Abstract
Objectives. Although the burden of diarrheal disease resulting from inadequate water quality, sanitation practices, and hygiene remains high, there is little understanding of the integration of these environmental control strategies. We tested a modeling framework designed to capture the interdependent transmission pathways of enteric pathogens.
Methods. We developed a household-level stochastic model accounting for 5 different transmission pathways. We estimated disease preventable through water treatment by comparing 2 scenarios: all households fully exposed to contaminated drinking water and all households receiving the water quality intervention.
Results. We found that the benefits of a water quality intervention depend on sanitation and hygiene conditions. When sanitation conditions are poor, water quality improvements may have minimal impact regardless of amount of water contamination. If each transmission pathway alone is sufficient to maintain diarrheal disease, single-pathway interventions will have minimal benefit, and ultimately an intervention will be successful only if all sufficient pathways are eliminated. However, when 1 pathway is critical to maintaining the disease, public health efforts should focus on this critical pathway.
Conclusions. Our findings provide guidance in understanding how to best reduce and eliminate diarrheal disease through integrated control strategies.
In the developing world, more than 1 billion people continue to lack an adequate supply of clean water and adequate disposal of excreta.1 Such statistics explain why the overall global burden of water-, sanitation-, and hygiene-related disease remains high2,3 even though oral rehydration therapy has led to reductions in mortality.4 Despite this demonstrated need for water, sanitation, and hygiene improvements,3 our understanding of integrated control strategies remains poor. Part of the reason is that most intervention studies have examined 1 intervention in isolation without considering other potential pathways of transmission.
There is increasing evidence that the efficacy of household water quality interventions depends on the level of sanitation within the targeted community.5–7 This dependency may explain why, although many household-level water quality intervention studies have shown impressive reductions in health burden,8 results have been highly variable. Some studies have shown reductions as high as 85%, and others have shown no reduction. Moreover, estimates of disease reduction may be inflated because of publication bias (positive results are more likely to be published than are negative results), lack of blinding (a study design feature in which participants do not know whether they are involved in the intervention or nonintervention arm of the study; only 1 of the 15 developing country studies reviewed by Fewtrell et al.8 were blinded), and lack of randomization (only 5 of the 15 studies reviewed by Fewtrell et al.8 were randomized).9
These interpretive challenges arise in part because enteric pathogens are transmitted through a complex set of interdependent pathways, including both contaminated food and water along with household- and community-level person-to-person routes; these various pathways have been codified in the F diagram, which classifies transmission pathways as mediated through food, fingers, fomites, flies, and so on10 (see Bern et al.,11 Huttly et al.,12 and Curtis et al.13 for reviews). The exposure factors summarized in the F diagram (e.g., general hygiene behaviors,14,15 fecal contamination,16–18 food contamination,19 and drinking water storage practices19) as well as more distal factors (e.g., day-care centers20 and socioeconomic factors21,22) are important to our understanding of these pathways.
Water may be contaminated through runoff and may expose individuals through drinking water or recreational, bathing, or washing activities, and food may be contaminated either through infected animals or from contact with contaminated water or soil. Inadequate hygiene may result in contamination of fomites in common living spaces23; infection may then be transmitted in many ways (e.g., through exposures in day-care centers or through sexual activity). Soil may be contaminated through improper management of excreta (poor sanitation). Cairncross et al.24 extended the F diagram by differentiating between infection transmission within households and within the public domain.
Other studies have addressed the interaction between different transmission pathways, suggesting that the risk associated with water contamination depends on the level of community sanitation.5,7,25 Although implicitly assuming that level of community sanitation modifies the association between water contamination and diarrheal disease, none of these studies have addressed the observation that the multiple transmission pathways and contagious nature of pathogens result in risks that are dependent on the disease status of the community.26–29 Many enteric pathogens can be transmitted from infectious human excreta to susceptible humans either directly or indirectly through the environment, and thus they are sustained through chains of transmission that may pass through combinations of pathways. The importance of each pathway depends on the pathogen and specific environmental conditions, and the efficacy of any given exposure-specific intervention strategy depends on the level of pathogen exposure from other pathways.
