Abstract
Matrix metalloproteinase (MMP) degradation of extracellular matrix is thought to play an important role in invasion, angiogenesis, tumor growth, and metastasis. Several studies have demonstrated that CD147/extracellular MMP inducer, a membrane-spanning molecule highly expressed in tumor cells, may be involved in the progression of malignancies by regulating expression of MMP in peritumoral stromal cells. In the present study we show that CD147 is expressed in microvesicles derived from epithelial ovarian cancer cells and that CD147-positive vesicles may promote an angiogenic phenotype in endothelial cells in vitro. Vesicles shed by human ovarian carcinoma cell lines OVCAR3, SKOV3, and A2780 expressed different levels of CD147 and stimulated proangiogenic activities of human umbilical vein endothelial cells (HUVECs) in a CD147-dependent fashion (OVCAR3 > SKOV3 > A2780). Moreover, vesicles shed by ovarian carcinoma cell line CABA I with low CD147 expression had no significant effect on the development of angiogenic phenotype in HUVECs. The treatment of OVCAR3 cells with small interfering RNA against CD147 suppressed the angiogenic potential of OVCAR3-derived microvesicles. However, transfection of CD147 cDNA into the CABA I cell line enabled CABA I-derived vesicles to induce angiogenesis and to promote MMP genes expression in HUVECs. We therefore conclude that vesicles shed by ovarian cancer cells may induce proangiogenic activities of HUVECs by a CD147-mediated mechanism.
Keywords: Shed membrane microvesicles, CD147, ovarian cancer, angiogenesis, matrix metalloproteinases
Introduction
CD147, also named extracellular matrix metalloproteinase (MMP) inducer or basigin, is a plasma membrane glycoprotein enriched on the surface of many malignant tumor cells [1–3]. Thus far, several studies have convincingly demonstrated that CD147 could play a crucial role in the progression of malignancies by regulating expression of vascular endothelial growth factor (VEGF) and MMPs in stromal cells [1,4]. Accordingly, it has been previously shown that CD147 interacts with fibroblasts and stimulates their production of MMP-1, MMP-2, MMP-3, and membrane type 1 MMP (MT1-MMP) [5,6]. CD147 is also believed to be involved in the interaction of the tumor with its stromal microenvironment by acting in a paracrine fashion on stroma cells to regulate the production of MMPs [4]. Interestingly, recent studies have provided evidence that microvesicular release of CD147 from tumor cells could play a role in tumor-stromal interactions through upregulation of MMPs production [7,8]. In addition, CD147 is also known to stimulate the production of MMPs in human umbilical vein endothelial cells (HUVECs) [9].
Matrix metalloproteinase degradation of extracellular matrix is thought to play an important role in invasion, angiogenesis, tumor growth, and metastasis [10]. In this regard, increased tumor aggressiveness has been associated with enhanced MMP expression both in vitro and in vivo [11]. Because stromal cells are the major source of MMPs in most of the human cancers analyzed so far, CD147 is deemed to be a critical determinant in cancer growth and dissemination [9,10,12]. In line with this possibility, CD147 expression in human breast and ovarian cancer has been related to a more invasive phenotype [3,13–15]. In addition, human breast cancer cells transfected with CD147 cDNA showed a significantly higher rate of tumor growth in a xenograft model [11].
Shedding of membrane vesicles is a vital phenomenon frequently observed in eukaryotic cells and suggested to be involved in several pathophysiologic processes such as angiogenesis, thrombosis, inflammation, and immunity [16,17]. In previous investigations, two types of membrane vesicles have been described, referred to as microvesicles and exosomes [17–19]. It is worth noting that microvesicles and exosomes are released by different cellular mechanisms, namely, microvesicles by surface shedding and exosomes from exocytosis of multivesicular bodies [18–20].
We have previously shown that tumor microvesicles may carry tumor-associated surface antigens and may be detected both in the serum and in ascites from patients with ovarian cancer [21]. Furthermore, a significant positive correlation was seen between tumor malignancy and both vesicle amount and vesicle-associated MMP-2 activity. Hence, human tumors constitutively release microvesicles, transporting a broad array of biologically active molecules, including cell surface receptors, adhesion molecules, and MMPs [22–26]. Growing interest has been focused on tumor-released microvesicles because they have the ability to modulate invasive capabilities [27–29] and to promote angiogenesis [30–32]. Previous studies have shown that human cancer cells (including lung carcinoma, colon carcinoma, and pancreatic cancers) produce large amounts of CD147-positive microvesicles [7,8]. However, the pathophysiologic role of CD147-positive vesicles has not been completely understood. Working from these assumptions, the aim of our study was to investigate the role of microvesicles released by human ovarian cancer cells in the angiogenic process by evaluating their effect on HUVEC phenotype.
