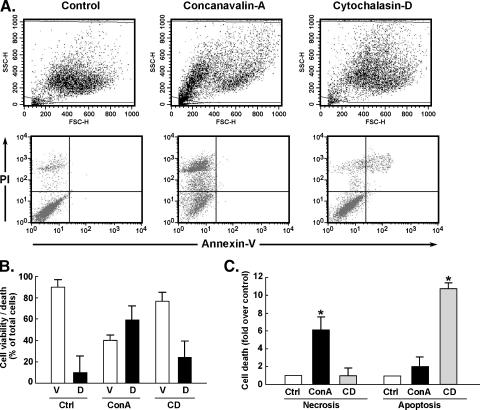
Differential Induction of Cell Necrosis and Cell Apoptosis by Cytoskeleton-Disrupting Agents
Concanavalin A (ConA) and cytochalasin D (CytoD) have been shown to disrupt the cytoskeleton architecture [20]. After cell staining with annexin V/propidium iodide (PI), flow cytometry was used to assess the extent of cell death induced by both agents. From the side scatter (SSC)/forward scatter (FSC) plots, the changes in cell “morphology” features that each agent induced are clearly visible (Figure 1A). When annexin V/PI cell staining was performed, ConA treatment resulted in a marked increase in necrosis (Figure 1A, bottom, upper left quadrant), whereas CytoD triggered late apoptosis (Figure 1A, bottom, upper right quadrant). Cell viability and total cell death were quantified (Figure 1B). Furthermore, cell necrosis and cell apoptosis (early and late) (Figure 1C) were also separately quantified to show the differential induction of necrosis by ConA and induction of apoptosis by CytoD.
Figure 1.
Differential induction of cell necrosis and cell apoptosis by cytoskeleton-disrupting agents. U87 glioblastoma cells were cultured as described in the Materials and Methods section until they reached ∼ 75% to 90% confluence. They were then serum-starved for 24 hours before the addition of 10 µg/ml ConA or 1 µmol/l CytoD (CD). Incubation was continued for another 24 hours. (A) Flow cytometry was then used to either assess light-scattering properties associated with changes in cell morphology (top panels) or cell death from annexin V/PI-stained cells (bottom panels). (B) Cell viability (white columns) was assessed as the percentage of total cells present in the lower left quadrants. Cell death (black columns) represents the combined cells present in necrosis, early/late apoptosis (black columns). (C) The respective cell death proportions attributable to either necrosis or apoptosis (early and late) are shown. Data are the averages ± SEM of four independent experiments. Statistical significance is represented by (*).
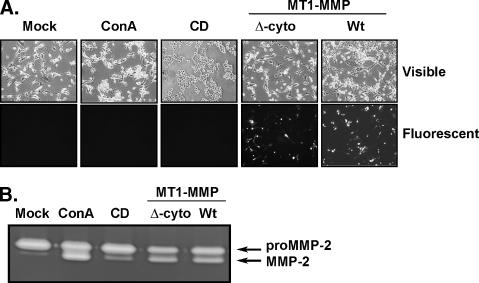
Recombinant MT1-MMP and Cytoskeleton-Disrupting Agents Induce proMMP-2 Activation
Latent proMMP-2 activation into its active MMP-2 form has been correlated with cell death [21,22]. Accordingly, we have previously shown that the cytoskeleton-disrupting agents ConA and CytoD triggered proMMP-2 activation in U87 glioma cells [23]. Using fluorescent microscopy, we validated overexpression of full-length wild-type (wt) recombinant MT1-MMP or the membrane-bound cytoplasmic-deleted recombinant MT1-MMP both fused to green fluorescent protein (GFP) (Figure 2A) and consequent proMMP-2 activation demonstrated by gelatin zymography (Figure 2B). Transfections of cells with cDNA encoding GFP alone did not affect the ability of ConA or CytoD to induce proMMP-2 activation (data not shown). These results suggest that, similar to cytoskeleton-disrupting agents ConA and CytoD, MT1-MMP-mediated proMMP-2 activation is also potentially linked to the control of cell survival in U87 glioma cells.
Figure 2.
Recombinant MT1-MMP and cytoskeleton-disrupting agents induce proMMP-2 activation. U87 glioblastoma cells were treated as described in the legend to Figure 1. Transfections of cDNA plasmids encoding full-length Wt-MT1-MMP and cytoplasmic domain - truncated MT1-MMP both fused to GFP were carried out as described in the Materials and Methods section. (A) Changes in cell morphology are shown through phase-contrast microphotography (visible light), whereas transfection efficiency was validated by fluorescence microscopy. (B) Gelatin zymography was carried out to assess the extent of proMMP-2 activation levels, as described in the Materials and Methods section, using conditioned media isolated from each of the serum-starved cell conditions.
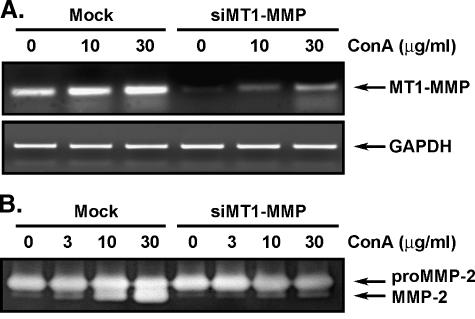
ConA-Mediated Activation of proMMP-2 Requires MT1-MMP
To assess the involvement of MT1-MMP in the proMMP-2 activation process and the necrotic effects of ConA, we specifically downregulated MT1-MMP gene expression using a specific MT1-MMP gene silencing strategy [24]. Cells were transfected with mismatched (Mock) or MT1-MMP-targeted small interfering RNA (siRNA) duplexes, as described in the Materials and Methods section, and then treated with increasing concentrations of ConA. Total RNA was isolated and reverse transcription-polymerase chain reaction (RTPCR) confirmed that MT1-MMP gene downregulation was successfully achieved (Figure 3A). Conditioned media from these conditions were also isolated to assess the MT1-MMP-mediated proMMP-2 activation by ConA. Gelatin zymography clearly showed significantly decreased proMMP-2 activation in the cells in which MT1-MMP gene expression had been knocked down (Figure 3B). This was quantified by scanning densitometry, showing close to 90% inhibition (data not shown). This clearly suggests that the ConA-mediated events that we previously observed involve MT1-MMP in proMMP-2 activation and are likely involved in cell death.
