Abstract
Objectives. We examined socioeconomic inequalities in initiation and cessation rates of smoking and the resultant inequality in smoking prevalence among 3 consecutive Italian birth cohorts.
Methods. We used data from the 1999–2000 Italian National Health Interview Survey, which included 28958 men and 29769 women who were born between 1940 and 1969. The association between smoking variables and level of education was assessed with logistic regression and life table analyses.
Results. Inequalities in the lifetime prevalence of smoking increased across the 3 birth cohorts in Italy. At age 40, lower-educated persons in the youngest cohort reported on average 1 to 5 years of additional exposure to regular smoking compared with higher-educated persons. Inequalities in smoking prevalence increased among both men and women because of widening inequalities in initiation rates. Among women, growing inequalities in cessation rates also played a role.
Conclusions. The relative contribution of initiation and cessation to socioeconomic inequalities in smoking rates varied by both gender and birth cohort. For the youngest birth cohort, policies that address inequalities in smoking should focus on both initiation and cessation.
The association between smoking and low socioeconomic status (SES) has become increasingly stronger in almost all industrialized countries.1–4 This is associated with a shift in the social distribution of smoking over time: higher-SES groups take up the habit before lower-SES groups, but ultimately, this pattern reverses. The transition has been completed in the United States and in several northern European countries, but it is still under way in southern Europe.1,5
Despite this evidence, antitobacco measures in industrialized countries have so far failed to address the widening social inequality in smoking over time, because they have mainly focused on decreasing the overall prevalence of smoking. In a recent review, Platt et al. suggested that a mix of interventions—such as increased taxation, availability of nicotine-replacement therapy, and measures that address the underlying economic and psychosocial determinants of smoking initiation and cessation—may reduce inequality in smoking prevalence.6 Amos suggested that the effectiveness of antitobacco interventions would be improved if gender differences and stage of the smoking epidemic (i.e., phase of diffusion of smoking within a population) were taken into account.7 However, it is unknown whether inequalities can be reduced by measures that affect initiation of smoking among adolescents or by measures that affect cessation among adults.
We wanted to disentangle the dynamics of inequalities in smoking with data from Italy, a country in which the social distribution of smoking has been rapidly changing.3 Our goal was to (1) describe socioeconomic inequalities in initiation and cessation rates of smoking and the resultant inequality in smoking prevalence among 3 consecutive Italian birth cohorts, and (2) identify changes among these birth cohorts. More specifically, our first objective was to describe the educational differences (how large the differences were and whether they varied by age) in the 3 indicators associated with tobacco use—probability of taking up the habit, probability of quitting, and prevalence of smoking—for each birth cohort and for both genders. A second specific objective was to estimate whether differences in either initiation or cessation contributed to the inequalities in lifetime prevalence of smoking and whether their relative contribution varied with gender and birth cohort.
METHODS
We used data from the 1999–2000 Italian Health Interview Survey of 140011 individuals who were randomly chosen within strata of geographical area, municipality, and household size (multistage sampling). The nonresponse rate was 13%. The design of similar surveys of civilian noninstitutionalized populations have been published elsewhere.3,8,9 Our study was restricted to individuals who were born during the following decades: 1940–1949, 1950–1959, and 1960–1969, which yielded a study sample of 28958 men and 29769 women.
In the case of missing values, the survey used an automatic procedure of data imputation10 for the following variables: gender, age, occupation, education, municipality size, and geographic area of residence. The proportion of missing values for the variables associated with smoking were very low in our study: 2% for smoking status, 2% for age at smoking initiation, and 7% for age at smoking cessation. The analysis of raw or imputed data showed very similar proportions of current, former, and never smokers. Good levels of agreement also were shown for the median ages at initiation and cessation of smoking (Istituto Nazionale di Statistica [ISTAT], unpublished data, 1998).