Materials and Methods
Cell Culture
HUVECs were isolated from umbilical cord veins and grown on 1% gelatin-coated flasks in DMEM supplemented with 10% fetal calf serum (FCS), 10% newborn calf serum, 2 mmol/l glutamine, 50 µg/ml endothelial cell growth factor (crude extract from bovine brain), penicillin, and streptomycin. Cells from the third to fifth passages of culture were used. The CABA I cell line was established from the ascitic fluid of an ovarian carcinoma patient not undergoing drug treatment [33]. CD147-transfected CABA I cells were maintained as monolayers in RPMI 1640 (Euroclone, Devon, UK) containing 5% FCS and 400 µg/ml G418. Human ovarian carcinoma cell lines A2780, OVCAR 3 and SKOV 3 were cultured in RPMI medium supplemented with 10% FCS.
Small Interfering RNA and Transfection
Transfection of CD147/green fluorescent protein cDNA into human CABA I ovarian cancer cells with low CD147 expression was performed by the method of Zucker et al. [11]. Cells were stably transfected using FuGene (Roche, Basel, Switzerland) as per manufacturer's protocol. G418-resistant clones were microscopically screened for green fluorescent protein fluorescence and positive clones were pooled to minimize clonal variations. Control transfectants carrying the pcDNA3 vector alone were generated. Identical results were obtained with nontransfected CABA I cells or CABA I cells transfected with pcDNA3 (data not shown). We silenced CD147 in OVACAR3 cells by using CD147 small interfering RNA (siRNA) (h) sc-35298, scrambled oligos sc-37007 as control, and sc-29528 siRNA transfection reagents (Santa Cruz Biotechnology, Santa Cruz, CA) according to the manufacturer's protocol. The same results were obtained with nonsilenced OVCAR3 cells or OVCAR3 cells transfected with scrambled oligos.
Flow Cytometric Analysis
Flow cytometry analysis was performed as previously described [33], with slight modifications. Briefly, cells were incubated on ice for 60 minutes with mouse anti-human CD147 primary antibody (8D6 mouse monoclonal IgG1, Santa Cruz Biotechnology; 1 µg/106 cells) in PBS containing 0.03% BSA. After washing with PBS, cells were incubated with fluorescein-labeled goat anti-mouse IgG (Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD) on ice for 30 minutes. The cells were analyzed for cell-associated fluorescence in a flow cytometer (FACScan, Becton Dickinson, Collaborative Research, Bedford, MA).
Isolation of Membrane Microvesicles from Cell-Conditioned Medium
Microvesicles were prepared as previously described [19]. Conditioned medium obtained as above was centrifuged at 600g for 15 minutes and then at 1500g for 15 minutes to remove cells and large debris. Supernatants were centrifuged at 100,000g for 1 hour at 4°C. Pelleted microvesicles were resuspended in PBS (pH 7.4). Vesicles were quantified based on measurements of vesicle-associated protein levels, using the method of Bradford (Bio-Rad, Milan, Italy), with BSA (Sigma, St. Louis, MO) as standard.
Cord Formation Assay
HUVECs (70,000/well) were seeded onto Matrigel-coated 24-well plates in endothelial growth medium containing 5% serum. Cells were stimulated by microvesicles added as indicated. After 2 and 24 hours, the formation of cords was photographed and independently scored by two blinded observers.
Invasion Assay
HUVEC invasion was assayed using modified Boyden chambers with polycarbonate PVP-free Nucleopore filters (pore size, 8 µm) [34]. Filters were coated with a thick layer of reconstituted basement membrane (0.05 mg/ml Matrigel, Becton Dickinson), which cells must degrade to migrate through the filter. Microvesicles were added as indicated. HUVECs were detached, washed in DMEM-0.1% BSA, resuspended in the same medium at a concentration of 5 x 105 cells/ml, and added to the upper compartment of the chamber. After 6 hours, filters were stained with 1% crystal violet in methanol, and migrated cells in 10 high-power fields were counted.
Zymography
Zymography was performed using SDS-polyacrylamide gels copolymerized with 1 mg/ml gelatin type B (Sigma). Microvesicles (2.5 µg) and fivefold concentrated HUVEC-conditioned medium were diluted in SDS-PAGE sample buffer under nonreducing conditions without heating. After electrophoresis, gels were washed twice for 30 minutes in 2.5% Triton X-100 at room temperature and incubated overnight in collagenase buffer (50 mmol/l Tris-HCl, pH 7.6, 10 mmol/l NaCl, 0.02% Brij 35, and 5 mmol/l CaCl2) at 37°C. Gels were stained with Coomassie Blue R 250 (Bio-Rad) in 30% methanol and 10% acetic acid for 2 hours and destained in the same solution without dye. Gelatinase activity was visualized as clear bands on a dark background, indicating proteolysis of the substrate. The supernatant of WM983A melanoma cells was used as a reference standard for MMP-2 and MMP-9.
Western Blot Analysis
Cells were lysed with lysis buffer containing 50 mmol/l Tris (pH 7.8), 150 mmol/l NaCl, and 1% NP40. Protein concentrations of cellular lysates and microvesicles were determined as described above. Cell lysates (40 µg) and microvesicle proteins (10 µg) were resolved by SDS 10% PAGE under reducing or nonreducing conditions and then transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Nonspecific binding sites were blocked by incubation with 10% nonfat dry milk in TBST at room temperature for at least 1 hour. The blots were incubated with a monoclonal antibody raised against human CD147 (8D6 mouse monoclonal IgG1, 1:500 dilution; Santa Cruz Biotechnology) for 1 hour, or with antibody raised against actin (SC-1616, Santa Cruz Biotechnology), followed by peroxidase-conjugated secondary antibody in blocking buffer. After washing, reactive bands were visualized by using a chemiluminescence detection kit (ECL, Amersham-Pharmacia; Biotech, Piscataway, NJ).
