Abstract
Using contact-dependent three-dimensional coculture systems and serum-free conditions, we compared the ability of estrogen receptor (ER)-α+ tamoxifen-sensitive premalignant (EIII8) or tumorigenic (MCF-7), ER-α+ tamoxifen-resistant (EIII8-TAMR) or ER-α− MDA-MB-231 breast cancer cells to interact and undergo epithelial morphogenesis on association with breast tumor-derived fibroblasts. Although all breast cancer cell lines interacted with tumor fibroblasts, EIII8 and its intrinsically tamoxifen-resistant counterpart EIII8-TAMR cells were most receptive and responded with dramatic, albeit, aberrant epithelial morphogenesis. EIII8 cells underwent epithelial morphogenesis when cocultured with fibroblasts from ER-α−/PgR− or ER-α+/PgR+ breast tumors; however, EIII8 cells cocultured with ER-α−/PgR− tumor-derived fibroblasts exhibited decreased tamoxifen sensitivity compared with cells cocultured with ER-α+/PgR+ tumor fibroblasts. Fibroblast-induced tamoxifen resistance was accompanied by mitogen-activated protein kinase and Akt hyperactivation, reduced sensitivity to U0126 or LY294002, and ER-α hyperphosphorylation in the activation function-1 domain. The intrinsic tamoxifen resistance of EIII8-TamR cells correlated with constitutive ER-α hyperphosphorylation that was unaffected by the tumor fibroblasts. Our results suggest that tumor fibroblast-induced tamoxifen resistance of EIII8 cells is not mediated by epidermal growth factor receptor or insulin-like growth factor (IGF)-1R axes because no correlation was found between expression levels of IGF-1, IGF-2, phosphorylated IGF-1R, or epidermal growth factor receptor, and tamoxifen sensitivity of EIII8 fibroblast cultures.
The estrogen receptor (ER) plays a prominent role in the control of breast epithelial cell proliferation, and expression of ER is used to identify patients who might benefit from anti-estrogen therapy. Transcription activation of estrogen responsive genes is mediated via two functional regions called activation function-1 (AF-1) and activation function-2 (AF-2) that modulate binding of ER to estrogen responsive elements (EREs). ER can also modulate the activities of other transcription factors, such as activator protein-1 (AP-1) or SP-1, by stabilizing their DNA binding. Tamoxifen inhibits AF-2, but not AF-1.1 Although most ER-α+ breast cancers initially respond to tamoxifen therapy, they eventually acquire resistance to tamoxifen,2 and a proportion of tumors despite being ER-α+ exhibit de novo resistance to tamoxifen.3 Posttranslational modification of ER such as by phosphorylation regulates ER activity. Serine residues at 105, 118, and 167 represent major phosphorylation sites in the AF-1 domain and are activated in vivo in a ligand-dependent and -independent manner by cyclin A/cdk2, mitogen-activated protein (MAP) kinase, or Akt, respectively.4,5 There is increasing evidence that growth of ER-α+ breast tumors and their transition to a hormone-independent state is influenced by activation of a growth factor signaling cascade, which in turn can induce ER-α activity through effects on ER phosphorylation.6 Overexpression of MAP kinase (MAPK) and Akt has been associated with tamoxifen resistance in breast cancer cell lines and tumors.7,8,9,10 The consensus Akt phosphorylation site RXRXXS/T is present in ER-α and not in ER-β,11 suggesting the possibility that Akt-induced changes in ER phosphorylation and activity are mediated through ER-α.
The human epidermal growth factor (EGF) receptor family and the type I insulin-like growth factor receptor (IGF-1R) receptor have been shown to regulate proliferation of breast cancer cells12 and phosphorylate ER in the AF-1 domain via activation of downstream kinases such as MAPK and Akt,13 thus playing an active role in the progression of ER-α+ breast cancer to a hormone-independent state. However, the majority of these studies have been performed in homotypic epithelial monolayers or two-dimensional cultures that rely entirely on autocrine or paracrine activation pathways originating from the breast cancer cells, a situation not entirely relevant in the physiological setting. Reciprocal cellular interactions between epithelial cells and fibroblasts play a key role in the morphogenesis, proliferation, and cytodifferentiation of both endocrine and nonendocrine target organs.14,15 The stroma provides vascular supply and specific soluble and extracellular matrix molecules that are required for tumor growth and progression. Several lines of evidence indicate that stromal cells play a central role via extracellular matrix remodeling in tumor invasion and dissemination.16,17,18 However, the direct impact of fibroblasts, a major stromal component, and their ensuing effects on paracrine/autocrine activation mechanisms, ER-α phosphorylation, and ER activity have not been examined.