We used simulation modeling to evaluate the effectiveness of water quality interventions under varying community sanitation and hygiene conditions, explicitly acknowledging that rates of infection depend on numbers of current and past infections. We characterized the specifics of this dependency by explicitly modeling transmission pathways, in effect yielding a dynamic version of the F diagram. Specifically, we determined (1) how the efficacy of water quality interventions depends on the level of both household- and community-level transmission and (2) the conditions under which water quality interventions, hygiene and sanitation improvements, or both are effective in reducing the burden of disease in a community.
METHODS
Community-Level Model
In constructing our model, we assumed that enteric pathogens can survive in the environment outside of a host; this fact dictates the possible pathways a waterborne pathogen can exploit in completing its transmission cycle (Figure 1a ▶). Because infectious diseases are transmissible, unlike many other conditions studied in analytic epidemiology, individuals may be indirectly at risk of an environmental exposure; for example, cohort A may be infected with a pathogen as a result of exposure to contaminated water (a water quality issue) and in turn may transmit this pathogen to cohort B through a food, hygiene, or sanitation pathway (Figure 1b ▶). In this manner, hygiene and sanitation can modulate the effects of drinking water contamination, and likewise drinking water contamination can modulate the effects of poor hygiene or sanitation.
FIGURE 1—
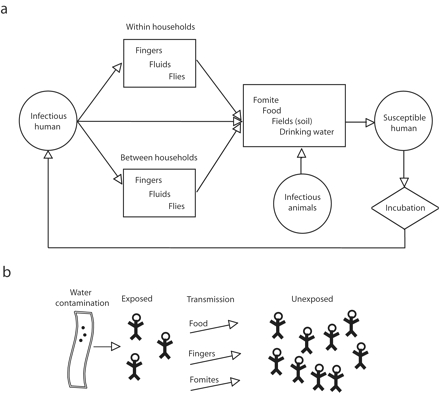
Diagram illustrating potential transmission routes for enteric (waterborne) pathogens (a) and how indirect exposure to contaminated drinking water can occur as a result of the multiple and interdependent nature of exposure pathways (b).
The model used in this study was a household-level model incorporating 5 transmission pathways (Figure 2 ▶. First, between-household transmission accounts for the movement of pathogens from an individual in one household to an individual in another household. This transmission can occur in communal settings (e.g., washing in rivers or schools) or in settings where a family member contaminates his or her hands in the community and brings that contamination into the household. Second, within-household transmission accounts for the movement of pathogens between 2 individuals residing in the same household. The magnitude of this transmission pathway is generally thought of as a function of hygiene.
FIGURE 2—
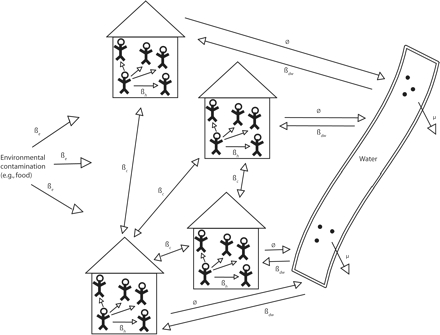
Schematic for a household-level infection transmission model.
Note. βc = between-household transmission; βd = within-household transmission; φ = contamination of water; βdw = exposure from contaminated water; βe = other sources.
Third, household-to-water transmission accounts for the contamination of water because of the inappropriate disposal of feces. The magnitude of this pathway is generally thought of as a function of sanitation and is often addressed through building latrines. Fourth, water-to-household transmission accounts for the movement of pathogens to humans as a result of exposure to pathogens in drinking water. Improving water quality controls this pathway. Finally, external transmission of pathogens to the community from an outside source accounts for pathogen introduction from upstream water flow, contaminated food, or an infectious individual exposed outside of the community. Any of the arrows linking households in Figure 2 ▶ can move through any of the paths shown in Figure 1 ▶.