Education is one of the most widely used and valid indicators of SES.11 Compared with other indicators—such as occupation, which can only be applied to those actively engaged in work—it has a substantial advantage because it allows the classification of all individuals in a population. Because the highest level of education is usually acquired early in life, its use as a proxy of SES makes it less likely that selection mechanisms will have an effect (e.g., implying that a person attains a lower educational level because he is a smoker). Furthermore, data about income were not available in the Italian Health Interview Survey. We grouped level of education into 2 categories according to the highest level successfully completed: those who had a high-school degree or higher (higher-educated) and those who had less than a high-school degree (lower-educated). The outcome measures were regular current smoking, age at initiation, and age at cessation. Table 1 ▶ shows the proportions of respondents who had a higher education, who started smoking before age 20 years, and who quit before age 30 years for each of the 3 cohorts studied.
TABLE 1—
Smoking and Educational Statistics for 3 Italian Cohorts, by Gender
| Birth Cohort | No. of Respondents | More Educated,a % | Started Smoking Before Age 20 Years, % | Quit Smoking Before Age 30 Years, % | |
| Men | 1940–1949 | 8 715 | 34.1 | 45.6 | 4.2 |
| 1950–1959 | 9 618 | 48.5 | 49.1 | 9.5 | |
| 1960–1969 | 10 625 | 52.0 | 43.1 | 13.8 | |
| Women | 1940–1949 | 9 108 | 23.8 | 11.7 | 3.3 |
| 1950–1959 | 9 663 | 44.1 | 25.3 | 10.3 | |
| 1960–1969 | 10 998 | 56.5 | 27.8 | 16.4 |
aPercentage of respondents who had upper-secondary or postsecondary education.
For each level of education and 5-year interval, we calculated smoking prevalence rates and initiation and cessation probabilities. The probability of being a smoker, calculated from age 10 years, was defined as the proportion of regular smokers in each age interval; in this quotient, the numerator was the number of respondents who had ever smoked in that interval, and the denominator comprised all respondents. The probability of smoking initiation, calculated from age 10 years, was defined for each age interval as the quotient between the number of individuals who started smoking in that age interval and the number of individuals who were at risk (nonsmokers at the beginning of the interval, excluding former smokers). The probability of smoking cessation, calculated from age 20 years, was defined as the proportion of smokers at the beginning of each age interval that quit during the same interval. The follow-up of respondents in the last 5-year interval was on average half compared with the other intervals; therefore, we approximately corrected the probability of both initiation and cessation during the last interval by multiplying these probabilities by 2.
We used 2 complementary methods to calculate summary measures of the inequality in initiation, cessation, and prevalence of smoking. First, we used logistic regression to compute the Relative Index of Inequalities (RII) with a 95% confidence interval (CI). Second, we applied the life table technique with the previously calculated age-specific probabilities. In the regression analysis, smoking-related variables were associated with relative position on the educational hierarchy. Thus, the RII took into account the fact that the distribution of level of education changed markedly among the 3 cohorts (Table 1 ▶).
The RII is a ratio that compares the odds of event for those at the bottom of the educational hierarchy compared with those at the top of it. To compute RIIs, we used a finer classification of educational attainment by distinguishing 4 ordinal categories on the basis of highest degree achieved: elementary education (5 years of education), middle school education (8 years), high-school education (12–13 years), and university education (16–18 years). In the regression models, both educational rank and age were entered as continuous covariates. The dependent variables were the odds for starting smoking, the odds for quitting smoking, and the odds for being a smoker at age 40 years. Details about this measure have been published elsewhere.12
We also calculated summary measures across all age groups for smoking initiation, cessation, and prevalence with an absolute measure of level of education. To summarize inequalities in smoking initiation and cessation, we calculated the cumulative probabilities of initiation and cessation with the previously computed age-specific probabilities of starting and quitting smoking. These measures were estimated with multiple-decrement life tables, and initiation and cessation were the only transition probabilities. For smoking prevalence, we calculated the average number of years of smoking on the basis of the age-specific prevalence rates of smoking. These measures summarize educational differences in smoking in concrete terms that can be interpreted from a life-course perspective.
We stratified all analyses by gender and by birth cohort. In the calculation, we took into account the multistage sampling design of the survey, with the municipalities—which were sampled by strata according to their population size—being the primary sampling units. Each observation had a weight that corresponded with the inverse of the probability of being sampled. We used Stata statistical software, version 8.2 (Stata Corp, College Station, Tex).