Real-Time Polymerase Chain Reaction
Total RNA was isolated by using SV Total RNA Isolation System (Promega, Madison, WI) and cDNA was synthesized from 5 µg RNA by using the ImProm-II Reverse Transcription System (Promega) according to the manufacturer's protocol. Each real-time polymerase chain reaction (PCR) reaction was prepared in triplicate and contained 2.0 µl of cDNA. PCR was carried out using SYBR-green detection of PCR products in real time (Roche). The sequences of the primers used for PCR were as follows: MT1-MMP forward, 5′-GAGCTCAGGGCAGTGGATAG-3′, MT1-MMP reverse 5′-GGTAGCCCGGTTCTACCTTC (215 bp); MMP-1 forward, 5′-AGGTCTCTGAGGGTCAAGCA-3′, MMP-1 reverse, 5′-CTGGTTGAAAAGCATGAGCA (111 bp); MMP-2 forward, 5′-CACTTTCCTGGGCAACAAAT-3′, MMP-2 reverse, 5′-TGATGTCATCCTGGGACAGA-3′ (257 bp); MMP-9 forward, 5′-TTGACAGCGACAAGAAGTGG, MMP-9 reverse, 5′-GCCATTCACGTCGTCCTTAT-3′ (179 bp); GADPH forward 5′-GGCCTCCAAGGAGTAAGACC-3′, GADPH reverse 5′-AGGGGTCTACATGGCAACTG-3′ (147 bp). A comparative ΔCt method was used to determine gene expression. Expression levels were normalized to the expression levels of the housekeeping gene GADPH.
Results
CD147 Expression in Ovarian Cancer Cell Lines and Shed Microvesicles
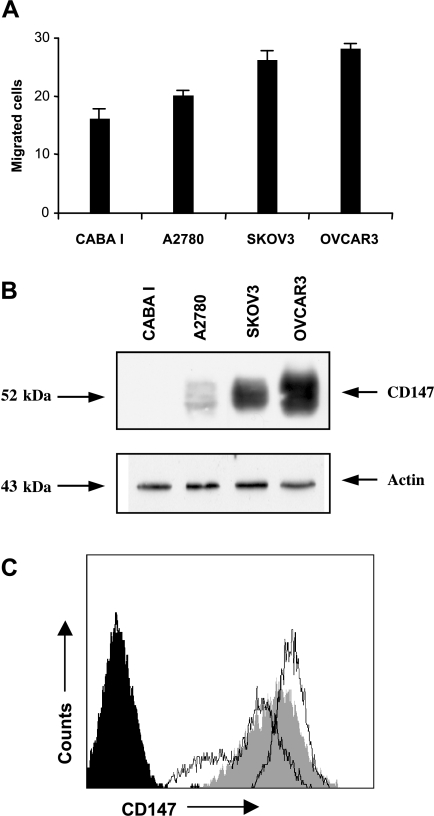
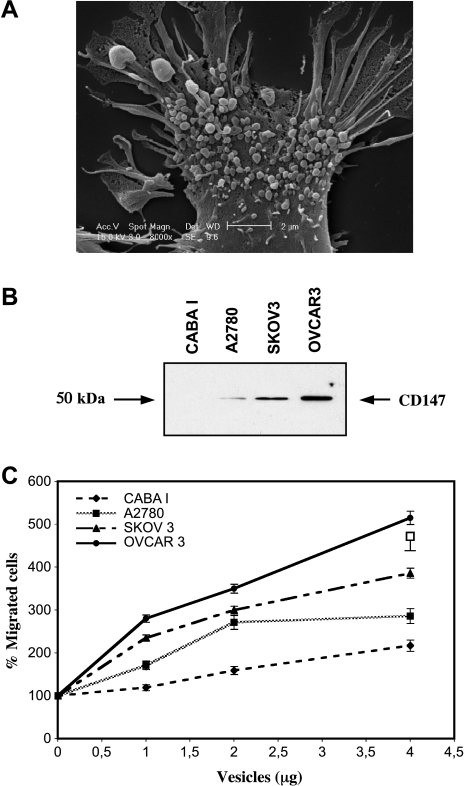
We examined four ovarian cancer cell lines with different invasion capability (CABA I, A2780, SKOV3, and OVCAR3 in order of increasing invasion activity; Figure 1A). All tested cell lines were positive for CD147 by Western blot analysis of cell lysates (Figure 1B), except CABA I, which show not detectable CD147, with increasing levels in the following order: CABA I, A2780, SKOV3, and OVCAR3 (Figure 1C). A representative image, obtained using scanning electron microscopy, of the vesicle shedding phenomenon from CABA I cells is shown in Figure 2A. The size of microvesicles ranged between 200 and 900 nm. To investigate whether CD147 is released from ovarian cancer cell lines through the shedding of microvesicles, membrane microvesicles were isolated by centrifugation and analyzed by Western blotting (Figure 2B). Vesicles shed by ovarian cancer cells were positive for CD147, with increasing levels in the following order: CABA I, A2780, SKOV3, and OVCAR3.
