Abstract
We present an overview of different methods for decomposing a multichannel spontaneous electroencephalogram (EEG) into sets of temporal patterns and topographic distributions. All of the methods presented here consider the scalp electric field as the basic analysis entity in space. In time, the resolution of the methods is between milliseconds (time-domain analysis), subseconds (time- and frequency-domain analysis) and seconds (frequency-domain analysis). For any of these methods, we show that large parts of the data can be explained by a small number of topographic distributions. Physically, this implies that the brain regions that generated one of those topographies must have been active with a common phase. If several brain regions are producing EEG signals at the same time and frequency, they have a strong tendency to do this in a synchronized mode. This view is illustrated by several examples (including combined EEG and functional magnetic resonance imaging (fMRI)) and a selective review of the literature. The findings are discussed in terms of short-lasting binding between different brain regions through synchronized oscillations, which could constitute a mechanism to form transient, functional neurocognitive networks.
Keywords: synchronous neural oscillations, microstates, transient binding, combined electroencephalogram–functional magnetic resonance imaging
1. Introduction
The number of neurons in the human brain is approximately equal to the number of transistors in a modern personal computer. Compared with the computer that operates in the GHz range, the brain has a much lower firing rate (below ca. 1000 Hz), but a massive advantage in the interconnectivity between its basic units: a single neuron can have up to 1000 connections to other neurons, and in the entire human brain, there are approximately 100 trillions of neuronal connections. While computers are certainly faster and more reliable in straight-forward ‘number crunching’ computational tasks, the brain outperforms computers in reliability and accuracy when dealing with complex tasks such as language understanding, object recognition or planning and strategy development. Thus, it is likely that the brain's advantage in these tasks must be in the cooperation of the units, rather than the processing speed in the units. Therefore, connectivity in the brain is probably one of the most fundamental and challenging fields of study at this time. Useful theories on ‘how the brain works’ will certainly have to account for how the brain establishes, organizes, maintains and modifies its internal connectivity structure in space and time.
Connectivity can be studied on many spatial and temporal scales. In space, it ranges from the axonal wiring within and between layers of cortical tissue to the connections between remote brain regions based on large fibre bundles. In time, it extends from the instantaneous functional connectivity established through active synapses of simple neural feedback loops that result in a millisecond by millisecond spiking pattern, to the genetically outlined macroscopic structure of white matter connections throughout the brain that are modified on a time-scale of generations. The accessibility of measurements of connectivity on these different scales is driven by the available measurements (electrophysiological, metabolic or structural imaging) and analysis methods. Today, these methods still do not provide enough information to reconstruct the whole connectivity matrix of the brain with sufficient resolution in time and space. However, the current state of research already allows for some conclusions and may be sufficient to speculate about specific features of brain connectivity (Friston et al. 2003).
The present paper will focus on the implications on brain connectivity that can be drawn from observations made in multichannel electroencephalograms (EEG) of healthy subjects. In space, it is on a global level, considering the entire brain as a single research object. In time, it covers a time range from a few milliseconds to a few seconds. We will introduce the basic constituting elements of the EEG in space and time and illustrate various methods for mapping such elements to EEG data. Some of the basic empirical findings obtained with these methods are presented as well as results obtained in combination with other methods (functional magnetic resonance imaging; fMRI). The aim is to demonstrate a consistent relationship of these findings to brain connectivity. This review does not claim to be a complete coverage of connectivity in the field of EEG research. It also does not attempt to vigorously exclude other possible hypotheses, but it emphasizes and illustrates the current view of the authors. The methods presented here are driven by observations in real EEG data and, therefore, the conclusions that we draw in terms of connectivity are data, rather than theory, driven. Furthermore, they are compatible with physically and physiologically plausible models of the generation of the EEG.
2. Basic elements of the EEG
In the following section, we introduce and discuss the basic ‘atomic’ spatial and temporal elements that we will use as a basis for the assessment and interpretation of functional connectivity in EEG.
In space, the atomic elements of the EEG are well defined; they are the scalp electric fields produced by the activity of an atomic electric element in the brain, which is a single current dipole. The physical theory that relates a single current dipole localized in the brain to the scalp electric field is well known and essentially determined by the volume conduction properties of the tissues in the head (brain, skull and scalp) and its geometry. The electric leadfield, the physical term that relates the intracerebral activity to scalp electric fields, has been shown to act as a spatial smoothing operator function. This implies that even single, point-like bipolar sources (i.e. a single dipole) in the brain produce bipolar fields that generally extend over the entire scalp surface. Furthermore, since volume conduction is instantaneous, the signal from any single source will affect all electrodes simultaneously, thus, a single source cannot account for time delays between electrodes. When several dipoles are active at the same time (i.e. several ‘atoms’ form a ‘molecule’), the measured scalp electric field is the sum of the independent scalp fields produced by those dipoles (see equation (2.1)). Therefore, at every single electrode, we may observe a mixture of activity resulting from several different sources. Furthermore it is also possible that the scalp electric fields of several active dipoles cancel each other out, such that the activity of these sources remains invisible for the EEG.
| (2.1) |
where v is the vector of scalp electric potentials, K is the leadfield matrix, j is the intracerebral current source density distribution and e is a noise term.