RESULTS
Figure 1 ▶ shows the age-specific probabilities of smoking initiation. These were similar among the higher-educated and lower-educated males who were born between 1940 and 1949, and they were markedly decreased among the higher-educated males in the youngest cohort. Among women, higher initiation rates were found among the higher-educated in the older cohorts and among the lower-educated in the youngest cohort.
FIGURE 1—
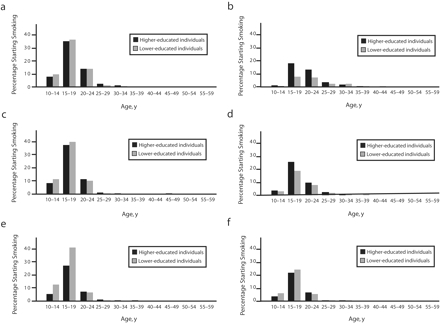
Age-specific probabilities of smoking initiation among 3 Italian birth cohorts: 1940–1949 for men (a) and women (b), 1950–1959 for men (c) and women (d), and 1960–1969 for men (e) and women (f).
Note. In 2000, data were available up to age 50 years for the 1950–1959 cohort and up to age 40 years for the 1960–1969 cohort.
Age-specific probabilities of quitting smoking are shown in Figure 2 ▶. In all 3 cohorts, higher-educated men showed an increased “risk” for quitting compared with lower-educated men up to age 49 years. Among women, socioeconomic differences increased in the youngest birth cohort. Again, the probability of quitting smoking was lower among lower-SES groups during early adulthood.
FIGURE 2—
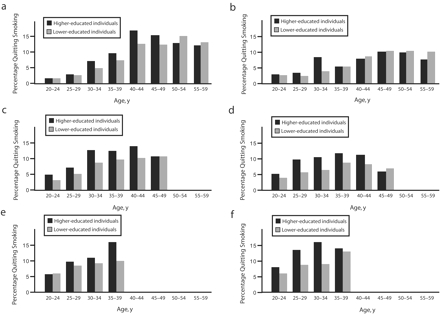
Age-specific probabilities of smoking cessation among 3 Italian birth cohorts: 1940–1949 for men (a) and women (b), 1950–1959 for men (c) and women (d), and 1960–1969 for men (e) and women (f).
Note. In 2000, data were available up to age 50 years for the 1950–1959 cohort and up to age 40 years for the 1960–1969 cohort.
Figure 3 ▶ shows the proportion of individuals who reported smoking on a regular basis for each 5-year interval between age 10 and 59 years. Prevalence rates clearly declined among males in the youngest birth cohort, but the decrease was larger among higher-educated men. Among women, higher prevalence rates were present among the higher-educated in the oldest cohort and among the lower-educated in the youngest cohort. The socioeconomic differences in the prevalence of smoking visibly increased with age among females in all 3 cohorts.
FIGURE 3—
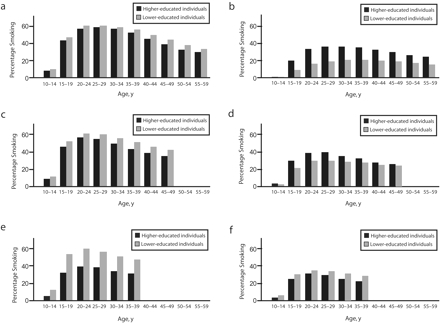
Age-specific probabilities of smoking among 3 Italian birth cohorts: 1940–1949 for men (a) and women (b), 1950–1959 for men (c) and women (d), and 1960–1969 for men (e) and women (f).
Note. In 2000, data were available up to age 50 years for the 1950–1959 cohort and up to age 40 years for the 1960–1969 cohort.