A prominent feature of this model structure is that transmission pathways are interdependent; for example, the rate of infection from exposure to contaminated water affects the rate of within- or between-household transmission. Likewise, the rate of within- or between-household transmission affects the rate of pathogens shed into the environment by infectious individuals. Thus, as transmission from person to person increases, the concentration of pathogens in the water increases, which in turn increases the risk of exposure to contaminated water. The dynamic process represented in this relationship is not accounted for in the standard risk models predominant in epidemiology, wherein risk of infection from 1 transmission pathway is assumed to be independent of rate of infection from other pathways. To represent this conceptual model in a mathematical framework, we used a discrete-event stochastic model (a model that estimates probability by allowing random variation in 1 or more variables over time) structure at the household level.29–31
We used 3 model-state variables, Si , Ii , and Ri , representing the numbers of susceptible individuals, infectious individuals, and individuals immune to further infection, respectively, for household i. For illustrative purposes, we chose to model a pathogen that confers complete immunity, a property that many types of enteric viruses share. In addition, Ni and N represent the total number of people living in household i (Ni = Si + Ii + Ri ) and in the community (N = ∑i Ni ), respectively.
Seven model parameters require identification: ρ, the per individual recovery rate; φ, the rate at which infected individuals shed viable pathogens into the water supply; μ, the mortality rate for pathogens in the water supply; r, the risk of infection per pathogen exposure; ɛ, the number of pathogens in the environment, not from drinking water but from other sources such as food; βh , the within-household rate of transmission; and βc , the between-household rate of transmission standardized according to the total community population (Table 1 ▶). In addition to these final 2 transmission rate parameters, the model included the rate of transmission from the environment (other than water), βe = rɛ, and the rate of transmission from water, βdw = rW(t ), where W(t ) is the number of waterborne pathogens at time t.
TABLE 1—
Parameter Values and Units Used in the Simulation Analysis
| Parameter Values | Units | |
| ρ | 10 | Days |
| φ | {0, 0.5, 1.0, 1.5, 2.0} | Pathogens/person/day |
| β c | {0, 0.005, 0.01, 0.02, 0.03, 0.04, 0.06, 0.1} | No. of transmission events/infected individual |
| β h | {0, 0.005, 0.01, 0.02, 0.03, 0.04, 0.06, 0.1} | No. of transmission events/infected individual |
| ɛ | 30 | Pathogens |
| μ | 1 | Pathogens/day |
| r | 0.000002 | Infections/pathogen |
Note. ρ = recovery rate; φ = rate at which infected individuals shed pathogens into the water supply; βc and βh = between-and within-household transmission rates; ɛ= level of environmental contamination; μ = pathogen die-off rate in the water supply; r = risk of infection per pathogen exposure.
On the basis of these state variables and parameters, 5 events are possible: (1) recovery from infection, (2) secondary infection from someone inside the household, (3) secondary infection from someone in the community, (4) infection from an environmental source other than drinking water, and (5) infection from drinking contaminated water. The first 4 events are assumed to occur in an exponential waiting time pattern; that is, rates remain constant between events in which the numbers of infected individuals change. The hazard for an infection event resulting from drinking water is dependent on the total number W(t) of viable pathogens in the water at a specific time; modeling the number of pathogens by a differential equation led to a time-dependent hazard for water-related infections (shown in the online supplement to this article).
Simulation Analysis
In this simulation, we chose to use an event-driven model in which recovery and infection events are scheduled for each household, assuming a simple Poisson process32,33 (details are presented in the online supplement to this article). We ran simulations using all combinations of φ, βc, and βh, for a total of 320 (5 × 8 × 8) parameter sets. To estimate the efficacy of a water quality intervention, we examined 2 scenarios: all households were either fully exposed to contaminated drinking water (A = 0) or all households received the water treatment intervention (A = 1).
We then defined the fraction of disease preventable through water treatment, known as the preventable fraction, as (IA = 1 − IA = 0)/IA = 1, where IA = 1 and IA = 0 represent the cumulative incidence estimates from the scenarios in which (1) there was no intervention and individuals were exposed to contaminated water (A = 0) and (2) a water quality intervention was implemented completely protecting the population from exposure to contaminated water (A = 1). Simulations were repeated 10 times for each parameter set and for both exposed and unexposed scenarios, resulting in a total of 6400 (320 × 2 × 10) simulations. Table 1 ▶ summarizes the values for the 7 parameters used in the simulation. We conducted all simulations and analyses on a Pentium III PC using MATLAB (Mathworks, Natick, Mass).