For the characterization of atomic elements in time and frequency, some models concerning the physiological mechanisms of the generation of EEG oscillations exist (Lopes da Silva 1991), but the knowledge is still sparse and cannot provide a basis for sufficiently well-determined models. Therefore, the current practice is to employ user-defined elements that are assumed to suit the specific research hypotheses and objectives. The most important time elements used for EEG analysis are briefly introduced below.
In quantitative EEG, the most widely used atomic elements in time are continuous sine and cosine functions (i.e. obtained by the Fast Fourier Transformation; FFT). This has provided many important, well-established and clinically relevant findings (John et al. 1977, 1994; Dumermuth & Molinari 1987) and helped to attribute specific brain functions to specific, narrowly defined EEG frequency bands (Fernandez et al. 2000). Sine and cosine functions are especially useful for investigating brain states that are assumed to have little and very slow fluctuations (e.g. sleep stages (Achermann & Borbely 2003), drug induced states (Saletu et al. 1987; Itil & Itil 1995), maturation (John et al. 1980), and neuropathology (Prichep et al. 1994).
However, many fluctuations of brain state occur at a subsecond time-scale. These events are ill-represented or cancel out when using the FFT. Time-domain analysis uses instantaneous (spike-like) delta-functions as atomic time events. In order to allow comparisons between conditions or subjects using delta-functions, one needs a common reference (zero) point in time. Therefore, time-domain EEG analysis has mainly been applied to event-related data, since events provide external, directly accessible reference points in time that allow to assess stimulus or response-locked brain activity. In spontaneous EEG, there are no external time reference points, therefore, pattern recognition approaches have been applied; these approaches will be discussed in §4 below.
While time-domain analysis provides a high time resolution and the FFT provides high frequency resolution, neither of the two analyses provides information about time and frequency together. In order to characterize events that occur in a certain time-window and oscillate at a certain frequency, that is, in order to identify transient, frequency-locked oscillatory states, the atomic elements of choice are Gabor-functions. Gabor-functions are Gaussian-modulated sinusoids. They have been used to identify sleep spindles (Zygierewicz et al. 1999), epileptic seizures (Franaszczuk et al. 1998), movement related synchronization and desynchronization (Alegre et al. 2003; Durka et al. 2004) and oscillations related to task induced cognitive states (Braeutigam et al. 2001; Gurtubay et al. 2001). Furthermore, we have proposed a multichannel topographic time–frequency analysis based on Gabor-functions that explains the EEG as transient oscillations of a small set of topographies (Koenig et al. 2001b).
How do these atomic elements in time and the atomic elements in space relate? In order to account for a multichannel EEG, the atomic spatial elements (topographies) and the atomic temporal elements (time-courses) must be combined to form spatio-temporal events. The amplitude of each topography is therefore assumed to change in time. This time-varying behaviour of a single topography is represented by one specific atomic temporal element. Given a set of topographies with a corresponding and temporal behaviour, the computation of the resulting scalp EEG measurements is straightforward: the contribution of each topography to the EEG is obtained by modulating (multiplying) that topography with its corresponding time-course. If there are several pairs of topographies with corresponding waveshapes, the resulting EEG is obtained by summing up the contributions of each of these pairs at each moment in time and at each electrode (Koenig et al. 2002a). In general, the EEG must therefore be considered as sum of the activity of different sources that mix in time and space (see equation (2.2)). An example of the modelling of EEG using two different topographies and their time-courses is shown in figure 1.
| (2.2) |
Y, m×r matrix of the multichannel EEG signal over time (channels are given columnwise); D, m×n matrix of signal time-courses (atomic events are given columnwise); V, n×r matrix of scalp electric potentials (topographies are given rowwise); E, m×r matrix of the multichannel signal noise (channel noises are given columnwise); m, number of samples in time; n, overall number of atomic temporal elements; r, overall number of electrodes.
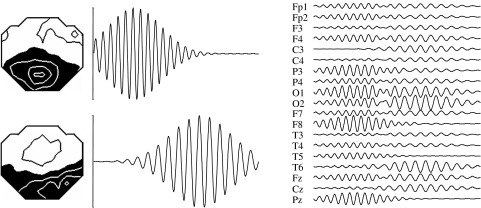
Figure 1.