The models for smoking initiation and smoking prevalence at age 40 years highlight the increasing socioeconomic inequalities among younger cohorts of both genders (Table 2 ▶). The RII for smoking initiation was 1.07 (95% CI = 0.87, 1.32) for males in the older cohorts, and it was 5.10 (95% CI = 4.12, 6.31) for the cohort that was born between 1960 and 1969. In all 3 cohorts, higher-educated individuals were more likely to quit smoking. Among females, there was a shift in the RIIs for starting smoking, from values below 1 (indicating a higher probability among the higher-educated) to values larger than 1 in the younger cohort, and a similar shift was observed for smoking prevalence. On the contrary, the likelihood of quitting smoking was higher among higher-educated women in all 3 cohorts, but this difference tended to increase in the younger cohorts.
TABLE 2—
Regression-Based Estimates of Inequalities in Smoking Initiation, Cessation, and Prevalence Among 3 Italian Birth Cohorts, by Gender
| Relative Index of Inequality (95% Confidence Interval) | |||
| Birth Cohort | Probability of Starting Smoking | Probability of Quitting Smoking | Probability of Being a Smoker at Age 40 Years |
| Men | |||
| 1940–1949 | 1.07 (0.87, 1.32) | 0.77 (0.69, 1.00) | 1.21 (1.00, 1.47) |
| 1950–1959 | 1.38 (1.11, 1.70) | 0.49 (0.37, 0.64) | 1.80 (1.42, 2.28) |
| 1960–1969 | 5.10 (4.12, 6.31) | 0.70 (0.53, 0.92) | 4.10 (3.39, 4.96) |
| Women | |||
| 1940–1949 | 0.25 (0.20, 0.32) | 0.87 (0.58, 1.32) | 0.32 (0.25, 0.41) |
| 1950–1959 | 0.43 (0.35, 0.53) | 0.46 (0.33, 0.65) | 0.69 (0.56, 0.86) |
| 1960–1969 | 1.42 (1.18, 1.72) | 0.39 (0.29, 0.52) | 1.99 (1.61, 2.45) |
Table 3 ▶ shows inequalities with absolute differences in the cumulative probability of smoking initiation and cessation and absolute differences in the number of years having smoked. The calculation for these measures is extended to age 40 years. The cumulative probability of starting smoking was nearly identical for lower-SES (0.52) and higher-SES (0.51) men who were born between 1940 and 1949; cumulative probabilities for similar men in the youngest birth cohort were 0.53 and 0.37, respectively. Higher-educated men in all 3 cohorts had a greater probability of quitting smoking, and this difference tended to increase among higher-educated men in the youngest cohort. On average, before reaching his 40th birthday, a lower-SES man who was born between 1940 and 1949 spent 0.8 more years smoking compared with a higher-educated man in the same cohort, and the educational difference (low minus high) among men who were born 2 decades later was 5.1 years.
TABLE 3—
Life Table–Based Estimates of Educational Inequalities in Smoking Initiation, Cessation, and Prevalence Among 3 Italian Birth Cohorts, by Gender
| Cumulative Probability of Starting Before Age 40 Yearsa | Cumulative Probability of Quitting Before Age 40 Yearsa | Years of Smoking Before Age 40 Yearsa | ||||||||
| Birth Cohort | Difference | Lower-Educated | Higher-Educated | Difference | Lower-Educated | Higher-Educated | Difference | Lower-Educated | Higher-Educated | |
| Men | 1940–1949 | 1.0 | 51.9 | 50.9 | −4.3 | 16.3 | 20.6 | 0.8 | 14.7 | 13.9 |
| 1950–1959 | 2.6 | 52.9 | 50.3 | −8.0 | 24.5 | 32.5 | 1.7 | 14.7 | 13.0 | |
| 1960–1969 | 15.4 | 52.6 | 37.2 | −6.5 | 29.6 | 36.0 | 5.1 | 14.2 | 9.1 | |
| Women | 1940–1949 | −6.9 | 28.3 | 35.2 | −5.2 | 14.2 | 19.4 | −3.7 | 4.4 | 8.2 |
| 1950–1959 | −8.0 | 29.7 | 37.8 | −9.8 | 22.6 | 32.3 | −1.9 | 7.0 | 9.0 | |
| 1960–1969 | 3.4 | 34.0 | 30.6 | −10.5 | 31.7 | 42.2 | 1.4 | 8.4 | 6.9 | |
a These measures were based on previously calculated age-specific probabilities.