Figure 3.
ConA-mediated activation is an MT1-MMP- mediated event. Because proMMP-2 activation is thought to proceed through an MT1-MMP-mediated event, U87 glioblastoma cells were transfected with siRNA against MT1-MMP or mismatched siRNA (Mock) for 48 hours, as described in the Materials and Methods section, before treatment with increasing concentrations of ConA. (A) Total RNA was isolated and MT1-MMP gene expression was assessed by RT-PCR as described in the Materials and Methods section. The gene expression level of GAPDH was used as the internal control. (B) Gelatin zymography was performed to assess the extent of proMMP-2 activation in the conditioned media of serum-starved Mock and siMT1-MMP cells treated with increasing concentrations of ConA.
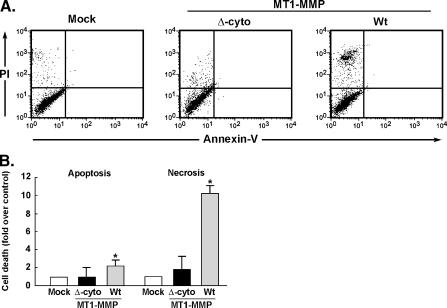
MT1-MMP Cytoplasmic Domain Is Responsible for Cell Death Signaling
Although extracellular catalytic inhibition of MT1-MMP only partially reversed its ability to trigger cell death [11], the implication of MT1-MMP in signaling intracellular cell death processes was thus evaluated. Transient transfection using cDNA plasmids encoding either the full-length or the membrane-bound cytoplasmic-deleted recombinant forms of MT1-MMP was performed in U87 glioma cells. Annexin V/PI staining was then performed and cell death (necrosis and apoptosis) was assessed by flow cytometry (Figure 4A). Overexpression of native MT1-MMP significantly triggered cell necrosis by more than 10 times, whereas cell apoptosis was also induced approximately twofold (Figure 4B, gray bars). Although still catalytically active at the cell surface (Figure 2B), the deletion of the MT1-MMP cytoplasmic domain significantly abrogated the induction of both cell necrosis and apoptosis (Figure 4B, black bars). These observations suggest that active MMP-2 is not responsible for cell death and that some MT1-MMP-mediated intracellular signaling is a prerequisite for the control of cell survival.
Figure 4.
MT1-MMP overexpression triggers cell death and is signaled through its intracellular cytoplasmic domain. U87 glioblastoma cells were transfected with cDNA encoding full-length Wt-MT1-MMP or its cytoplasmic domain-truncated form as described in the Materials and Methods section. (A) Flow cytometry was used to assess the extent of cell death in annexin V/PI-stained cells. (B) Quantification of cell death caused by either combined early and late apoptosis (upper and lower right quadrants) or necrosis (upper left quadrants) was performed for cells transfected with cytoplasmic domain-truncated MT1-MMP (Δ-cyto) or full-length Wt-MT1-MMP.
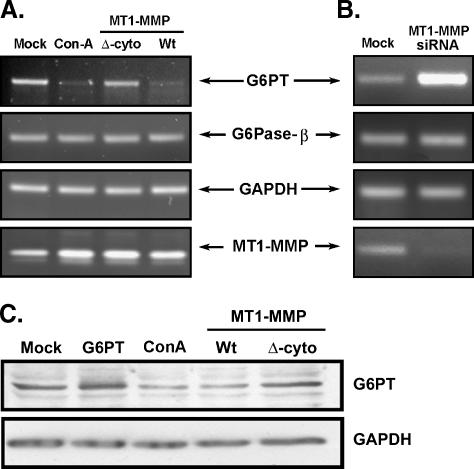
MT1-MMP Overexpression and ConA Treatment Downregulate G6PT Gene Expression
To investigate the intracellular events involved in MT1-MMP- and ConA-mediated cell death, we examined the prosurvival microsomal glucose-6-phosphate translocase (G6PT) as a potential link. Cytoskeleton disruption is often linked to ER stress [25,26], and silencing of G6PT, a microsomal resident protein, has recently been shown to induce cell death in U87 glioma cells [14]. We thus isolated total RNA from ConA-treated cells and from MT1-MMP-transfected cells because cell necrosis was a common event in both conditions. RT-PCR was performed as described in the Materials and Methods section and we found that G6PT gene expression was significantly reduced in ConA-treated and in the MT1-MMP-transfected cells (Figure 5A). Interestingly, in agreement with its inability to trigger cell death, deletion of MT1-MMP's cytoplasmic domain was also ineffective in reducing G6PT gene expression. Gene expression of glucose-6-phosphatase (G6Pase)-β, the only other component of the G6Pase system that was expressed in U87 cells [13] and of GAPDH remained unaffected and can be considered as unaffected internal controls (Figure 5A). ConA treatment and MT1-MMP overexpression resulted, as expected, in an increase in MT1-MMP transcript levels. Altogether, this demonstrates that necrosis-inducing conditions, such as those triggered by ConA or overexpression of recombinant MT1-MMP, are molecularly linked to the prosurvival functions of G6PT. Interestingly, when MT1-MMP gene expression was silenced, the expression of G6PT increased significantly in comparison to the mismatched siRNA-transfected cells (Mock), suggesting that MT1-MMP exerted a repressive effect on G6PT gene regulation (Figure 5B). Modulation of G6PT gene expression was further confirmed at the protein level. We showed that ConA treatment or Wt-MT1-MMP overexpression downregulated G6PT protein expression (Figure 5C). Thus, our results show that G6PT gene regulation is signaled by the intracellular MT1-MMP cytoplasmic domain.