Figure 1.
(A) Comparison of the invasive potential of three ovarian cancer cell lines. Migration activity (representative of three experiments) was calculated as the mean number of migrated cells observed in ten high-power fields (mean ± SD of triplicates). (B) Western blot analysis of CD147. (C) Flow cytometric analysis of CD147. Right profile, white area, OVACR3; gray profile, SKOV3; central profile, white area, A2780; black profile, CABA I.
Figure 2.
(A) Western blot analysis of microvesicle-associated CD147. (B) Effect of microvesicles derived from tumor cells on endothelial cell invasiveness. HUVECs were stimulated by microvesicles shed by ovarian cancer cells (1, 2, and 4 µg protein). Migration activity (representative of three experiments) was calculated as the mean number of migrated cells observed in 10 high-power fields (mean ± SD of triplicates). Supernatant of NIH-3T3, used as a reference attractant, stimulated invasion with a value of 480%.
Effect of Tumor CD147-Positive Microvesicles on HUVECs
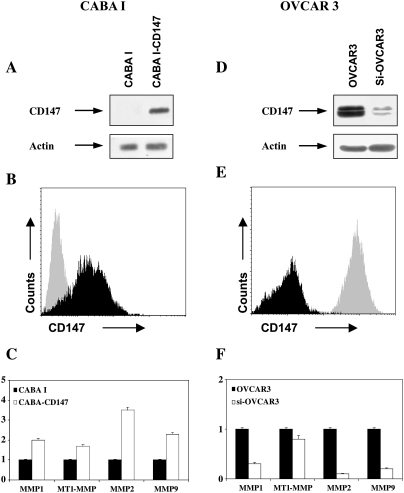
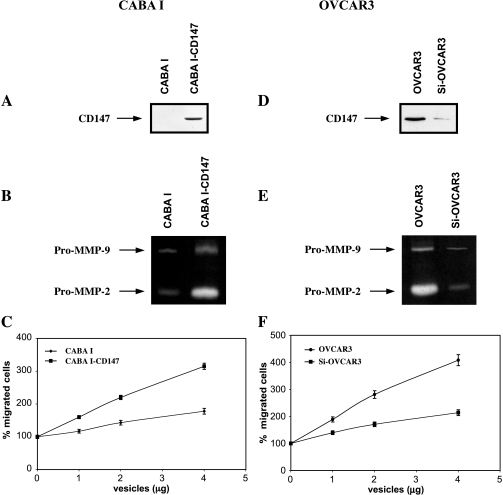
In vitro experiments using a Matrigel invasion assay showed that CD147-positive microvesicles shed by ovarian cancer cell lines enhanced HUVEC invasiveness. There was a direct correlation between CD147 expression in microvesicles and endothelial cell invasiveness (OVCAR3 > SKOV3 > A2780 > CABA I; Figure 2C). Microvesicles were also able to induce HUVEC proliferation (data not shown). Vesicles shed by ovarian carcinoma cell line CABA I with nondetectable CD147 expression had no significant effect on the development of angiogenic phenotype in HUVECs. We thus hypothesized that vesicles shed by ovarian cancer cells may induce proangiogenic activities of HUVEC by a CD147-mediated mechanism. To shed more light on this issue, we transfected CD147 cDNA into noninvasive CABA I human ovarian cell line to generate CABA I cells with high CD147 expression (Figure 3A). Expression of the CD147-GPF fusion protein was confirmed by flow cytometry (Figure 3B) and confocal microscopy (data not shown). We used real-time PCR analysis to examine MMP induction in CD147-transfected CABA I cells. CD147-transfected cells showed enhanced MMP-1, MT1-MMP, MMP-2, and MMP-9 mRNA compared with vector-transfected CABA I cells. The most prominent gene induced was MMP-2 (Figure 3C). CABA I-shed microvesicles, after the CD147 transfection, became CD147 positive (Figure 4A). Zymographic analysis of vesicle-associated MMP confirmed higher expression of pro-MMP-2 and pro-MMP-9 in microvesicles shed by CD147-transfected CABA I compared with vector-transfected cells (Figure 4B). Moreover, CD147-positive microvesicles exhibited a greater capacity to induce migration of HUVECs compared with those generated from vector-transfected cells (Figure 4C). To further investigate the potential regulatory role of vesicle-associated CD147 on HUVEC phenotype, CD147-positive OVCAR3 cells were transfected with a specific siRNA against CD147 to silence its expression. Western blot analysis of cell extracts from cells treated with siRNA showed a lower CD147 expression (∼ sixfold) compared with the parental OVCAR3 cells (Figure 3D). The result was confirmed by FACS (Figure 3E) and confocal microscopy analyses (data not shown). Real-time PCR was performed to quantify MMP gene expression in cells transfected with siRNA relative to the parental OVCAR3 population. Cells with silenced CD147 expression showed a marked inhibition in the MMP-1, MT1-MMP, MMP-2, and MMP-9 gene expression compared with vector-transfected CABA I cells. A weak effect on MT1-MMP mRNA was also noticed (Figure 3F). Microvesicles shed by OVCAR3 cells with silenced CD147 showed a reduced CD147 expression compared with parental cell-derived microvesicles (Figure 4D). Similarly, microvesicles isolated from OVCAR3 cells with silenced CD147 expression were less effective inducers of pro-MMP-2 and pro-MMP-9 synthesis in HUVECs compared with parental cell-derived microvesicles (Figure 4E). Moreover, microvesicles derived from OVCAR3 cells with silenced CD147 expression were less effective at inducing the migration of HUVECs (Figure 4F).