Among women, the probability of starting smoking was larger among higher-educated women in the older cohorts; in the youngest cohort, it was greater among lower-educated women. Similar to men, higher-educated women were more likely than lower-educated women to quit smoking in all 3 cohorts, and the difference increased from 5.2% among women who were born between 1940 and 1949 to 10.5% among women who were born 2 decades later. When the age span is extended to the 60th birthday (1940–1949 cohort), inequalities in cessation of smoking were somewhat attenuated among women (data not shown). Absolute differences in the average number of years having smoked changed from negative values (higher prevalence among higher-educated) in the older cohorts to positive values in the youngest birth cohort.
DISCUSSION
We found increasing inequalities in smoking prevalence among 3 successive birth cohorts in Italy, with a distinct gender pattern. Among males, the rising inequality was mainly the result of widening inequalities in initiation. Among females, this was the result of progressively higher uptake rates among lower-SES groups and growing differences in cessation rates. Thus, the relative contribution of initiation and cessation to socioeconomic inequalities in smoking prevalence rates varied according to both gender and birth cohort.
Evaluation of Data Problems
The accuracy of self-reported data on smoking has been questioned13; additionally, increased underreporting has been seen in Italy.14,15 However, this would have affected our estimates of the educational difference only if the misreporting was dependent on level of education. In this regard, 1 study found no significant differences in the misclassification of smokers between socioeconomic groups.16
Two other potential sources of error—data and selection bias—are associated with the retrospective design of this research. In our study, we reconstructed the lifetime smoking experience of respondents on the basis of questions about the timing of smoking initiation and smoking cessation. Thus, recall of either age at starting smoking or age at quitting smoking may have been problematic, especially in the case of older respondents. Some studies found high levels of agreement between retrospective and contemporaneous measures of smoking status, with greater differences emerging with longer recall periods.17 However, other research has shown that retrospective reports on smoking habits produce prevalence rates similar to those from contemporaneous reports.18 Larger inconsistencies were found among light smokers, and among heavy smokers and nonsmokers, the inconsistencies were smaller.19
The second potential bias is the selection of respondents for the 1999–2000 survey, because low-educated smokers may have suffered from higher mortality rates. The result would have been an underestimation of the average number of years having smoked experienced by lower-educated individuals. To evaluate the joint effect of recall bias and selective mortality, we compared the smoking data recorded in the 2000 survey with data that was collected in the 1990–1991 Italian Health Interview Survey. For each survey, we calculated the proportion of respondents who were born between 1910 and 1969 and who had ever smoked before 1990 (data not shown). Although the 2 samples were independently drawn and misclassification of smoking may have occurred in both surveys, prevalence rates matched reasonably well in most cases. The prevalence of ever smoking was larger in retrospective (1999–2000 survey) compared with contemporaneous (1990–1991 survey) reports, except among lower-educated males and higher-educated females in the oldest birth cohort, which suggests that selective mortality played a role among older respondents but not among subjects in our study.
There also was a notable contrast among those who were born between 1960 and 1969. Prevalence estimates from the retrospective data were larger than estimates from the contemporaneous data, with a difference ranging from 9% to 13%. This may have been the result of smoking habits that were not well established in 1990 and 1991, when respondents were in their twenties, or efforts to conceal habits and give “desirable” answers to the questionnaire. However, the difference in prevalence rates between the 2 surveys was similar for both educational groups; therefore, our estimates of inequalities were not substantially biased.
Comparison With Previous Studies
To our knowledge, this study is the first to describe the socioeconomic differences (how large the differences were and whether they varied by age) in the prevalence, initiation, and cessation of smoking in Italy, with a focus on the lifetime smoking trajectories of individuals in 3 consecutive birth cohorts.
Previous studies have reported the changing association between smoking and SES by birth cohort in the United States and several European countries. These studies examined either initiation or prevalence of smoking. In the United States, the proportion of smokers was higher among White lower-educated males than among higher-educated males in all birth cohorts from the past century. Among White women, higher prevalence rates emerged among the lower-educated who were born between 1930 and 1939.20 The same pattern was found among other racial/ethnic groups, with some variations regarding educational differences (how large the differences were and whether they varied by age).