Left are two EEG topographies, seen from above, nose up. In the middle a (constructed) time-course over 2 s is shown for the two topographies. The two topographies and time-courses overlap in space and time. On the right side, the resulting EEG is displayed. The resulting EEG is a mixture of both activities.
The inverse problem, the decomposition of a real EEG into its spatial and temporal components, is not unique; one may construct many sets of topographies and waveshapes that can account for the measured EEG. Therefore, it becomes necessary to impose additional criteria that select a specific solution, for example, orthogonality (John et al. 1993) or independence (Makeig et al. 1997), which optimize statistical properties of the decomposition. We have begun to use another criterion that is initially driven by observations made in real EEG recordings and not by a priori statistical needs which will be covered in more detail in the discussion. The formalism of considering the EEG as a combination of topographies and time-courses will then allow us to derive certain conclusions about connectivity.
3. FFT methods
The most frequently used analysis method for the study of connectivity has probably been the computation of coherence between pairs of electrodes. Although there is a considerable body of literature based on that methodology (Rappelsberger & Petsche 1988; Nunez et al. 1997), the underlying assumption that a statistical relation between the signals at two electrodes indicates a functional relation of the brain regions below those electrodes implies a source model that lacks a stringent validation. In order to overcome this problem, the computation of coherence has recently been based upon the intracerebral current source densities obtained with distributed inverse solutions (Lehmann et al. in press). However, computing coherence based on distributed inverse solutions still requires a priori hypotheses about the sites that possibly interact and the distribution of the putative sources.
Another conceptually different approach to connectivity has been based on the observation that frequency transformed multichannel EEG can, in general, be accounted well by models that assume, for each frequency, a single phase across electrodes (Lehmann & Michel 1990). Therefore, for a given frequency, the model (called FFT-Approximation) consists of a single sinusoidal oscillation that is defined by its frequency and phase, and by a single topographic map that weights the oscillation for each electrode. Given the frequency, the phase of the oscillation and its weight at each electrode are optimized to account for a maximal proportion of the EEGs total variance at that frequency:
| (3.1) |
t1,…,tm, m sample points in time; Td, diagonal matrix diag(t1,…,tm); 1m, vector consisting of m ones; W, m×n matrix of the frequencies w1,…,wn with columns w11m,…,wn1m; P, m×n matrix of the phases p1,…,pn with columns p11m,…,pn1m.
If applied to whole-scalp EEG, the unexpected property of this model is how well it works. Assuming randomness, one would expect that such a model would explain somewhat more than 50% of the total variance of the EEG. The typical result of the FFT approximation, however, accounts for around 80% of the variance (Lehmann & Michel 1990). Thus, when an EEG is frequency transformed, the largest part of the variance of the data is compatible with a single phase model. Figure 2 shows the topography of a single phase map compared with a conventional power map.
Figure 2.
Left: topographic map that is optimized for a frequency-domain single phase model of a sample EEG, at a frequency of 11.5 Hz. In this example, the map explained 80.9% of the total variance at that frequency. Black and white map areas indicate opposite polarity. Middle: the power map corresponding to the single phase model on the left. Right: conventional power map of the same data at 11.5 Hz. The power map derived from the single phase model is very similar to the conventional power map.
So, in terms of connectivity, what are the implications of such a model? Physically, there are two possible interpretations:
There is a single source in the brain that accounts for the entire proportion of EEG that has a common phase. Physiologically, the assumption of a single source may apply to pathological cases such as epilepsy or tumours, but is unlikely to be the case in functional physiological brain states.
There are several sources active that operate in a phase-locked, zero-delay mode, such that they appear as a single meta-generator that hence produces a single electric field. The assumption that there are several EEG generators that operate with independent phases would predict large phase differences across electrodes and would therefore not be able to account for the typically observed predominance of a single phase across electrodes.
The conclusion we draw from this observation is that, in the proportion of brain activity visible in the EEG, the brain electric oscillations at a given frequency synchronize across a major proportion of the active sources. Synchronization of brain activity has long been observed on a millimetre spatial scale (e.g. Eckhorn et al. 1988; Gray & Singer 1989; Singer & Gray 1995) and a possible mechanism to explain how the brain detects unique combinations of all possible stimulus features has been suggested (the binding problem, Singer & Gray 1995; Livingstone 1996). The synchrony observed in whole-scalp, multichannel EEG thus suggests that similar mechanisms may occur at a global level, and that different brain regions connect through synchronous, rhythmic discharges to form transient, functional, neurocognitive networks (Mesulam 1990).