Figure 5.
Both MT1-MMP overexpression and ConA treatment downregulate G6PT gene and protein expression. (A) Total RNA was isolated from untreated (Mock), ConA-treated, or U87 glioblastoma cells transfected with either the cytoplasmic domain-truncated MT1-MMP (Δ-cyto) or full-length Wt-MT1-MMP cDNA. RT-PCR was performed to assess the changes in G6PT, G6Pase-β, GAPDH, or MT1-MMP gene expression in each condition. (B) MT1-MMP gene expression was specifically downregulated in U87 glioblastoma cells transfected with siMT1-MMP but not in mismatched siRNA-transfected cells (Mock) as described in the Materials and Methods section. Total RNA and RT-PCR were performed as in (A). (C) Cell lysates were isolated from U87 cells transfected with cDNA encoding G6PT, Wt-MT1-MMP, or Δ-cyto MT1-MMP, or treated with ConA. Western blotting and immunodetection was performed with anti-G6PT and anti-GAPDH antibodies.
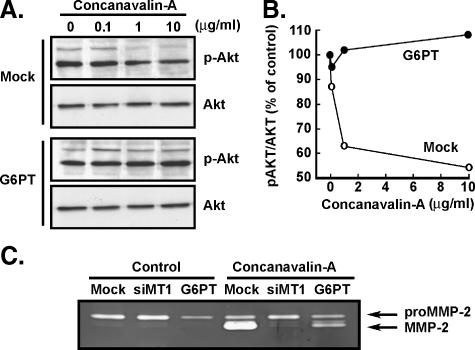
G6PT Overexpression Antagonizes the ConA-Mediated Lethal Effect
To characterize the molecular mechanism linking MT1-MMP to G6PT, we next assessed whether constitutively expressed recombinant G6PTcould overcome the lethal effect of ConA. MT1-MMP synthesis by a mechanism that involves phosphatidylinositol-3 kinase (PI3K)/Akt/mTOR was recently highlighted [27] and, thus, Akt phosphorylation state was explored. U87 cells were transiently transfected with cDNA encoding G6PT and then treated with the cell death inducer ConA. Immunodetection of total and phosphorylated Akt was performed on Mock and G6PT-transfected cell lysates. We observed that ConA decreased the basal levels of phosphorylated Akt by up to 50%, although not of total Akt protein expression, in Mock-transfected cells (Figure 6A). Transient overexpression of recombinant G6PT completely reversed the effects of ConA on Akt phosphorylation (Figure 6B). Despite being more associative than mechanistic, these observations confirm the G6PT prosurvival activity [14] and show that targeting PI3K/Akt signaling by ConA induces apoptosis [28]. Finally, we showed by zymography that G6PT overexpression significantly antagonized ConAmediated proMMP-2 activation (Figure 6C), an effect that may involve inhibiting MT1-MMP functions.
Figure 6.
G6PT overexpression antagonizes the ConA-mediated lethal effect. (A) Mock or G6PT-transfected U87 cells were treated with increasing ConA concentrations and cell lysates were used to immunodetect the levels of total or phosphorylated Akt (p-Akt). (B) Scanning densitometry was used to quantify the Akt immunoreactive bands, and results were expressed as the ratio of p-Akt over Akt. (C) Conditioned media isolated from serum-starved Mock, siMT1-MMP (siMT1), or G6PT-transfected U87 cells were used to perform gelatin zymography.
Discussion
Gliomas remain a great challenge in oncology today as they account for more than 50% of all brain tumors and are by far the most common primary brain tumors in adults [29]. More importantly, the mechanisms involved in the resistance of migrating glioblastoma cells to chemotherapy or to radiationinduced cell death have long been recognized [30] and still receive much attention in order to optimize future cellular targets for the treatment of glioblastomas [31]. A relationship between cell migration and apoptosis was highlighted by the observations that resistance to apoptosis is closely linked to tumorigenesis, but that paradoxically migrating tumor cells can also still be induced to die by nonapoptotic mechanisms such as necrosis [32]. In fact, tissue necrosis is a characteristic feature of malignant gliomas, in particular glioblastoma, and is most likely the consequence of rapidly increasing tumor mass that is inadequately oxygenated by the preexisting vasculature [33]. Because tumor cells respond to hypoxic stress by upregulating a variety of genes involved in glucose uptake, glycolysis, and angiogenesis, all essential to maintaining nutrient availability and intracellular ATP levels [34], the intracellular metabolic compartments regulating cell survival and invasiveness are of particular interest. Besides mitochondria and lysosomes, the ER is very important in this respect, as it is now recognized as an important sensor of cellular stress and plays a key role in the release and activation of death factors such as cathepsins, calpains, and other proteases through intracellular calcium flux [35]. For instance, migrating glioblastoma cells have recently been shown to overexpress death-associated protein-3 [36]. Therefore, new routes should be investigated as possible issues to combat apoptotic-resistant migrating glioblastoma cells.