In contrast to individuals who were born before 1920, lower-educated Finnish men who were born during the 1920s had higher initiation and lifetime prevalence rates compared with their higher-educated counterparts. Educational difference among males in Denmark and Spain appeared 10 and 20 years later, respectively. Among European women, the reversal in all these countries was 1 or 2 decades later.4,21,22 Our data are consistent with these findings and show that the inversion of the social gradient in smoking initiation emerged among Italian men and women 2 to 4 decades later than both the US and northern European countries. This trend coincides with the north-south diffusion of the smoking epidemic in Europe.1,5
Previous studies of smoking cessation have used the quit ratio, which is defined as the ratio—at 1 point in time—between former smokers and ever smokers (current + former smokers). In the United States, quit ratios increased between 1950 and 1990, but the increase was lower among the lower-educated and Black populations.23 Quitting smoking has become more frequent during the past 4 decades in most European countries as well, especially among higher-educated individuals,24,25 which is similar to what we found in Italy.
However, the life course perspective and the use of cumulative probabilities in our study have some advantages that quit ratios do not have. We showed there are educational differences in the timing of cessation by age group, which occurs at earlier ages among those who have higher levels of education. Additionally, we were able to analyze cessation and initiation rates in parallel ways and thus, show that the widening of inequalities in smoking prevalence was mainly the result of trends in smoking initiation (rather than cessation), especially among men.
Explanations
Several factors may have determined the increasing inequalities in initiation rates across the 3 cohorts. The first reports that associated smoking tobacco with cancer were published during the 1950s, and evidence of the negative impact of smoking on health grew during the following decades. In Italy, however, health information campaigns were delayed because a comprehensive strategy against smoking had not been developed.26 It was not until the mid-1980s that local administrations made a substantial effort to educate the public about tobacco use prevention.27 The gap among educational groups in initiation rates became particularly large within the cohort that was born during the 1960s, which makes it plausible that these campaigns were most effective among higher-educated individuals. A similar development occurred in the United States, where a significant negative correlation between education and smoking initiation was found only after information about the hazards of smoking was diffused.28
However, another factor is likely to have played an important role in the large rise of initiation rates among lower-educated women: the adoption of smoking as a symbol of independence and success, which is influenced by the mass media, tobacco advertising, and the examples of higher-SES women.29 Recently, tobacco companies have identified lower-SES women as a particularly promising “market segment,”30 and they are promoting cigarettes as a way to relieve stress, improve performance, and lose weight and as a means of social acceptance.31 Thus, the greater vulnerability of lower socioeconomic groups to these various messages and influences combined with a more skeptical attitude toward the occurrence of disease in the future may explain the increasing inequalities in smoking initiation that we found.
We have shown that cessation rates increased among the cohorts born after 1950, but this rate was lower among the lower-educated individuals. Increased knowledge about the adverse consequences of smoking and the different policy measures that have been progressively implemented over a 30-year span (e.g., banning tobacco advertising, no smoking in public places, health warnings on cigarette packs)26 may have contributed to the decision to quit smoking. However, higher-educated individuals, who are generally more health-conscious, seem to have been more responsive to these messages compared with lower-educated individuals.
It also has been reported that despite similar levels of motivation to stop, lower-SES smokers are less successful in their attempts to quit,32 and they have a lower level of social support and a lower level of confidence in their ability to quit. Inequalities in cessation of smoking were particularly large among females, possibly because lower-SES women often perceive smoking as an effective strategy for coping with stress and difficult living circumstances.33 When disadvantages accumulate over the life course, the risk for becoming a persistent smoker increases.34
Lower-educated individuals reported a lower probability of quitting compared with higher-educated individuals during early adulthood, but not afterwards, possibly because of a higher incidence of tobacco-related health problems with older age. Recent hospitalization, development of coronary heart disease, and severely impaired lung function35,36 are in fact important predictors of smoking cessation and occur most frequently among lower-SES men.