RESULTS
The fraction of disease preventable through water treatment varied from a few percentage points to more than 75%. This variation was completely explained by 3 transmission pathways: water contamination (φ), household transmission (βh), and community-level (or between-household) transmission (βc ; Figure 3 ▶).
FIGURE 3—
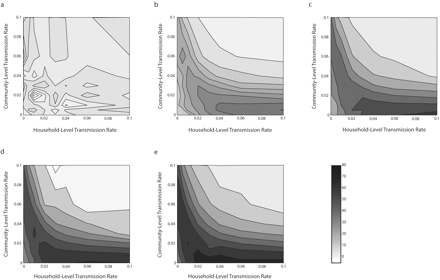
Contours of preventable fractions associated with improving water quality for different rates of household-level and community-level transmission.
Note. Each contour plot involves a different contamination rate (φ): 0 (a), 0.5 (b), 1.0 (c), 1.5 (d), and 2.0 (e).
When water contamination levels were low, the preventable fraction associated with water treatment was small. This was true regardless of household or community transmission levels, as depicted by the light contours in Figure 3a ▶ (note that the contour patterns shown in Figure 3a ▶ ranged from −8.3% to 7.2% and that this range was because of stochastic variation in the model simulations). In general, as the level of water contamination increased, so did the preventable fraction, as depicted by the darker contours; however, for higher levels of contamination, the predicted preventable fraction was also a function of transmission levels (Figure 3b–e ▶).
When transmission values were high (βc and βh > 0.06), the preventable fraction was small regardless of the level of contamination (Figure 3b–e ▶, upper right-hand corners). The findings shown in Figure 3b–e ▶ can be interpreted by considering 3 questions: (1) When there are zero to low levels of community transmission (βc < 0.01), how does household transmission affect the preventable fraction? (2) When there are zero to low levels of household transmission (βh < 0.01), how does community transmission affect the preventable fraction? (3) When there is a high level of community transmission (βc > 0.08), how does household transmission affect the preventable fraction? We discuss each of these issues in turn.
In the case of zero to low levels of community transmission, we found that whenever the household-level transmission rate increased, the preventable fraction also increased. That is, when there was little community transmission, household transmission acted primarily to amplify the waterborne process, which was the target of our intervention; in addition, each time a waterborne case was directly prevented, all of the household cases that would have resulted from it were also prevented—resulting in a higher preventable fraction. Yet, no matter how high the household transmission level is, it cannot result in self-sustained endemic conditions. In this sense, it does not represent a competing pathway; in the absence of community transmission, we found that increased household transmission always corresponded to a higher preventable fraction.
Conversely, in the absence of household transmission, we discovered that increased community transmission resulted in an entirely different qualitative pattern. In the case of low levels of household and community transmission, we found that as community transmission began to increase, the preventable fraction (Figure 3c–e ▶) increased at first but eventually decreased. When community transmission levels alone were too low to sustain endemicity, each case of waterborne disease resulted in a finite chain of cases, and the same amplification mechanism observed for household transmission acted here to increase the preventable fraction as well. Eventually, however, community transmission alone was able to sustain endemic conditions and became an entirely sufficient competing pathway, partially replacing water contamination as a source of infection and reducing the effectiveness of the intervention.
Finally, we found that when community-level transmission rates were high, increasing household transmission levels actually reduced the preventable fraction as well, in contrast to the case of zero to low levels of community transmission, in which increasing household transmission increased the preventable fraction. This occurred because household transmission amplified community transmission as well as waterborne transmission. When household transmission increased, the ability of community transmission to sustain endemic infection levels also increased. Under these conditions, in which household transmission contributed to a competing sufficient process, Figure 3 ▶ suggests that household transmission’s amplification of community-level spread outweighs its amplification of water-related cases.
A parametric sensitivity analysis suggested that the qualitative features of Figure 3c ▶ are robust to varying assumptions of pathogen die-off rates in water (as described in the online supplement to this article). In addition, these features seem to be robust to varying assumptions of infectivity, duration of infectiousness, and levels of contamination in the environment.
DISCUSSION
Public health policy recommendations are urgently needed to provide guidance for developing countries on how to make informed decisions regarding the most effective interventions for lowering the incidence of diarrheal diseases. Although promotion of oral rehydration therapy has resulted in significant decreases in mortality, enteric pathogens continue to cause a substantial disease burden.3 Environmental interventions, consisting of water quality, sanitation, hygiene, and food-based interventions, remain as crucial tools in further decreasing disease burden.