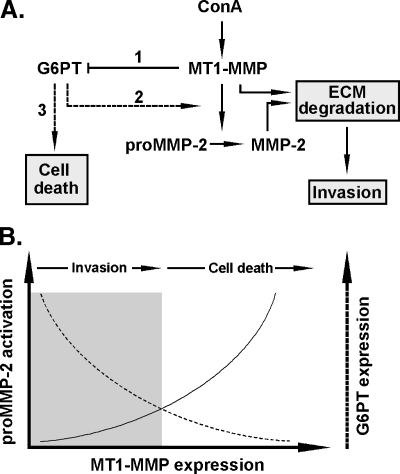
Given the ER localization of G6PTand the crucial role that the ER plays as a metabolic compartment, we suggest that G6PT is a key mediator in the regulation of cancer cell survival and ECM degradation signaling. In fact, regulation of G6PT expression may function as a “bioswitch” (Figure 7A) enabling cells to promote either migration or cell death processes. Switching from one state to another may occur in response to external stimuli, such as hypoxia, or as a result of intracellular metabolic changes [37]. Intracellular regulation of Ca2+ flux and cytosolic ATP and G6P levels are among the parameters that G6PT may modulate in the transformed proliferating cells. As such, metabolic profiling of cell growth and death in cancer is already used to identify the changes in glucose utilization for macromolecule synthesis in cancer [38,39]. Among the several brain tumor-derived cell lines tested, G6PT expression was found the highest in the highly infiltrating and angiogenic U87 glioma cells [13]. This potentially suggests that metabolic adaptive capacity, in part through G6PT, may regulate the invasive phenotype of aggressive cancer cells. Documenting the pleiotropic roles of G6PT in cancer cells will thus help optimize or design new antitumor therapies.
Figure 7.
G6PT as a bioswitch intermediate in the regulation of proMMP-2 activation and cell death signaling. (A) Summarized scheme of the events that lead to MT1-MMP- mediated signaling in proMMP-2 activation and in cell death. ConA upregulates MT1-MMP gene and protein expression, which in turn downregulates G6PT expression (1). Low levels of G6PT release the inhibitory effect on MT1-MMP- mediated proMMP-2 activation (2), which altogether leads to ECM degradation and cell invasion. When a specific balance is reached between MT1-MMP and G6PT expression (i.e., high MT1-MMP expression, low G6PT expression), cell death signaling is then activated (3). (B) Our data show that MT1-MMP expression leads to both proMMP-2 activation (solid line) and to concomitant downregulation of G6PT expression (dashed line). Conceptually, we suggest that a balance between the early invasive processes that are initiated by the increased MT1-MMP expression correlate with proMMP-2 activation to hydrolyze the ECM and promote cell migration (left, shaded area). Concurrently, G6PT expression decreases until it reaches a threshold where its inhibitory effect on proMMP-2 activation is released, which then leads to cell death (right area). The intersection between the G6PT expression curve and that of the proMMP-2 activation is the bioswitch that reflects the balance between cell invasion and cell death signaling.
We previously showed that inhibiting G6PT function by chlorogenic acid or by ATP-depleting agents such as 2-deoxyglucose in brain tumor-derived cells did not directly affect MT1-MMP catalytic function but still resulted in decreased invasiveness [13]. Moreover, cancer cells frequently display high rates of aerobic glycolysis compared with their nontransformed counterparts, and the possible applications of 2-deoxyglucose in anticancer therapies further support the theory that inhibiting G6PT function in cancer cells could decrease tumor progression. Some evidence also suggests that ER stress-inducing agents are useful as cancer agents and that excessive ER stress leads to apoptosis. These agents include glycosylation inhibitors (e.g., tunicamycin and 2-deoxyglucose), agents that deplete ER Ca2+ (e.g., the sarcoendoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin and various ionophores) and agents that induce reductive stress (dithiotreitol and β-mercaptoethanol) [40].
We also found that cytoskeleton remodeling is among the first events in a cascade of activation that leads to MT1-MMP-mediated downregulation of brain cancer cell survival, in part through PI3K/Akt-mediated plasma membrane to nucleus signaling. Interestingly, part of the switch between cell migration and cell death comes under the control of the PTEN/Akt/PI3K/mTOR pathway [30]. Survival through PI3K/Akt signaling is complex [41] and the activity of the PI3K/Akt pathway is, in fact, often upregulated in brain tumors as a result of excessive stimulation by growth factor receptors and Ras [42]. Moreover, glioblastomas frequently carry mutations in the PTEN tumor suppressor gene, whose tumor suppressor properties are closely related to its inhibitory effect on the PI3K-dependent activation of Akt signaling [43]. The activation of the PI3K pathway is significantly associated with increasing tumor grade, lower levels of apoptosis, and an adverse clinical outcome in the case of human gliomas [44]. All these factors indicate that aberrant PI3K/Akt signaling means that both cell proliferation in glioma cells and cell migration have become abnormal. A number of publications have already reported that an aberrantly activated PI3K/Akt pathway renders tumor cells resistant to cytotoxic insults, including those related to anticancer drugs [45,46]. In light of these results and because G6PT was able to reverse the cytotoxic effects of ConA, it is tempting to suggest that functional targeting of G6PT such as by the use of chlorogenic acid or by its analogs, the most potent G6PT inhibitors, could augment the effectiveness of chemotherapy on glioma cells.