Conclusions
Policy interventions should be more sensitive to the needs of the most disadvantaged. “Denormalizing” the use of tobacco—banning smoking in public places and workplaces, prohibiting all direct and indirect forms of tobacco promotion, tailoring communication for lower-SES groups, and targeting smoking cessation services geographically—may eventually decrease inequalities in smoking.37 Although the relative contribution of initiation and cessation to socioeconomic differences in the lifetime prevalence of smoking varied by both gender and birth cohort, inequalities were present in both initiation and cessation of smoking, and they increased among younger birth cohorts. For the youngest birth cohort, policies that address inequalities in smoking should focus on both initiation and cessation.
Acknowledgments
We thank the European Network on Smoking Prevention for financially supporting this study. We are grateful to Francesca Vannoni of the Epidemiology Unit, Piedmont Region, Italy, and 2 anonymous reviewers for their valuable comments on previous drafts of the article. We also thank Laura Iannucci of Istituto Nazionale di Statistica, who carried out supplementary analyses of the National Health Interview Survey.
Human Participant Protection Data acquired from ISTAT were anonymous, which ensured individual subjects’ protection.
Peer Reviewed
Contributors B. Federico conducted the data analyses and prepared the original draft. A. E. Kunst originated the study and supervised all phases of its implementation. G. Costa provided data and reviewed drafts of the article. All authors developed research questions and interpreted study findings.
References
- 1.Cavelaars AE, Kunst AE, Geurts JJ, et al. Educational differences in smoking: international comparison. BMJ. 2000;320:1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce JP, Fiore MC, Novotny TE, et al. Trends in cigarette smoking in the United States. Educational differences are increasing. JAMA. 1989;261:56–60. [PubMed] [Google Scholar]
- 3.Faggiano F, Versino E, Lemma P. Decennial trends of social differentials in smoking habits in Italy. Cancer Causes Control. 2001;12:665–671. [DOI] [PubMed] [Google Scholar]
- 4.Schiaffino A, Fernandez E, Borrell C, et al. Gender and educational differences in smoking initiation rates in Spain from 1948 to 1992. Eur J Public Health. 2003;13:56–60. [DOI] [PubMed] [Google Scholar]
- 5.Edwards R. The problem of tobacco smoking. BMJ. 2004;328:217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platt S, Amos A, Gnich W, et al., Strategies to reduce socioeconomic inequalities in health. In: Bakker MJ, Mackenbach JP, eds. Reducing Inequalities in Health: A European Perspective. London, UK, and New York, NY: Routledge; 2002:44–45.
- 7.Amos A. Women and smoking. Br Med Bull. 1996;52:74–89. [DOI] [PubMed] [Google Scholar]
- 8.La Vecchia C, Decarli A, Pagano R. Prevalence of cigarette smoking among subsequent cohorts of Italian males and females. Prev Med. 1986;15:606–613. [DOI] [PubMed] [Google Scholar]
- 9.Pagano R, La Vecchia C, Decarli A. Smoking in Italy, 1994. Tumori. 1996;82:309–313. [DOI] [PubMed] [Google Scholar]
- 10.Barcaroli G, D’Aurizio L, Luzi O, et al. Metodi e software per il controllo e la correzione dei dati. Rome, Italy: ISTAT; 1998.