The amount of common phases in EEG oscillations can be quantified for the different frequencies using a measure called global field synchronization (GFS; Koenig et al. 2001a, 2005). In line with the hypothesis that GFS relates to the existence of synchronously oscillating neurocognitive networks, GFS has been shown to decrease in correlation with increasing cognitive impairment in patients with Alzheimer's disease (Koenig et al. 2005) and schizophrenia (Koenig et al. 2001a).
4. Time-domain methods
The large and important frontal areas of the human brain are not associated with the representation of specific sensory inputs or motor outputs. These regions form circuits with other brain regions that do contain specific internal representations of perceptions (association cortex) and actions (premotor regions). Those circuits allow the human brain to make complex reflections and abstractions of the outer world without the corresponding external input. This ongoing, self-driven brain information processing is likely to occur at the same time-scale as the processing of external stimuli (i.e. on a subsecond time-scale). It is likely to be informative about key issues such as cognitive development and mental disorders. For the understanding of spontaneous brain activity and the underlying mental processes, it is thus necessary to obtain measurements and apply methods that provide a similar subsecond time resolution. This time resolution is initially available in EEG, but the traditional FFT-based EEG analysis strategies are ‘timeless’ and have offered little to exploit this.
In order to overcome this shortcoming, it is thus necessary to identify spontaneously occurring changes of short-lasting brain states in the EEG. The rationale upon which this can be achieved is a simple one. Since changes of the configuration of the EEG scalp field must be caused by changes of the active intracerebral sources, changes of the field assumingly also indicate changes of the functional state. By using a time-varying, global index of momentary field configuration change, one can thus attempt to assess changes of functional brain state. It has been observed that the measured field configurations remain stable for time periods of on average, approximately 100 ms, before they rapidly change into another, often very different, configuration (Lehmann et al. 1987), which is somewhat counterintuitive to our subjective experience of continuous stream of thought (William James). These periods of stable field configuration have been called microstates and were hypothesized to constitute basic building blocks of human information processing, or ‘atoms of thought’ (Lehmann 1990). EEG microstate topography has been shown to vary with different cognitive modes (Lehmann et al. 1998; Muller et al. 2005), maturational states (Koenig et al. 2002b) and abnormal conditions including dementia (Dierks et al. 1997; Strik et al. 1997) or schizophrenia (Koenig et al. 1999; Lehmann et al. 2005).
The interpretation of the observed microstates in terms of connectivity is similar to the one given for the frequency-domain EEG. Since a microstate is defined by a single, stable topography, it may only have been generated either by a single source (which is unlikely for a normally active brain), or by a set of sources that operate synchronously. Any other type of activity would have induced continuous changes in the EEG topography. The observation of microstates thus suggests the existence of subsecond global functional networks in the brain, established through a synchronized firing of the neural elements constituting the network. Apart from the apparently discontinuous transitions between microstates, the observed microstate configurations seem to concentrate in a limited number of prototypical maps and can be efficiently classified (Wackermann et al. 1993; Pascual-Marqui et al. 1995, see equation (4.1) and figure 3). Cluster analysis of microstate topographies has typically shown that in resting, eyes closed EEG, four clusters of microstate topography are sufficient to explain more than 80% of the total variance over time periods of minutes, and reproduce well, even across large populations (Koenig et al. 2002b). Thus, there seems to be a limited subset of microstate topographies that predominate in normal EEGs. Therefore, the predominance of a few microstate topographies suggests that there are also preferred patterns of neural coactivation (i.e. a non-random covariance matrix of the activity of different brain regions). Since the typical microstate topographies reproduce well across subjects, we assume that they are mediated by a genetically predetermined, long-term structural connectivity of the brain that selectively facilitates the coactivation of specific brain regions.
| (4.1) |
with the constraint that the signal time-courses have to have pairwise disjoint supports: For all k,l=1,…,n we have , if k≠1; a1,…,an, n columns of D; sp(ak), support of the signal time-course ak, defined as the sample points in time. Where ak does not vanish, let ak=(a1k,…,amK), then
Figure 3.
The tracings show a sample 2 s, eyes closed, resting EEG displayed against average reference. In the lower part, the assignment of the same EEG to four microstate class topographies is shown. The black areas indicate the assignment of the momentary EEG topography to the microstate topography shown on the right. The height of the area indicates the amplitude of the momentary EEG field (global field power). Continuous time periods assigned to a single topography can clearly be seen.
Finally, the observed transition probabilities between the different microstate topographies clearly deviate from what one would expect from a uniformly random process (Wackermann et al 1993; Lehmann et al. 2005, in press). This suggests that there are not only connectivity structures that facilitate the coactivation of brain regions within a microstate, but that there is another sequential connectivity where one type of brain state or mental operation facilitates the appearance of a later, different brain state with assumingly different functions.