Maintenance of cytoarchitecture is required for cell survival because its perturbation by CytoD- or ConA-mediated MT1-MMP mechanisms diminished cell survival and were correlated to proMMP-2 activation [21,22] (this study). In fact, silencing of the MT1-MMP gene prevented ConA from activating proMMP-2. Moreover, we showed that the intracellular domain of MT1-MMP is an absolute requirement for transducing the intracellular signaling that leads to cell death. Although the exact identity of the amino acid residues from the MT1-MMP intracellular domain remains to be addressed, speculations about the Tyr573, Cys574, and Val582 have been put forward as important for MT1-MMP signaling [47,48]. Similarly, a caspase-dependent mechanism has recently been associated with MT1-MMP function in endothelial cell morphogenic differentiation [49]. This suggests that MT1-MMP acts as a potential cell death sensor/effector that signals ECM degradation processes to be activated. Interestingly, hypoxia increased levels of MT1-MMP and the MT1-MMP transcription factor regulator Egr-1 in bone marrow-derived stromal cells [50], a condition that led to cell death [51]. Moreover, ConA was found ineffective in activating proMMP-2 or inhibiting G6PT gene expression in bone marrow stromal cells isolated from Egr-1-/- mouse [11].
The fact that G6PT overexpression inhibited ConAinduced proMMP-2 activation, but not cell death, further suggests that complex differential regulation takes place and highlights the pleiotropic intracellular functions of G6PT. Moreover, this observation also provides insight into the cellular event chronology, confirming that MT1-MMP-mediated activity and signaling are among the first steps that inhibit G6PT expression, ultimately leading to cell death. Interestingly, our data are consistent with some of the abnormal polymorphonuclear neutrophil phenotypes observed in glycogen storage disease type 1b, a clinical condition where the G6PT gene and/or protein activity is defective [52,53]. In fact, it has been hypothesized that G6PT might function as a G6P receptor/sensor [53] or that it could favor calcium sequestration in the ER lumen [17]. Finally, although no effects in response to MT1-MMP or cytoskeleton disruption were observed on the ER-embedded G6Pase-b, recent evidence regarding G6Pase-β involvement in cell survival was demonstrated in neutrophils, as disruption of the G6Pase-β gene expression also led to cell death, an event suggestive of a vital interaction between G6PT and G6Pase-β [54].
In summary, we highlight new functions of MT1-MMP in cell death signaling that may potentially be linked to those of the ER-embedded functions of G6PT. In fact, we believe that this signaling axis may not be exclusive to one cell line, but rather may regulate cell mobilization processes through metabolic and/or cell survival control such as similarly demonstrated for bone marrow-derived stromal cells [11]. Our study further shows a molecular axis linking the invasive phenotype of brain tumor cells to their potential metabolic control by G6PT and supports the notion of an MT1-MMP/G6PT bioswitch (Figure 7B) that could regulate glucose homeostasis and thus restrain cancer cell proliferation, inhibit ECM degradation, or induce cell death. By revealing tumor-specific metabolic shifts in tumor cells, metabolic profiling studies will further enable drug developers to identify the metabolic steps that control cell proliferation, thus aiding the identification of new anticancer targets and screening of lead compounds for antiproliferative metabolic effects.
Materials and Methods
Materials
SDS and BSA were purchased from Sigma (Oakville, ON, Canada). Cell culture medium was obtained from Life Technologies (Burlington, ON, Canada). Electrophoresis reagents were purchased from Bio-Rad (Mississauga, ON, Canada). The enhanced chemiluminescence (ECL) reagents were from Amersham Pharmacia Biotech (Baie d'Urfé, QC, Canada). The polyclonal antibodies against Akt and phospho-Akt were purchased from Cell Signaling (Danvers, MA). All other reagents were from Sigma-Aldrich Canada.
Cell Culture and Transfection Method
The U87 glioblastoma cell line was purchased from American Type Culture Collection (Manassas, VA) and cultured in Eagle's minimum essential medium (MEM) containing 10% (vol/vol) FBS (HyClone Laboratories, Logan, UT) and 2 mmol/l glutamine at 37°C under a humidified atmosphere containing 5% carbon dioxide. U87 glioblastoma cells were transiently transfected with the cDNA constructs encoding either the membrane-bound cytoplasmic domain-truncated MT1-MMP (Δ-cyto) where the last 20 amino acid residues were deleted, or the full-length Wt-MT1-MMP fused to GFP [55], or with 20 nmol/l siRNA (see below) using Lipofectamine 2000 (Invitrogen, Burlington, ON, Canada). The occurrence of MT1-MMP-specific gene knockdown was evaluated by semiquantitative RT-PCR and validated by assessing MT1-MMP-mediated proMMP-2 activation. Mock transfections of U87 cultures with pcDNA (3.1+) or cDNA encoding GFP expression vectors alone were used as controls.
RNA Interference
RNA interference experiments were performed using Lipofectamine 2000. An siRNA against MT1-MMP (siMT1-MMP) and mismatch siRNA were synthesized by EZBiolab Inc. (Westfield, IN) and annealed to form duplexes. The sequence of the siMT1-MMP used in this study is as follows: 5′-CCA--CCAGAAGCUGAAGGUAGAAdTdT-3′ (sense) and 5′-UUCUACCUUCAGCUUCUGGdTdT-3′ (antisense) [24]. The diminution of MT1-MMP expression, as assessed by RTPCR, ranged routinely from 75% to 90% (not shown).
Total RNA Isolation and RT-PCR Analysis
Total RNA was extracted from cultured U87 cells using TRIzol reagent (Invitrogen). One microgram of total RNA was used for first-strand cDNA synthesis followed by specific gene product amplification with the One-Step RT-PCR Kit (Invitrogen). Primers for G6PT, G6Pase-β, MT1-MMP, and GAPDH were all derived from human sequences and validated [13]. PCR conditions were optimized so that the gene products were examined at the exponential phase of their amplification and the products were resolved on 1.8% agarose gels containing 1 µg/ml ethidium bromide.