Integrative Intervention Strategies
Much is known about the natural history of infection transmission and the basic risk factors associated with disease, but little is known about how different transmission pathways interact to determine the ultimate efficacy of an intervention. VanDerslice and Briscoe7 provided observational data on the interaction between sanitation and water risks; Esrey showed a similar effect using Demographic and Health Surveys data,5 as did Gundry et al.6 in summarizing previously published intervention trial results. The range of efficacies seen in water intervention trials illustrates that when sanitation levels are poor, water quality projects may have minimal public health effect.
Our model provides a framework for understanding how to develop and assess public health interventions that seek to reduce diarrheal disease. In particular, we modeled how environmental-, household-, and community-level transmissions interact to determine intervention effectiveness and how this interaction can explain differences in the efficacies that have been observed for water quality interventions.
Our analysis quantifies the public health effect that can be obtained by intervening on 1 transmission pathway, for example, water quality, and shows how this effect depends on the magnitude of other transmission pathways such as those associated with sanitation and hygiene, a finding consistent with previous studies. Specifically, when community sanitation is poor, water quality improvements may have minimal impact, regardless of the amount of water contamination. Under these conditions of high community transmission, community-level sanitation must be considered a necessary intervention and possibly a sufficient one depending on the level of water contamination.
Unfortunately, strikingly few sanitation intervention studies are available to test the hypotheses generated in this model analysis.8 Additional health impact studies are needed to examine the role of sanitation in transmission, and future intervention designs need to consider the various pathways of transmission to better identify the set of interventions that are necessary and sufficient to lessen the burden resulting from diarrheal diseases.
Interpretation of Findings
When 1 pathway is critical to maintaining diarrheal disease, public health efforts should focus on this critical pathway, whether it involves improvements in water quality, sanitation, or hygiene. Under conditions in which each pathway alone is sufficient to maintain disease at high levels, however, single-pathway interventions will have minimal benefit, and ultimately an intervention will be successful only if all of the sufficient pathways are eliminated. To validate this concept, future intervention designs should therefore stratify treatment groups according to the intensity of transmission of alternative pathways.
For example, a water quality intervention might stratify treatment assignment according to communities with poor versus good sanitation. This design would provide important information on the degree to which benefits of water quality interventions will vary depending on sanitation conditions, a hypothesis put forth in our model analyses as well as a variety of other studies.5–7 It should be kept in mind that these model analyses serve only as guides to optimal intervention strategy design. Ultimately, any intervention strategy must be developed in the context of the perceived and actual needs of the target population.
In our current model structure, hygiene and sanitation are broadly defined as practices associated with transmission within households and between households. A more detailed model structure is needed to inform specific interventions such as those targeted toward hand washing, water storage, or keeping a house clean. The broad definitions used here, however, illustrate the interdependencies of transmission pathways.
Increasing transmission can either amplify or attenuate risks, depending on the outcome of interest and on levels of transmission.27,29,34 Attenuation occurs when transmission places unexposed populations indirectly at risk.34 In our scenario, this takes place through community transmission, in which unexposed households (those with water treatment) can place exposed households (those without water treatment) at risk. Thus, community transmission will attenuate the efficacy of a water quality intervention, because indirect cases may not share the same treatment device as their index cases.
Alternatively, amplification of risks occurs through chains of transmission events that multiply the effect of an exposure event.27 In our scenario, this takes place primarily through household transmission, in which, for any given household that is exposed to water contamination, transmission within the household will amplify risk. Amplification of risk can also occur through community transmission among households with the same treatment assignment.
The causal link between infectious individuals and susceptible individuals, generally mediated through environmental and social processes, involves a network of contacts. These network contacts include a number of important population-level features, such as the effect of community sanitation on risk, that are missed by an individual analytic approach; that is, a system-level perspective is needed when risks manifest themselves within a causal web of multiple and interdependent social, economic, biological, and environmental processes. In the case of enteric pathogens, these interdependent pathways are a result of both the ability of the pathogens to exploit a variety of exposure pathways and their contagious properties that place susceptible individuals at risk of infection from infectious individuals (Figure 1 ▶).