5. Time–frequency methods
Frequency-domain EEG data is based on stationary sinusoidal functions that provide maximal frequency resolution, but no information about time. Microstate analysis is based on delta functions that provide maximal information in time, but no frequency information. The above sections on frequency- and time-domain EEGs have shown that both frequency and time are important dimensions for the study of brain connectivity, and that it is probably useful to study them simultaneously, while maintaining the topographical aspects.
In order to obtain simultaneous, quantitative information about time, frequency and topography of EEG data, we have developed a time–frequency decomposition procedure that is based on a large set of spatially distributed Gabor-functions, that is, on transient oscillations with a determined frequency and time window (Koenig et al. 2001b). To be compatible with physically valid source models, each of the possible Gabor-functions appears simultaneously at all electrodes, with different weighting factors (equation (5.1)). Again, the weighting factors thus constitute a topography, observed during a known time and frequency window.
| (5.1) |
where D is a complete dictionary of Gabor-functions,
g, time window function; V, q×r matrix, that is a generalization of the matrix V introduced in equation (2.2), where the number of topographies were bound to the number of dictionary words n. Now, an arbitrary number of topographies q can be selected; Kd, diagonal matrix diag(K1,…,Kn), where a diagonal element Kk normalizes the discrete norm of the kth signal time-course in the dictionary D; T, n×m matrix with columns t11n,…,tm1n; U, m×n matrix of real translations u1,…,um with columns u11m,…,un1m; S, diagonal matrix diag(s1,…,sn) of positive scales s1,…,sn; C, n×q matrix of coefficients establishing a relation between D and V, where in each row of C, all but one value is zero; ⋅, pointwise matrix multiplication.
Similar to what has been observed in time-domain microstates, the entire set of topographies that results from applying large sets of Gabor-functions to normal EEG data can be efficiently accounted for by a small set of prototypical topographies. When the time–frequency decomposition of an EEG is mapped onto these prototypical topographies, separate and transient oscillatory events with stable field topography appear (see figure 4).
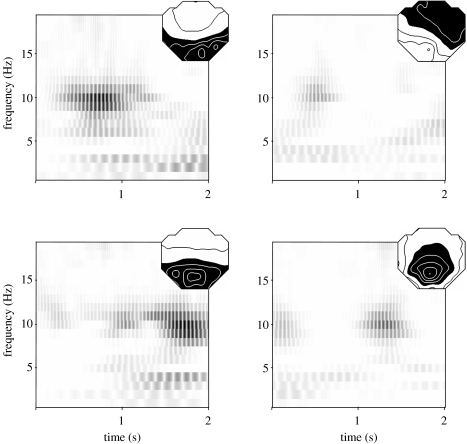
Figure 4.
Time frequency decomposition of the EEG shown in figure 3. The four panels show the time frequency planes (Wigner plots) for the optimal four classes of topography obtained. The vertical axis indicates frequency and the horizontal axis indicates time. The energy at each time frequency point is indicated by the darkness. The corresponding topographies are shown as isocontour potential maps. The decomposition explained 83.7% of the total variance of the EEG.
The interpretation of this observation in terms of connectivity is again similar to those for microstates and the frequency-transformed EEG findings. Assuming that the observed activity is unlikely to result from a single source, and knowing that a stable topography cannot account for time-lags in the activity of different sources, we conclude that the transient oscillatory events with a stable topography result from the transient binding of neural generators through synchronous oscillations. In a recent study (Koenig et al. 2004), we have investigated the haemodynamic correlates of such transient and spatially stable oscillations. A spontaneous 96 channel EEG was recorded in an MR scanner during the acquisition of fMRI-BOLD images sensitive to haemodynamic changes. After an extensive artefact removal procedure, the EEG data was parsed into 2 s epochs and the topographic time–frequency decomposition was applied to all of these epochs. Taking the haemodynamic response function of the fMRI into account, the spontaneous occurrence of such spatially stable, synchronized and transient oscillations with a single topography was used as predictor of the fMRI-BOLD signals using a general linear model. In agreement with the hypothesis that the brain regions that generate these synchronized oscillations represent an extended network, we found activations of several separate brain regions (figure 5). Thus, the most likely explanation for these activations is that the activity of these regions was locked in time and frequency (Koenig et al. 2004).
Figure 5.
Spectral power of three prototype topographies over a 6 min recording with corresponding fMRI-BOLD correlates. In each column of the figure, the upper part shows the spectral power (horizontal axis: time; vertical axis: frequency). On the right of each spectrogram is a plot of the mean intensity over time (i.e. the average spectrum of the signal). The topographies are also shown. The lower parts show the sites where there were changes of fMRI-BOLD activity that correlated significantly and positively with the fluctuations of each topography in the lower alpha band (8.5–10.5 Hz, grey area on spectrogram). (Coordinates in BrainVoyager system coordinates.)