Figure 3.
(A, D) Western blot analysis of CD147 in CABA I and OVAR3 ovarian cancer cell lines. (B) Flow cytometry analysis of CD147 in CABA I: gray profile indicates control cells, whereas black profile indicates CD147-transfected cells. (E) Flow cytometry analysis of CD147 in OVCAR3 cells. Gray profile indicates control cells, whereas black profile indicates cells with silenced CD147 expression. (C, F) RT-PCR analysis of ovarian cancer cell lines for MMP-1, -2, and -9 and MT1-MMP mRNA expression. Control cells were arbitrarily set at 1.
Figure 4.
(A, D) Western blot analysis of microvesicle-associated CD147 in ovarian cancer cells. (B, E) Zymographic analysis of tumor microvesicle-associated MMP-2 and MMP-9. (C, F) Effect of tumor cell-shed microvesicles on invasiveness of cultured endothelial cells. HUVECs were stimulated by microvesicles shed by CABA I cells with and without transfection with CD147 cDNA (C) as well as by vesicle shed by OVCAR3 cells with and without silenced CD147 expression (D) (1, 2, and 4 µg protein). Migration activity (representative of three experiments) was calculated as the mean number of migrated cells observed in 10 high-power fields (mean ± SD of triplicates).
Effects of Tumor-Shed Microvesicles on MMP Production by HUVECs
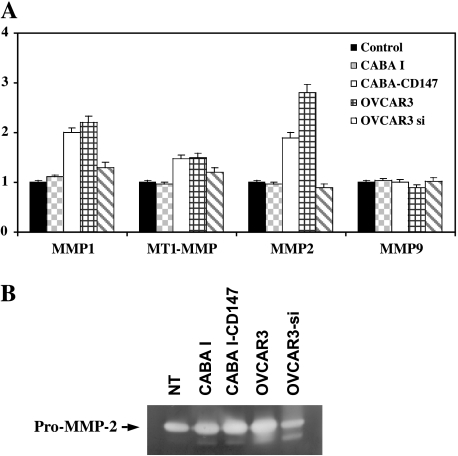
To further explore the mechanisms whereby shed microvesicles can mediate tumor-endothelium interactions, reverse transcription-PCR (RT-PCR) analysis was used to monitor MMP synthesis in HUVECs treated with tumor-released microvesicles. CABA I cell-derived microvesicles did not induce MMP expression in HUVECs. In contrast, microvesicles shed by CD147-transfected CABA I cells were able to increase MT1-MMP (∼1.5-fold), MMP-1 (∼2-fold), and MMP-2 (∼1.9-fold) mRNA transcription. However, vesicles shed by OVCAR3 cells induced upregulation of MT1-MMP (∼1.5-fold), of MMP-1 (∼2.2-fold), and MMP-2 (∼2.8-fold) mRNA. MMP-9 gene expression was not affected by microvesicles derived from OVCAR3 cells (Figure 5A). No induction of MMP was observed in HUVECs after treatment with microvesicles shed by OVCAR3 cells with silenced CD147 expression. Each conditioned medium was collected and examined by gelatin zymography. The conditioned media from HUVECs treated with OVCAR3-derived microvesicles showed increased MMP-2 activity (Figure 5B), whereas no evidence of MMP-9 induction was found (data not shown).
Figure 5.
(A) RT-PCR analysis of MMP expression in HUVECs after exposure to tumor-derived microvesicles. MMP mRNA expression of vesicleuntreated HUVECs was arbitrarily set at 1. (B) Gelatin zymography of HUVEC-conditioned medium after addition of microvesicles.