Although the specific model we analyzed was designed to examine the presence of a single generic enteric pathogen and to focus on a single community, it can be adapted to examine specific pathogens within specific contexts either at a community or a regional level. The transmission properties we assessed were robust to changing demographic and biological attributes of the transmission process. These changes may alter the quantitative aspects of transmission dynamics, but the qualitative features remain, and the methods we developed can be extended to assess the efficacy of an integrated control and prevention strategy in a particular setting.
Conclusions
Our analysis contributes to the dialogue on how to develop optimal integrated control strategies by providing insight into the causes of the variability observed in the effectiveness of different interventions; for example, differing baseline sanitation, hygiene, and water quality conditions may be sufficient to explain observed variations across water quality studies. Further simulation studies can extend the methods we have developed to address more detailed control strategy issues such as assessing the impact of specific sanitation and hygiene interventions in isolation and in combination with water quality interventions. We believe that simulation methods such as those described here will provide important guidance in efforts to understand how to best reduce and eliminate needless infections and deaths due to diarrheal disease.
Acknowledgments
This work was funded by the National Institute of Allergy and Infectious Diseases (grant RO1-AI050038).
Note. The funding agency had no role in the design of this study.
Human Participant Protection No protocol approval was needed for this study.
We thank Jamie Bartram for initial conversations that stimulated this project and James Koopman for valuable comments on the article.
Peer Reviewed
Contributors J. N. S. Eisenberg originated and supervised the study and led the writing. J. C. Scott completed the analysis. T. Porco assisted in model development and analysis.
References
- 1.Meeting the MDG Drinking Water and Sanitation Target: A Mid-Term Assessment of Progress. Geneva, Switzerland: World Health Organization and United Nations Children’s Fund; 2004.
- 2.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003; 361:2226–2234. [DOI] [PubMed] [Google Scholar]
- 3.Pruss A, Kay D, Fewtrell L, Bartram J. Estimating the burden of disease from water, sanitation, and hygiene at a global level. Environ Health Perspect. 2002; 110:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 5.Esrey SA. Water, waste, and well-being: a multi-country study. Am J Epidemiol. 1996;143:608–623. [DOI] [PubMed] [Google Scholar]
- 6.Gundry S, Wright J, Conroy R. A systematic review of the health outcomes related to household water quality in developing countries. J Water Health. 2004;2:1–13. [PubMed] [Google Scholar]
- 7.VanDerslice J, Briscoe J. Environmental interventions in developing countries: interactions and their implications. Am J Epidemiol. 1995;141:135–144. [DOI] [PubMed] [Google Scholar]
- 8.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM Jr. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. [DOI] [PubMed] [Google Scholar]
- 9.Juni P, Altman D, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawata K. Water and other environmental interventions—the minimum investment concept. Am J Clin Nutr. 1978;31:2114–2123. [DOI] [PubMed] [Google Scholar]
- 11.Bern C, Martines J, deZoysa I, Glass RJ. The magnitude of the global problem of diarrhoeal disease: a ten year update. Bull World Health Organ. 1992;70: 705–714. [PMC free article] [PubMed] [Google Scholar]
- 12.Huttly SR, Morris SS, Pisani V. Prevention of diarrhoea in young children in developing countries. Bull World Health Organ. 1997;75:163–174. [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis V, Cairncross S, Yonli R. Domestic hygiene and diarrhea—pinpointing the problem. Trop Med Int Health. 2000;5:22–32. [DOI] [PubMed] [Google Scholar]