Gelatin Zymography
Gelatin zymography was used to assess the extent of proMMP-2 activation in conditioned media. Briefly, a 20-µl aliquot of the culture medium was subjected to SDSPAGE in a gel containing 0.1 mg/ml gelatin. The gels were then incubated in 2.5% Triton X-100 and rinsed in nanopure distilled water. Gels were further incubated at 37°C for 20 hours in 20 mmol/l NaCl, 5 mmol/l CaCl2, 0.02% Brij-35, 50 mmol/l Tris-HCl buffer (pH 7.6), then stained with 0.1% Coomassie Brilliant Blue R-250 and destained in 10% acetic acid, 30% methanol in water. Gelatinolytic activity was detected as unstained bands on a blue background.
Immunoblotting Procedures
Proteins from control and treated cells were separated by SDS-PAGE. After electrophoresis, proteins were electrotransferred to polyvinylidene difluoride membranes, which were then blocked for 1 hour at room temperature with 5% nonfat dry milk in Tris-buffered saline (150 mmol/l NaCl, 20 mmol/l Tris-HCl, pH 7.5) containing 0.3% Tween 20 (TBST). Membranes were further washed in TBST and incubated with the primary antibodies (1:1000 dilution) in TBST containing 3% BSA and 0.02% NaN3, followed by a 1-hour incubation with horseradish peroxidase-conjugated anti-rabbit IgG (1:2500 dilution) in TBST containing 5% nonfat dry milk. Immunoreactive material was visualized by enhanced chemiluminescence (Amersham Biosciences, Baie d'Urfée, QC, Canada).
Analysis of Cell Death by Flow Cytometry
Cell death was assessed by flow cytometry as described previously [14]. Adherent and floating cells were harvested by trypsin digestion and gathered to produce a single cell suspension. The cells were pelleted by centrifugation and washed with PBS. Then, 105 cells were pelleted and suspended in 200 µl of buffer solution and stained with annexin V-fluorescein isothiocyanate and PI according to the manufacturer's protocol (BD Biosciences, Mississauga, ON, Canada). The cells were diluted by adding 300 µl of buffer solution and processed for data acquisition and analysis on a (Becton Dickinson, San Jose, CA) FACS Calibur flow cytometer using CellQuest Pro software. The x- and y-axes indicate the fluorescence of annexin V and PI, respectively. It was possible to detect and quantitatively compare the percentages of gated populations in all of the four regions delineated. In the early stages of apoptosis, phosphatidylserine is well known to translocate to the outer surface of the plasma membrane, which still remains physically intact. As annexin V binds to phosphatidylserine but not to PI, and the dye is incapable of passing the plasma membrane, it is excluded in early apoptosis (annexin V positive/PI negative). Cells in late apoptosis are stained with annexin V and PI (annexin V positive/PI positive). Necrotic cells have lost the integrity of their plasma membrane and are predominantly stained with PI (annexin V negative/PI positive).
Statistical Data Analysis
Data are representative of three or more independent experiments. Statistical significance was assessed by Student's unpaired t test and probability values less than .05 were considered significant; an asterisk (*) identifies such significance in each figure.
Abbreviations
- ConA
concanavalin A
- CytoD
cytochalasin D
- ECM
extracellular matrix
- ER
endoplasmic reticulum
- G6P
glucose-6-phosphate
- G6Pase
glucose-6-phosphatase
- G6PT
G6P transporter
- MMP
matrix metalloproteinase
- PI
propidium iodide
- siRNA
small interfering RNA
Footnotes
This study was funded by grant 1R01CA113553-01A1 (J.C.) and by the Natural Sciences and Engineering Research Council of Canada (NSERC) (B.A.). B.A. holds a Canada Research Chair in Molecular Oncology from the Canadian Institutes of Health Research.
References
- 1.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 2.Koumenis C. ER stress, hypoxia tolerance and tumor progression. Curr Mol Med. 2006;6:55–69. doi: 10.2174/156652406775574604. [DOI] [PubMed] [Google Scholar]
- 3.Csala M, Banhegyi G, Benedetti A. Endoplasmic reticulum: a metabolic compartment. FEBS Lett. 2006;580:2160–2165. doi: 10.1016/j.febslet.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vedrenne C, Hauri HP. Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic. 2006;7:639–646. doi: 10.1111/j.1600-0854.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 6.Remacle AG, Rozanov DV, Baciu PC, Chekanov AV, Golubkov VS, Strongin AY. The transmembrane domain is essential for the microtubular trafficking of membrane type-1 matrix metalloproteinase (MT1-MMP) J Cell Sci. 2005;118:4975–4984. doi: 10.1242/jcs.02610. [DOI] [PubMed] [Google Scholar]
- 7.Sato H, Takino T, Miyamori H. Roles of membrane-type matrix metalloproteinase-1 in tumor invasion and metastasis. Cancer Sci. 2005;96:212–217. doi: 10.1111/j.1349-7006.2005.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher E, Mayer G, Londono I, Bendayan M. Expression and localization of MT1-MMP and furin in the glomerular wall of shortand long-term diabetic rats. Kidney Int. 2006;69:1570–1577. doi: 10.1038/sj.ki.5000316. [DOI] [PubMed] [Google Scholar]
- 9.McLennan SV, Fisher E, Martell SY, Death AK, Williams PF, Lyons JG, Yue DK. Effects of glucose on matrix metalloproteinase and plasmin activities in mesangial cells: possible role in diabetic nephropathy. Kidney Int Suppl. 2000;77:S81–S87. doi: 10.1046/j.1523-1755.2000.07713.x. [DOI] [PubMed] [Google Scholar]