- 11.Winkleby MA, Jatulis DE, Frank E, et al. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health. 1992;82:816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackenbach JP, Kunst AE. Measuring the magnitude of socio-economic inequalities in health: an overview of available measures illustrated with two examples from Europe. Soc Sci Med. 1997;44:757–771. [DOI] [PubMed] [Google Scholar]
- 13.Patrick DL, Cheadle A, Thompson DC, et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84:1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallus S, Colombo P, Scarpino V, et al. Smoking in Italy, 2002. Tumori. 2002;88:453–456. [DOI] [PubMed] [Google Scholar]
- 15.La Vecchia C. Smoking in Italy, 1949–1983. Prev Med. 1986;15:274–281. [DOI] [PubMed] [Google Scholar]
- 16.Suadicani P, Hein HO, Gyntelberg F. Serum validated tobacco use and social inequalities in risk of ischaemic heart disease. Int J Epidemiol. 1994;23: 293–300. [DOI] [PubMed] [Google Scholar]
- 17.Krall EA, Valadian I, Dwyer JT, et al. Accuracy of recalled smoking data. Am J Public Health. 1989;79: 200–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenkel D, Lillard DR, Mathios A. Smoke or fog? The usefulness of retrospectively reported information about smoking. Addiction. 2003;98:1307–1313. [DOI] [PubMed] [Google Scholar]
- 19.Kenkel DS, Lillard DR, Mathios AD. Accounting for misclassification error in retrospective smoking data. Health Econ. 2004;13:1031–1044. [DOI] [PubMed] [Google Scholar]
- 20.Escobedo LG, Peddicord JP. Smoking prevalence in US birth cohorts: the influence of gender and education. Am J Public Health. 1996;86:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laaksonen M, Uutela A, Vartiainen E, et al. Development of smoking by birth cohort in the adult population in eastern Finland 1972–97. Tob Control. 1999;8: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osler M, Holstein B, Avlund K, et al. Socioeconomic position and smoking behaviour in Danish adults. Scand J Public Health. 2001;29:32–39. [PubMed] [Google Scholar]
- 23.Gilpin EA, Pierce JP. Demographic differences in patterns in the incidence of smoking cessation: United States 1950–1990. Ann Epidemiol. 2002;12:141–150. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez E, Schiaffino A, Garcia M, Borras JM. Widening social inequalities in smoking cessation in Spain, 1987–1997. J Epidemiol Community Health. 2001;55:729–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morabia A, Costanza MC, Bernstein MS, et al. Ages at initiation of cigarette smoking and quit attempts among women: a generation effect. Am J Public Health. 2002;92:71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galeone D. Il contesto normativo nazionale [ Tobacco control: Italian laws]. Ital Heart J. 2001;2 (suppl 1): 19–21. [PubMed] [Google Scholar]
- 27.Mangiaracina G. Percorsi sociali e strategie non istituzionali. Available at: http://www.iss.it/sitp/ofad/docu/0001.pdf. Accessed August 30, 2005.
- 28.Farrell P, Fuchs VR. Schooling and health: the cigarette connection. J Health Econ. 1982;1:217–230. [DOI] [PubMed] [Google Scholar]
- 29.Amos A, Haglund M. From social taboo to “torch of freedom”: the marketing of cigarettes to women. Tob Control. 2000;9:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbeau EM, Leavy-Sperounis A, Balbach ED. Smoking, social class, and gender: what can public health learn from the tobacco industry about disparities in smoking? Tob Control. 2004;13:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook BL, Wayne GF, Keithly L, et al. One size does not fit all: how the tobacco industry has altered cigarette design to target consumer groups with specific psychological and psychosocial needs. Addiction. 2003; 98:1547–1561. [DOI] [PubMed] [Google Scholar]
- 32.Osler M, Prescott E. Psychosocial, behavioural, and health determinants of successful smoking cessation: a longitudinal study of Danish adults. Tob Control. 1998;7:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham H. Women’s smoking and family health. Soc Sci Med. 1987;25:47–56. [DOI] [PubMed] [Google Scholar]
- 34.Jefferis BJ, Power C, Graham H, et al. Changing social gradients in cigarette smoking and cessation over two decades of adult follow-up in a British birth cohort. J Public Health (Oxf). 2004;26:13–18. [DOI] [PubMed] [Google Scholar]
- 35.Godtfredsen NS, Prescott E, Osler M, et al. Predictors of smoking reduction and cessation in a cohort of danish moderate and heavy smokers. Prev Med. 2001; 33:46–52. [DOI] [PubMed] [Google Scholar]
- 36.Freund KM, D’Agostino RB, Belanger AJ, et al. Predictors of smoking cessation: the Framingham Study. Am J Epidemiol. 1992;135:957–964. [DOI] [PubMed] [Google Scholar]
- 37.Kunst AE, Giskes K, Mackenbach JP. SocioEconomic Inequalities in Smoking in the European Union. Applying an Equity Lens to Tobacco Control Policies. Bruxelles, Belgium: ENSP; 2004:52–60.