- 14.Kaltenthaler EC, Drasar BS. Understanding of hygiene behaviour and diarrhoea in two villages in Botswana. J Diarrhoeal Dis Res. 1996;14:75–80. [PubMed] [Google Scholar]
- 15.Huttly SR, Lanata CF. Feces, flies, and fetor: findings from a Peruvian shantytown. Rev Panam Salud Publica. 1998;4:75–79. [DOI] [PubMed] [Google Scholar]
- 16.Bukenya GB, Nwokolo N. Transient risk factors for acute childhood diarrhoea in an urban community of Papua New Guinea. Trans R Soc Trop Med Hyg. 1990;84:857–860. [DOI] [PubMed] [Google Scholar]
- 17.Han AM, Moe K. Household feacal contamination and diarrhoea risk. J Trop Med Hyg. 1990;93: 333–336. [PubMed] [Google Scholar]
- 18.Yeager BA, Huttly SR, Bartolini R, Rojas M, Lanata CF. Defecation practices of young children in a Peruvian shanty town. Soc Sci Med. 1999;49:531–541. [DOI] [PubMed] [Google Scholar]
- 19.Simango C, Dindiwe J, Rukure G. Bacterial contamination of food and household stored drinking water in a farmworker community in Zimbabwe. Centr Afr J Med. 1992;38:143–149. [PubMed] [Google Scholar]
- 20.Sempértegui F, Estrellá B, Egas J, et al. Risk of diarrheal disease in Ecuadorian day-care centers. Pediatr Infect Dis J. 1995;14:606–612. [DOI] [PubMed] [Google Scholar]
- 21.Mock NB, Sellers TA, Abdoh AA, Franklin RR. Socioeconomic, environmental, demographic and behavioral factors associated with occurrence of diarrhea in young children in the Republic of Congo. Soc Sci Med. 1993;36:807–816. [DOI] [PubMed] [Google Scholar]
- 22.Yeager BA, Lanata CF, Lazo F, Verastegui H, Black RE. Transmission factors and socioeconomic status as determinants of diarrhoeal incidence in Lima, Peru. J Diarrhoeal Dis Res. 1991;9:186–193. [PubMed] [Google Scholar]
- 23.Stanton BF, Clemens JD, Clements JD. Soiled saris: a vector of disease transmission? Trans R Soc Trop Med Hyg. 1986;80:485–488. [DOI] [PubMed] [Google Scholar]
- 24.Cairncross S, Blumenthal U, Kolsky P, Moraes L, Tayeh A. The public and domestic domains in the transmission of disease. Trop Med Int Health. 1996;1: 27–34. [DOI] [PubMed] [Google Scholar]
- 25.Esrey SA, Potash JB, Roberts L, Shiff C. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull World Health Organ. 1991; 69:609–621. [PMC free article] [PubMed] [Google Scholar]
- 26.Koopman JS, Longini IM Jr, Jacquez JA, et al. Assessing risk factors for transmission of infection. Am J Epidemiol. 1991;133:1199–1209. [DOI] [PubMed] [Google Scholar]
- 27.Koopman JS, Longini IM Jr. The ecological effects of individual exposures and nonlinear disease dynamics in populations. Am J Public Health. 1994;84: 836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenberg JN, Lei X, Hubbard AH, Brookhart MA, Colford JM Jr. The role of disease transmission and conferred immunity in outbreaks: analysis of the 1993 Cryptosporidium outbreak in Milwaukee, Wisconsin. Am J Epidemiol. 2005;161:62–72. [DOI] [PubMed] [Google Scholar]
- 29.Eisenberg JN, Lewis BL, Porco TC, Hubbard AH, Colford JM Jr. Bias due to secondary transmission in estimation of attributable risk from intervention trials. Epidemiology. 2003;14:442–450. [DOI] [PubMed] [Google Scholar]
- 30.Ball F. Stochastic and deterministic models for SIS epidemics among a population partitioned into households. Math Biosci. 1999;156:41–67. [DOI] [PubMed] [Google Scholar]
- 31.Becker NG, Dietz K. The effect of household distribution on transmission and control of highly infectious diseases. Math Biosci. 1995;127:207–219. [DOI] [PubMed] [Google Scholar]
- 32.Porco TC, Small PM, Blower SM. Amplification dynamics: predicting the effect of HIV on tuberculosis outbreaks. J Acquir Immune Defic Syndr. 2001;28: 405–498. [DOI] [PubMed] [Google Scholar]
- 33.Bratley P, Fox BL, Schrage LE. A Guide to Simulation. 2nd ed. New York, NY: Springer-Verlag; 1987.
- 34.Halloran ME, Struchiner CJ. Causal inference in infectious diseases. Epidemiology. 1995;6:142–151. [DOI] [PubMed] [Google Scholar]