Effects of Tumor Microvesicles on HUVEC Cord Formation
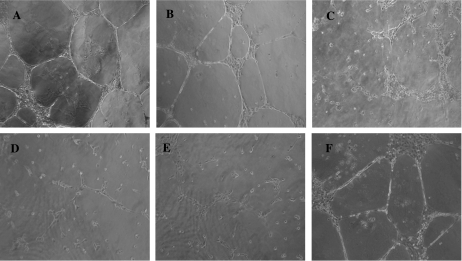
We have already shown that HUVEC microvesicles may stimulate formation of capillary-like structures in an autocrine manner [16]. To investigate whether microvesicles shed by ovarian cancer cells can exert similar effects, we used a three-dimensional matrix (Matrigel) to analyze the outgrowth of human microvascular endothelial cells in capillary-like tubular structures. After 24 hours in complete medium containing 10% serum, more than 95% of the HUVECs were organized into capillary-like structures (Figure 6A). In contrast, cells maintained in low-serum (5%) medium were unable to form a tube network (Figure 6D). Treatment of HUVEC maintained in low-serum medium with microvesicles shed by OVCAR3 resulted in the formation of cords as observed with complete medium (Figure 6B). However, microvesicles shed from CABA I cells were not able to induce capillary-like tube formation (Figure 6C). No cord formation was observed after treatment of HUVECs with microvesicles shed by OVCAR3 cells with silenced CD147 expression (Figure 6E), whereas microvesicles shed by CD147-transfected CABA I cells were able to induce the formation of capillary-like tubular structures (Figure 6F). These results suggest that tube formation by HUVEC maintained in low-serum medium is crucially dependent on CD147 expression in tumor-shed microvesicles.
Figure 6.
Effect of microvesicles on the formation of capillary-like structures by endothelial cells. HUVECs were plated on Matrigel in complete medium containing 10% serum (A), medium with 5% serum (D), medium with 5% serum containing OVCAR3-derived microvesicles (B), medium with 5% serum containing microvesicles derived from OVCAR3 with silenced CD147 expression (E), medium with 5% serum containing microvesicles shed by CABA I cells (C), and medium with 5% serum containing microvesicles shed by CABA I cells transfected with CD147 cDNA (F). The photographs were taken after 24 hours of incubation (original magnification, x100).
Discussion
CD147 is highly expressed on the surface of tumor cells and stimulates surrounding fibroblasts to produce MMPs in a paracrine fashion [35,36]. Furthermore, it has recently been demonstrated that CD147 stimulates expression of VEGF in both tumor and stromal compartments [9]. These findings have made CD147 an important molecule in tumor progression, including cancer cell growth, invasion, metastasis, and angiogenesis [5,11].
Because CD147 is a marker of poor clinical outcome in serous ovarian cancer patients [3], in this report we sought to determine the role of CD147-harboring microvesicles derived from ovarian cancer cells as mediators of tumor/endothelial cell cross-talk. A positive correlation was seen between the cell expression of CD147 and ovarian cancer cell invasive ability. Although CABA I cells had the lowest invasive potential, they retained some ability to invade Matrigel, thereby indicating that other factors play a role in their invasiveness (e.g., MMPs, urokinase-like plasminogen activator) [37,38]. Furthermore, all tested cell lines shed membrane microvesicles into the culture medium. Of note, microvesicle-bound CD147 was directly proportional to the expression of CD147 in ovarian cancer cell lines as assessed by Western blot analysis. Notably, the presence of double bands of CD147 in cell extracts from all cell lines was not observed in microvesicles. Because the microvesicles arise from lipid rafts, we suppose that this is due to a selective partitioning of the CD147 protein in these membrane areas.
The development and progression of tumors result from the concerted activity of tumor cells with neighboring cells including fibroblasts and endothelial cells [39,40]. In this regard, alterations in the stromal microenvironment, including angiogenesis, modified extracellular matrix composition, and unbalanced protease activity are essential regulatory factors of tumor growth and invasion. CD147 expressed on cancer cells has been proven to stimulate the adjacent stromal cells to produce several MMPs. Although this process precedes any direct cell-cell contact, it currently remains unclear whether CD147 is released in soluble form in the tumor microenvironment [4] or associated to microvesicles shed from cancer cells [7].
Shedding of membrane microvesicles in vivo and in vitro from the cell surface may be observed in both normal and tumor cells [19,41]. Under physiological conditions, normal cells release only a limited amount of microvesicles in response to specific stimuli [16]. In contrast, vesicle shedding by tumor cells is largely an uncontrolled process whereby numerous vesicles are constitutively shed from the entire cell surface [19,22]. Moreover, tumor-derived microvesicles carry angiogenic factors [30,42] that may enhance angiogenesis by promoting endothelial cell migration and tubulogenesis [31,32].
We have previously shown that microvesicles shed by HUVECs may induce cord formation of arterial endothelial cells in an autocrine manner [16]. In this study, we provide evidence that vesicles shed by human ovarian carcinoma cell lines OVCAR3, SKOV3, and A2780 expressed different levels of CD147 and stimulated proangiogenic activities of HUVECs in a CD147-dependent fashion (OVCAR3 > SKOV3 > A2780). Moreover, vesicles shed by ovarian carcinoma cell line CABA I with low CD147 expression had no significant effect on the development of angiogenic phenotype in HUVECs. The treatment of OVCAR3 cells with siRNA against CD147 suppressed the angiogenic potential of OVCAR3-derived microvesicles. However, transfection of CD147 cDNA into the CABA I cell line enabled CABA I-derived vesicles to induce an angiogenic phenotype in cultured HUVECs. These effects of tumor-derived microvesicles on in vitro angiogenesis were likely to occur, at least in part, through transcriptional upregulation of endothelial cell MMPs.