6. Conclusions
We have shown several ways to decompose an EEG into single topographic and time–frequency elements. These methods optimize properties of the decomposition that are not driven by statistical criteria, but derived from empirical observations in the EEG data. Across the presented methods, the criteria employed here can be resumed as criteria of sparseness of topographies. In the frequency analysis, a single topography was used to account for the activity at a single frequency. In the microstate analysis, the EEG was accounted for by a small set of topographies that were alternatively present across time. In the time–frequency analysis, there were a small set of topographies that efficiently accounted for the EEG oscillations across time and frequency.
Suggesting sparseness of topographies as a suitable criterion for the decomposition of brain electric scalp data implies that there must be some kind of sparseness in the generating brain activity. Theoretically, this sparseness may be present in space. However, this would suggest that most of the time, only a single, focal region of the cortex is active; an assumption that is not plausible for higher-order cognitive processes in a normally functioning brain (Mesulam 1990). The other possibility where sparseness may occur in the brain is in the time-course of the activity of brain regions, which would indicate that several brain regions display the same activation pattern for a certain time. These common activation patterns may constitute a mechanism to establish a transient connectivity between different brain regions and cognitive domains. If the capacities of each of these different brain regions are sufficient to achieve large parts of the cognitive demands by themselves, the common, synchronized processing of information may constitute a kind of ‘cognitive four-wheel-drive’ that explains the robustness of cognition.
The limitations of the conclusions drawn here are framed by the limitations of EEG data. First, we were arguing on a global level, considering the entire brain as single research object. Scalp EEG has a low spatial resolution so EEG data is not well suited to extend the study of connectivity to a scale below centimetres. Intracranial recordings provide a better spatial resolution but usually do not cover the entire brain, and are only available in patients with a neurological problem (in humans). fMRI also has a good spatial resolution but neural activity is visible only through a slow haemodynamic response, which introduces blurring in time. Furthermore, coactivation patterns found in fMRI might be confounded by the dynamics and regulation of blood-flow in the brain itself, without relation to neural activity.
Second, there are parts of the brain that do not produce a detectable EEG change when active. These regions may nevertheless play an important role in organizing brain activity. This applies namely to the thalamus, that itself is silent in EEG. The thalamus is tightly connected to the neocortex, where most of the EEG signals are assumed to originate. Thalamo-cortical networks have been suggested as mechanisms for some of the most prominent EEG oscillations (Lopes da Silva 1991). Combined EEG and fMRI studies have indeed shown that the power of the EEG alpha rhythm correlates with thalamic activation measured using the fMRI (Goldman et al. 2002; Martinez-Montes et al. 2004). This indicates that structures that do not do produce an EEG signal itself are functionally involved in the generation of synchronized EEG oscillations. Using EEG alone, a complete picture of functional brain connectivity is thus unlikely to emerge. Finally, the material presented here is mostly based on EEG data recorded in healthy resting subjects. The question remains whether other mental states, such as different sleep states, arousal, drug-induced states or neuropsychiatric disorders, change the functional connectivity of the brain.
Acknowledgments
This study has been supported by a travel fellowship for L. Melie-García from the Swiss National Foundation.
Footnotes
One contribution of 21 to a Theme Issue ‘Multimodal neuroimaging of brain connectivity’.
References
- Achermann P, Borbely A.A. Mathematical models of sleep regulation. Front Biosci. 2003;8(Suppl.):S683–S693. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- Alegre M, Labarga A, Gurtubay I.G, Iriarte J, Malanda A, Artieda J. Movement-related changes in cortical oscillatory activity in ballistic, sustained and negative movements. Exp. Brain Res. 2003;148:17–25. doi: 10.1007/s00221-002-1255-x. [DOI] [PubMed] [Google Scholar]
- Braeutigam S, Bailey A.J, Swithenby S.J. Phase-locked gamma band responses to semantic violation stimuli. Brain Res. Cogn. Brain Res. 2001;10:365–377. doi: 10.1016/s0926-6410(00)00055-0. [DOI] [PubMed] [Google Scholar]
- Dierks T, Jelic V, Julin P, Maurer K, Wahlund L.O, Almkvist O, Strik W.K, Winblad B. EEG-microstates in mild memory impairment and Alzheimer's disease: possible association with disturbed information processing. J. Neural Transm. 1997;104:483–495. doi: 10.1007/BF01277666. [DOI] [PubMed] [Google Scholar]
- Dumermuth G, Molinari L. Spectral analysis of the EEG. Some fundamentals revisited and some open problems. Neuropsychobiology. 1987;17:85–99. doi: 10.1159/000118345. [DOI] [PubMed] [Google Scholar]