- 10.Van de Werve G, Lange A, Newgard C, Mechin MC, Li Y, Berteloot A. New lessons in the regulation of glucose metabolism taught by the glucose 6-phosphatase system. Eur J Biochem. 2000;267:1533–1549. doi: 10.1046/j.1432-1327.2000.01160.x. [DOI] [PubMed] [Google Scholar]
- 11.Currie JC, Fortier S, Sina A, Galipeau J, Cao J, Annabi B. MT1-MMP downregulates the glucose-6-phosphate transporter expression in marrow stromal cells: a molecular link between proMMP-2 activation, chemotaxis and cell survival. J Biol Chem. 2007;282:8142–8149. doi: 10.1074/jbc.M610894200. [DOI] [PubMed] [Google Scholar]
- 12.Gaut JR, Hendershot LM. The modification and assembly of proteins in the endoplasmic reticulum. Curr Opin Cell Biol. 1993;5:589–595. doi: 10.1016/0955-0674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- 13.Belkaid A, Currie JC, Desgagnes J, Annabi B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006;6:7–12. doi: 10.1186/1475-2867-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belkaid A, Copland IB, Massillon D, Annabi B. Silencing of the human microsomal glucose-6-phosphate translocase induces glioma cell death: potential new anticancer target for curcumin. FEBS Lett. 2006;580:3746–3752. doi: 10.1016/j.febslet.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 15.Linder S, Shoshan MC. Lysosomes and endoplasmic reticulum: targets for improved, selective anticancer therapy. Drug Resist Updat. 2005;8:199–204. doi: 10.1016/j.drup.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Chintala SK, Tonn JC, Rao JS. Matrix metalloproteinases and their biological function in human gliomas. Int J Dev Neurosci. 1999;17:495–502. doi: 10.1016/s0736-5748(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 17.Chen PY, Csutora P, Veyna-Burke NA, Marchase RB. Glucose-6-phosphate and Ca2+ sequestration are mutually enhanced in microsomes from liver, brain, and heart. Diabetes. 1998;47:874–881. doi: 10.2337/diabetes.47.6.874. [DOI] [PubMed] [Google Scholar]
- 18.Carew JS, Nawrocki ST, Krupnik YV, Dunner K, Jr, McConkey DJ, Keating MJ, Huang P. Targeting endoplasmic reticulum protein transport: a novel strategy to kill malignant B cells and overcome fludarabine resistance in CLL. Blood. 2006;107:222–231. doi: 10.1182/blood-2005-05-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Dunner K, Jr, Huang P, Abbruzzese JL, McConkey DJ. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005;65:11658–11666. doi: 10.1158/0008-5472.CAN-05-2370. [DOI] [PubMed] [Google Scholar]
- 20.Allenberg M, Weinstein T, Li I, Silverman M. Activation of procollagenase IV by cytochalasin D and concanavalin A in cultured rat mesangial cells: linkage to cytoskeletal reorganization. J Am Soc Nephrol. 1994;4:1760–1770. doi: 10.1681/ASN.V4101760. [DOI] [PubMed] [Google Scholar]
- 21.Hinoue A, Takigawa T, Miura T, Nishimura Y, Suzuki S, Shiota K. Disruption of actin cytoskeleton and anchorage-dependent cell spreading induces apoptotic death of mouse neural crest cells cultured in vitro. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:130–137. doi: 10.1002/ar.a.20150. [DOI] [PubMed] [Google Scholar]
- 22.Preaux AM, D'ortho MP, Bralet MP, Laperche Y, Mavier P. Apoptosis of human hepatic myofibroblasts promotes activation of matrix metalloproteinase-2. Hepatology. 2002;36:615–622. doi: 10.1053/jhep.2002.35279. [DOI] [PubMed] [Google Scholar]
- 23.Gingras D, Page M, Annabi B, Beliveau R. Rapid activation of matrix metalloproteinase-2 by glioma cells occurs through a posttranslational MT1-MMP- dependent mechanism. Biochim Biophys Acta. 2000;1497:341–350. doi: 10.1016/s0167-4889(00)00071-9. [DOI] [PubMed] [Google Scholar]
- 24.Neth P, Ciccarella M, Egea V, Hoelters J, Jochum M, Ries C. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24:1892–1903. doi: 10.1634/stemcells.2005-0503. [DOI] [PubMed] [Google Scholar]
- 25.Grzanka D, Domaniewski J, Grzanka A, Zuryn A. Ultraviolet radiation (UV) induces reorganization of actin cytoskeleton in CHOAA8 cells. Neoplasma. 2006;53:328–332. [PubMed] [Google Scholar]
- 26.Seyb KI, Ansar S, Bean J, Michaelis ML. beta-Amyloid and endoplasmic reticulum stress responses in primary neurons: effects of drugs that interact with the cytoskeleton. J Mol Neurosci. 2006;28:111–123. doi: 10.1385/jmn:28:2:111. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, Brodt P. Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling. Oncogene. 2003;22:974–982. doi: 10.1038/sj.onc.1206197. [DOI] [PubMed] [Google Scholar]
- 28.Kim D, Cheng GZ, Lindsley CW, Yang H, Cheng JQ. Targeting the phosphatidylinositol-3 kinase/Akt pathway for the treatment of cancer. Curr Opin Investig Drugs. 2005;6:1250–1258. [PubMed] [Google Scholar]
- 29.Glioma Meta-analysis Trialists (GMT) Group, author. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. The Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 30.Berens ME, Giese A. “... those left behind.” Biology and oncology of invasive glioma cells. Neoplasia. 1999;1:208–219. doi: 10.1038/sj.neo.7900034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 32.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 33.Raza SM, Lang FF, Aggarwal BB, Fuller GN, Wildrick DM, Sawaya R. Neurosurgery. Vol. 51. 2002. Necrosis and glioblastoma: a friend or a foe? A review and a hypothesis; pp. 2–12. [DOI] [PubMed] [Google Scholar]