Recently, we have demonstrated that tumor-shed vesicles transport VEGF and that the bioavailability of angiogenic factor depends on vesicle rupture induced by acidic pH in the microenvironment [42,43]. However, it remains unclear whether vesicle-associated CD147 may directly interact with the plasma membrane of the target cells. Another possibility is that CD147 availability could involve release of this molecule from bursting vesicles. Further studies are thus warranted to shed more light on the molecular mechanisms underlying this phenomenon.
In conclusion, we have shown that vesicles shed by ovarian cancer cells may induce proangiogenic activities of HUVECs by a CD147-mediated mechanism. Taken together, our findings point to CD147 as an important player in tumorinduced angiogenesis and, therefore, a potential target for novel therapeutic approaches.
Footnotes
This work was supported by grants from the Italian Ministry of University and Scientific and Technological Research (MURST) to A.P. and V.D.
References
- 1.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. [PubMed] [Google Scholar]
- 2.Toole BP. Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr Top Dev Biol. 2003;54:371–389. doi: 10.1016/s0070-2153(03)54015-7. [DOI] [PubMed] [Google Scholar]
- 3.Davidson B, Goldberg I, Berner A, Kristensen GB, Reich R. EMMPRIN (extracellular matrix metalloproteinase inducer) is a novel marker of poor outcome in serous ovarian carcinoma. Clin Exp Metastasis. 2003;20:161–169. doi: 10.1023/a:1022696012668. [DOI] [PubMed] [Google Scholar]
- 4.Tang Y, Kesavan P, Nakada MT, Yan L. Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res. 2004;2:73–80. [PubMed] [Google Scholar]
- 5.Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61:2276–2281. [PubMed] [Google Scholar]
- 6.Guo H, Zucker S, Gordon MK, Toole BP, Biswas C. Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem. 1997;272:24–27. [PubMed] [Google Scholar]
- 7.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;23:956–963. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- 8.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Branski P, Ratajczak MZ, Zembala M. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caudroy S, Polette M, Nawrocki-Raby B, Cao J, Toole BP, Zucker S, Birembaut P. EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis. 2002;19:697–702. doi: 10.1023/a:1021350718226. [DOI] [PubMed] [Google Scholar]
- 10.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 11.Zucker S, Hymowitz M, Rollo EE, Mann R, Conner CE, Cao J, Foda HD, Tompkins DC, Toole BP. Tumorigenic potential of extracellular matrix metalloproteinase inducer. Am J Pathol. 2001;158:1921–1928. doi: 10.1016/S0002-9440(10)64660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193–3199. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 13.Reiland J, Kempf D, Roy M, Denkins Y, Marchetti D. FGF2 binding, signaling and angiogenesis are modulated by heparanase in metastatic melanoma cancer. Neoplasia. 2006;8:596–606. doi: 10.1593/neo.06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morimoto-Tomita M, Uchimura K, Bistrup A, Lum DH, Egeblad M, Boudreau N, Werb Z, Rosen SD. SULF-2, a proangiogenic heparan sulfate endosulfatase, is upregulated in breast cancer. Neoplasia. 2005;7:1001–1010. doi: 10.1593/neo.05496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reimers N, Zafrakas K, Assmann V, Egen C, Riethdorf L, Riethdorf S, Berger J, Ebel S, Janicke F, Sauter G, et al. Expression of extracellular matrix metalloproteases inducer on micrometastatic and primary mammary carcinoma cells. Clin Cancer Res. 2004;10:3422–3428. doi: 10.1158/1078-0432.CCR-03-0610. [DOI] [PubMed] [Google Scholar]
- 16.Taraboletti G, D'Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9 and MT1-MMP as membrane vesicles-associated components by endothelial cells. Am J Pathol. 2002;160:673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 18.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 19.Dolo V, Ginestra A, Ghersi G, Nagase H, Vittorelli ML. Human breast carcinoma cells cultured in the presence of serum shed membrane vesicles rich in gelatinolytic activities. J Submicrosc Cytol Pathol. 1994;26:173–180. [PubMed] [Google Scholar]
- 20.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 21.Ginestra A, Miceli D, Dolo V, Romano FM, Vittorelli ML. Membrane vesicles in ovarian cancer fluids: a new potential marker. Anticancer Res. 1999;19:3439–3445. [PubMed] [Google Scholar]
- 22.Dolo V, Adobati E, Canevari S, Picone MA, Vittorelli ML. Membrane vesicles shed into the extracellular medium by human breast carcinoma cells carry tumor-associated surface antigens. Clin Exp Metastasis. 1995;13:277–286. doi: 10.1007/BF00133483. [DOI] [PubMed] [Google Scholar]