- Durka P.J, Zygierewicz J, Klekowicz H, Ginter J, Blinowska K.J. On the statistical significance of event-related EEG desynchronization and synchronization in the time–frequency plane. IEEE Trans. Biomed. Eng. 2004;51:1167–1175. doi: 10.1109/TBME.2004.827341. [DOI] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck H.J. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol. Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Fernandez T, et al. Specific EEG frequencies at specific brain areas and performance. NeuroReport. 2000;11:2663–2668. doi: 10.1097/00001756-200008210-00012. [DOI] [PubMed] [Google Scholar]
- Franaszczuk P.J, Bergey G.K, Durka P.J, Eisenberg H.M. Time–frequency analysis using the matching pursuit algorithm applied to seizures originating from the mesial temporal lobe. Electroencephalogr. Clin. Neurophysiol. 1998;106:513–521. doi: 10.1016/s0013-4694(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Friston K.J, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Goldman R.I, Stern J.M, Engel J, Jr, Cohen M.S. Simultaneous EEG and fMRI of the alpha rhythm. NeuroReport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C.M, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl Acad. Sci. USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtubay I.G, Alegre M, Labarga A, Malanda A, Iriarte J, Artieda J. Gamma band activity in an auditory oddball paradigm studied with the wavelet transform. Clin. Neurophysiol. 2001;112:1219–1228. doi: 10.1016/s1388-2457(01)00557-0. [DOI] [PubMed] [Google Scholar]
- Itil T.M, Itil K.Z. Quantitative EEG brain mapping in psychotropic drug development, drug treatment selection, and monitoring. Am. J. Ther. 1995;2:359–367. doi: 10.1097/00045391-199505000-00013. [DOI] [PubMed] [Google Scholar]
- John E.R, et al. Neurometrics. Science. 1977;196:1393–1410. doi: 10.1126/science.867036. [DOI] [PubMed] [Google Scholar]
- John E.R, Ahn H, Prichep L, Trepetin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980;210:1255–1258. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- John E.R, Easton P, Prichep L.S, Friedman J. Standardized varimax descriptors of event related potentials: basic considerations. Brain Topogr. 1993;6:143–162. doi: 10.1007/BF01191080. [DOI] [PubMed] [Google Scholar]
- John E.R, Prichep L.S, Alper K.R, Mas F.G, Cancro R, Easton P, Sverdlov L. Quantitative electrophysiological characteristics and subtyping of schizophrenia. Biol. Psychiatry. 1994;36:801–826. doi: 10.1016/0006-3223(94)90592-4. [DOI] [PubMed] [Google Scholar]
- Koenig T, Lehmann D, Merlo M.C, Kochi K, Hell D, Koukkou M. A deviant EEG brain microstate in acute, neuroleptic-naive schizophrenics at rest. Eur. Arch. Psychiatry Clin. Neurosci. 1999;249:205–211. doi: 10.1007/s004060050088. [DOI] [PubMed] [Google Scholar]
- Koenig T, Lehmann D, Saito N, Kuginuki T, Kinoshita T, Koukkou M. Decreased functional connectivity of EEG theta-frequency activity in first-episode, neuroleptic-naive patients with schizophrenia: preliminary results. Schizophr. Res. 2001;50:55–60. doi: 10.1016/s0920-9964(00)00154-7. [DOI] [PubMed] [Google Scholar]
- Koenig T, Marti-Lopez F, Valdes-Sosa P. Topographic time–frequency decomposition of the EEG. NeuroImage. 2001;14:383–390. doi: 10.1006/nimg.2001.0825. [DOI] [PubMed] [Google Scholar]
- Koenig T, Hubl D, Muller T. Decomposing the EEG in time, space and frequency: a formal model, existing methods, and new proposals. In: Hirata K, editor. International Congress Series. Elsevier; Amsterdam: 2002. pp. 317–321. [Google Scholar]
- Koenig T, Prichep L, Lehmann D, Sosa P.V, Braeker E, Kleinlogel H, Isenhart R, John E.R. Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. NeuroImage. 2002;16:41–48. doi: 10.1006/nimg.2002.1070. [DOI] [PubMed] [Google Scholar]
- Koenig, T., Melie-Garcia, L., Hubl, D, Valdes-Sosa, P., and Dierks, T. 2004 Multichannel time frequency analysis of spontaneous EEG and fMRI correlates. In Proceedings of the Mediterranean Conference on Medical and Biological Engineering 2004 (Medicon)
- Koenig T, Prichep L, Dierks T, Hubl D, Wahlund L, John E.R, Jelic V. Decreased EEG synchronization in Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2005;26:165–171. doi: 10.1016/j.neurobiolaging.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Lehmann D. Brain electric microstates and cognition: the atoms of thought. In: John E.R, editor. Machinery of the mind. Birkhäuser; Boston: 1990. pp. 209–224. [Google Scholar]
- Lehmann D, Michel C.M. Intracerebral dipole source localization for FFT power maps. Electroencephalogr. Clin. Neurophysiol. 1990;76:271–276. doi: 10.1016/0013-4694(90)90022-c. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Ozaki H, Pal I. EEG alpha map series: brain micro-states by space-oriented adaptive segmentation. Electroencephalogr. Clin. Neurophysiol. 1987;67:271–288. doi: 10.1016/0013-4694(87)90025-3. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Strik W.K, Henggeler B, Koenig T, Koukkou M. Brain electric microstates and momentary conscious mind states as building blocks of spontaneous thinking: I. Visual imagery and abstract thoughts. Int. J. Psychophysiol. 1998;29:1–11. doi: 10.1016/s0167-8760(97)00098-6. [DOI] [PubMed] [Google Scholar]
- Lehmann, D. et al 2005 EEG microstate duration and syntax in acute, medication-naïve, first-episode schizophrenia: a multi-center study. Psychiatry Res.138, 141–156. [DOI] [PubMed]
- Lehmann, D., Faber, P. L., Gianotti, L. R., Kochi, K., Pascual-Marqui, R. D. In press b EEG coherency computed from scalp data and from LORETA model sources. Brain Topogr.