- 34.Ziemer LS, Koch CJ, Maity A, Magarelli DP, Horan AM, Evans SM. Hypoxia and VEGF mRNA expression in human tumors. Neoplasia. 2001;3:500–508. doi: 10.1038/sj.neo.7900195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–3162. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 36.Mariani L, Beaudry C, McDonough WS, Hoelzinger DB, Kaczmarek E, Ponce F, Coons SW, Giese A, Seiler RW, Berens ME. Death-associated protein 3 (Dap-3) is overexpressed in invasive glioblastoma cells in vivo and in glioma cell lines with induced motility phenotype in vitro. Clin Cancer Res. 2001;7:2480–2489. [PubMed] [Google Scholar]
- 37.Slepchenko BM, Terasaki M. Bio-switches: what makes them robust? Curr Opin Genet Dev. 2004;14:428–434. doi: 10.1016/j.gde.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Boros LG, Cascante M, Lee WN. Metabolic profiling of cell growth and death in cancer: applications in drug discovery. Drug Discov Today. 2002;7:364–372. doi: 10.1016/s1359-6446(02)02179-7. [DOI] [PubMed] [Google Scholar]
- 39.Ramos-Montoya A, Lee WN, Bassilian S, Lim S, Trebukhina RV, Kazhyna MV, Ciudad CJ, Noe V, Centelles JJ, Cascante M. Pentose phosphate cycle oxidative and nonoxidative balance: a new vulnerable target for overcoming drug resistance in cancer. Int J Cancer. 2006;119:2733–2741. doi: 10.1002/ijc.22227. [DOI] [PubMed] [Google Scholar]
- 40.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 41.Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7:356–368. doi: 10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newton HB. Molecular neuro-oncology and the development of targeted therapeutic strategies for brain tumors. Part 5: Apoptosis and cell cycle. Expert Rev Anticancer Ther. 2005;5:355–378. doi: 10.1586/14737140.5.2.355. [DOI] [PubMed] [Google Scholar]
- 43.Knobbe CB, Reifenberger G. Genetic alterations and aberrant expression of genes related to the phosphatidyl-inositol-3′-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol. 2003;13:507–518. doi: 10.1111/j.1750-3639.2003.tb00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, Loeffler JS. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22:1926–1933. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 45.Shingu T, Yamada K, Hara N, Moritake K, Osago H, Terashima M, Uemura T, Yamasaki T, Tsuchiya M. Synergistic augmentation of antimicrotubule agent - induced cytotoxicity by a phosphoinositide 3-kinase inhibitor in human malignant glioma cells. Cancer Res. 2003;63:4044–4047. [PubMed] [Google Scholar]
- 46.Joy AM, Beaudry CE, Tran NL, Ponce FA, Holz DR, Demuth T, Berens ME. Migrating glioma cells activate the PI3-K pathway and display decreased susceptibility to apoptosis. J Cell Sci. 2003;116:4409–4417. doi: 10.1242/jcs.00712. [DOI] [PubMed] [Google Scholar]
- 47.Labrecque L, Nyalendo C, Langlois S, Durocher Y, Roghi C, Murphy G, Gingras D, Beliveau R. Src-mediated tyrosine phosphorylation of caveolin-1 induces its association with membrane type 1 matrix metalloproteinase. J Biol Chem. 2004;279:52132–52140. doi: 10.1074/jbc.M409617200. [DOI] [PubMed] [Google Scholar]
- 48.Anilkumar N, Uekita T, Couchman JR, Nagase H, Seiki M, Itoh Y. Palmitoylation at Cys574 is essential for MT1-MMP to promote cell migration. FASEB J. 2005;19:1326–1328. doi: 10.1096/fj.04-3651fje. [DOI] [PubMed] [Google Scholar]
- 49.Langlois S, Di Tomasso G, Boivin D, Roghi C, Murphy G, Gingras D, Beliveau R. Membrane type 1-matrix metalloproteinase induces endothelial cell morphogenic differentiation by a caspasedependent mechanism. Exp Cell Res. 2005;307:452–464. doi: 10.1016/j.yexcr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, Eliopoulos N, Galipeau J, Beliveau R. Hypoxia promotes murine bone-marrow- derived stromal cell migration and tube formation. Stem Cells. 2003;21:337–347. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]
- 51.Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation - induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 52.Leuzzi R, Banhegyi G, Kardon T, Marcolongo P, Capecchi PL, Burger HJ, Benedetti A, Fulceri R. Inhibition of microsomal glucose-6-phosphate transport in human neutrophils results in apoptosis: a potential explanation for neutrophil dysfunction in glycogen storage disease type 1b. Blood. 2003;101:2381–2387. doi: 10.1182/blood-2002-08-2576. [DOI] [PubMed] [Google Scholar]
- 53.Hiraiwa H, Pan CJ, Lin B, Moses SW, Chou JY. Inactivation of the glucose 6-phosphate transporter causes glycogen storage disease type 1b. J Biol Chem. 1999;274:5532–5536. doi: 10.1074/jbc.274.9.5532. [DOI] [PubMed] [Google Scholar]
- 54.Cheung YY, Kim SY, Yiu WH, Pan CJ, Jun HS, Ruef RA, Lee EJ, Westphal H, Mansfield BC, Chou JY. Impaired neutrophil activity and increased susceptibility to bacterial infection in mice lacking glucose-6-phosphatase-beta. J Clin Invest. 2007;117:784–793. doi: 10.1172/JCI30443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao J, Chiarelli C, Kozarekar P, Adler HL. Membrane type 1-matrix metalloproteinase promotes human prostate cancer invasion and metastasis. Thromb Haemost. 2005;93:770–778. doi: 10.1160/TH04-08-0555. [DOI] [PubMed] [Google Scholar]