- 23.Dolo V, Ginestra A, Cassara D, Violini S, Lucania G, Torrisi MR, Nagase H, Canevari S, Pavan A, Vittorelli ML. Selective localization of matrix metalloproteinase 9, beta1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Res. 1998;58:4468–4474. [PubMed] [Google Scholar]
- 24.Dolo V, D'Ascenzo S, Violini S, Pompucci L, Festuccia C, Ginestra A, Vittorelli ML, Canevari S, Pavan A. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis. 1999;17:131–140. doi: 10.1023/a:1006500406240. [DOI] [PubMed] [Google Scholar]
- 25.Albanese J, Meterissian S, Kontogiannea M, Dubreuil C, Hand A, Sorba S, Dainiak N. Biologically active Fas antigen and its cognate ligand are expressed on plasma membrane-derived extracellular vesicles. Blood. 1998;91:3862–3874. [PubMed] [Google Scholar]
- 26.Zitvogel L, Fernandez N, Lozier A, Wolfers J, Regnault A, Raposo G, Amigorena S. Dendritic cells or their exosomes are effective biotherapies of cancer. Eur J Cancer. 1999;35(Suppl 3):S36–S38. doi: 10.1016/s0959-8049(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 27.Ginestra A, Monea S, Seghezzi G, Dolo V, Nagase H, Mignatti P, Vittorelli ML. Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. J Biol Chem. 1997;272:17216–17222. doi: 10.1074/jbc.272.27.17216. [DOI] [PubMed] [Google Scholar]
- 28.Angelucci A, D'Ascenzo S, Festuccia C, Gravina GL, Bologna M, Dolo V, Pavan A. Vesicle-associated urokinase plasminogen activator promotes invasion in prostate cancer cell lines. Clin Exp Metastasis. 2000;18:163–170. doi: 10.1023/a:1006778000173. [DOI] [PubMed] [Google Scholar]
- 29.Poste G, Nicolson GL. Arrest and metastasis of blood-borne tumor cells are modified by fusion of plasma membrane vesicles from highly metastatic cells. Proc Natl Acad Sci USA. 1980;77:399–403. doi: 10.1073/pnas.77.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taverna S, Ghersi G, Ginestra A, Rigogliuso S, Pecorella S, Alaimo G, Saladino F, Dolo V, Dell'Era P, Pavan A, et al. Shedding of membrane vesicles mediates fibroblast growth factor-2 release from cells. J Biol Chem. 2003;278:51911–51919. doi: 10.1074/jbc.M304192200. [DOI] [PubMed] [Google Scholar]
- 31.Kim CW, Lee HM, Lee TH, Kang C, Kleinman HK, Gho YS. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002;62:6312–6317. [PubMed] [Google Scholar]
- 32.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 33.Dolo V, Ginestra A, Violini S, Miotti S, Festuccia C, Miceli D, Migliavacca M, Rinaudo C, Romano FM, Brisdelli F, et al. Ultrastructural and phenotypic characterization of CABA I, a new human ovarian cancer cell line. Oncol Res. 1997;9:129–138. [PubMed] [Google Scholar]
- 34.Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990;111:765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bordador LC, Li X, Toole B, Chen B, Regezi J, Zardi L, Hu Y, Ramos DM. Expression of emmprin by oral squamous cell carcinoma. Int J Cancer. 2000;85:347–352. [PubMed] [Google Scholar]
- 36.Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002;99:520–528. doi: 10.1002/ijc.10390. [DOI] [PubMed] [Google Scholar]
- 37.Millimaggi D, Festuccia C, Angelucci A, D'Ascenzo S, Rucci N, Flati S, Bologna M, Teti A, Pavan A, Dolo V. Osteoblast-conditioned media stimulate membrane vesicle shedding in prostate cancer cells. Int J Oncol. 2006;28:909–914. [PubMed] [Google Scholar]
- 38.Reichardt W, Hu-Lowe D, Torres D, Weissleder R, Bogdanov A., Jr Imaging of VEGF receptor kinase inhibitor-induced antiangiogenic effects in drug-resistant human adenocarcinoma. Neoplasia. 2005;7:847–853. doi: 10.1593/neo.05139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Said NA, Najwer I, Socha MJ, Fulton DJ, Mok S, Motamed K. SPARC inhibits LPA-mediated mesothelial-ovarian cancer cell crosstalk. Neoplasia. 2007;9:23–35. doi: 10.1593/neo.06658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zechmann CM, Woenne EC, Brix G, Radzwill N, Ilg M, Bachert P, Peschke P, Kirsch S, Kauczor HU, Delorme S, et al. Impact of stroma on growth, microcirculation and metabolism of experimental prostate tumors. Neoplasia. 2007;9:57–67. doi: 10.1593/neo.06688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor DD, Black PH. Shedding of plasma membrane fragments. In: Steinberg M, editor. Developmental Biology. New York: Plenum Press; 1986. pp. 33–57. [DOI] [PubMed] [Google Scholar]
- 42.Taraboletti G, D'Ascenzo S, Giusti I, Marchetti D, Borsotti P, Millimaggi D, Giavazzi R, Pavan A, Dolo V. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006;8:96–103. doi: 10.1593/neo.05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiffelers RM, Metselaar JM, Fens MH, Janssen AP, Molema G, Storm G. Liposome-encapsulated prednisolone phosphate inhibits growth of established tumors in mice. Neoplasia. 2005;7:118–127. doi: 10.1593/neo.04340. [DOI] [PMC free article] [PubMed] [Google Scholar]