- Livingstone M.S. Oscillatory firing and interneuronal correlations in squirrel monkey striate cortex. J. Neurophysiol. 1996;75:2467–2485. doi: 10.1152/jn.1996.75.6.2467. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F. Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalogr. Clin. Neurophysiol. 1991;79:81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- Makeig S, Jung T.P, Bell A.J, Ghahremani D, Sejnowski T.J. Blind separation of auditory event-related brain responses into independent components. Proc. Natl Acad. Sci. USA. 1997;94:10 979–10 984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Montes E, Valdes-Sosa P.A, Miwakeichi F, Goldman R.I, Cohen M.S. Concurrent EEG/fMRI analysis by multiway partial least squares. NeuroImage. 2004;22:1023–1034. doi: 10.1016/j.neuroimage.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann. Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Muller T.J, Koenig T, Wackermann J, Kalus P, Fallgatter A, Strik W, Lehmann D. Subsecond changes of global brain state in illusory multistable motion perception. J. Neural Transm. 2005;112:565–576. doi: 10.1007/s00702-004-0194-z. [DOI] [PubMed] [Google Scholar]
- Nunez P.L, Srinivasan R, Westdorp A.F, Wijesinghe R.S, Tucker D.M, Silberstein R.B, Cadusch P.J. EEG coherency. I: statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr. Clin. Neurophysiol. 1997;103:499–515. doi: 10.1016/s0013-4694(97)00066-7. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R.D, Michel C.M, Lehmann D. Segmentation of brain electrical activity into microstates: model estimation and validation. IEEE Trans. Biomed. Eng. 1995;42:658–665. doi: 10.1109/10.391164. [DOI] [PubMed] [Google Scholar]
- Prichep L.S, John E.R, Ferris S.H, Reisberg B, Almas M, Alper K, Cancro R. Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol. Aging. 1994;15:85–90. doi: 10.1016/0197-4580(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Rappelsberger P, Petsche H. Probability mapping: power and coherence analyses of cognitive processes. Brain Topogr. 1988;1:46–54. doi: 10.1007/BF01129339. [DOI] [PubMed] [Google Scholar]
- Saletu B, Anderer P, Kinsperger K, Grunberger J. Topographic brain mapping of EEG in neuropsychopharmacology. Part II. Clinical applications (pharmaco EEG imaging) Methods Find. Exp. Clin. Pharmacol. 1987;9:385–408. [PubMed] [Google Scholar]
- Singer W, Gray C.M. Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Strik W.K, Chiaramonti R, Muscas G.C, Paganini M, Mueller T.J, Fallgatter A.J, Versari A, Zappoli R. Decreased EEG microstate duration and anteriorisation of the brain electrical fields in mild and moderate dementia of the Alzheimer type. Psychiatry Res. 1997;75:183–191. doi: 10.1016/s0925-4927(97)00054-1. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Lehmann D, Michel C.M, Strik W.K. Adaptive segmentation of spontaneous EEG map series into spatially defined microstates. Int. J. Psychophysiol. 1993;14:269–283. doi: 10.1016/0167-8760(93)90041-m. [DOI] [PubMed] [Google Scholar]
- Zygierewicz J, Blinowska K.J, Durka P.J, Szelenberger W, Niemcewicz S, Androsiuk W. High resolution study of sleep spindles. Clin. Neurophysiol. 1999;110:2136–2147. doi: 10.1016/s1388-2457(99)00175-3. [DOI] [PubMed] [Google Scholar]