Using a physiologically relevant three-dimensional model system that permits establishment of reciprocal epithelial-fibroblast interactions resembling those in vivo, we have previously demonstrated that breast tumor fibroblasts play a dominant role in growth and aberrant morphogenesis of EIII8 premalignant breast epithelial cells.19 Here, we demonstrate that breast tumor fibroblasts establish similar heterotypic interactions with premalignant (EIII8), tumorigenic (MCF-7), or metastatic (MDA-MB-231) breast cancer cells but induce dramatic epithelial morphogenesis, albeit aberrant, only in EIII8 or EIII8-TAMR (the tamoxifen-resistant counterpart of EIII8 cells) cultures. Our analysis also shows that although ER-α+ tamoxifen-sensitive EIII8 cells responded similarly with respect to discernible epithelial morphogenesis in contact-dependent cocultures to fibroblast subsets derived from ER-α−/PgR− versus ER-α+/PgR+ breast tumors, EIII8 cells cocultured with fibroblasts derived from ER-α−/PgR− tumors display decreased sensitivity to tamoxifen compared with those cocultured with ER-α+/PgR+ tumor-derived fibroblasts that maintained their tamoxifen sensitivity. This fibroblast-induced acquisition of tamoxifen resistance of EIII8 cells is accompanied by decreased sensitivity to inhibition by MEK1/2 and phosphoinositide kinase-3 (PI3K)/Akt inhibitors, U0126 or LY294002, respectively, and hyperphosphorylation of ER-α+ in the AF-1 domain. Similar analysis of EIII8-TAMR cells shows that their intrinsic tamoxifen resistance correlates with constitutive ER-α hyperphosphorylation that is unaffected by the tumor fibroblasts. Interestingly, EIII8-TAMR cells cultured with ER-α−/PgR− tumor fibroblasts also exhibited diminished sensitivity to U0126 and LY294002 compared with those cultured with fibroblasts from ER-α+/PgR+ tumor fibroblasts. Our results also suggest that acquisition of fibroblast-induced tamoxifen resistance of EIII8 cells is mediated by growth factor signaling pathways other than those activated via the EGFR or IGF-1R axes.
Materials and Methods
Cell Lines and Primary Cultures
The MCF10AT system is a xenograft model of progressive human proliferative breast disease in which the progression of a T24-Ha-ras-transformed derivative of MCF10A,10 namely, MCF10AneoT,20 can be followed in immunodeficient mice from a histologically precancerous stage to development of frank invasive carcinoma.21,22 The present studies used MCF10AT1-EIII8 and MCF10AT1-EIII8-TAMR cells, referred to as EIII8 or EIII8-TAMR, respectively. EIII8 cells are premalignant epithelial cells that were derived from MCF10AT1 xenografts arising in E2-supplemented animals23 and respond to E2 with increased growth.24 EIII8-TAMR cells are the tamoxifen-resistant counterpart of EIII8 cells and were derived from MCF10AT1 xenografts arising in animals that received tamoxifen supplementation.25 EIII8 and EIII8-TAMR cells were maintained in phenol red-free Dulbecco’s modified Eagle’s medium (DMEM)-F12 medium supplemented with 0.1 μg/ml cholera toxin, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, 0.02 μg/ml EGF, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2.5% horse serum. MCF-7 and MDA-MB-231 breast cancer cells were maintained in phenol red-free DMEM/F-12 supplemented with insulin (10 μg/ml) and 5% fetal calf serum, or DMEM/F-12/containing 5% fetal bovine serum, respectively. Charcoal-stripped serum was not used because it reduced both the viability and proliferative capacity of the cells. The only sera used routinely were those that were unable to support the growth of the estrogen-dependent cell line MCF-7, indicating the absence of biologically significant levels of E2 or other estrogenic compounds.
Breast tumor tissues were acquired after protocol review and approval by the Wayne State University Human Investigation Committee (protocol no. 0409000436). Primary cultures of human breast fibroblasts were established from tumors that were >90% positive for ER-α and progesterone receptor (PgR) or negative for ER-α and PgR. Fibroblasts were isolated and characterized as previously described.19 Fibroblasts were cultured in DMEM/F12 supplemented with 10% fetal calf serum routinely up to 10 to 12 passages and were used at passages 4 to 6.
Heterotypic Three-Dimensional Coculture of Epithelial Cells and Fibroblasts
EIII8, MCF-7, or MDA-MB-231 cells (50 × 103) were mixed with an equivalent number of human breast fibroblasts (ER-α−/PgR− or ER-α+/PgR+ tumor-derived) and seeded onto chamber slides coated with growth factor-reduced and phenol red-free Matrigel.19 Cultures were performed in serum-free basal growth medium (SFM; Life Technologies, Inc., Grand Island, NY) supplemented with 10 ng/ml EGF, 20 ng/ml basic fibroblast growth factor, and 10 μg/ml fibronectin. Cocultures were maintained up to 2 weeks, and morphological development was analyzed by phase-contrast microscopy.
Quantitation of Effects of ER-α−/PgR− or ER-α+/PgR+ Tumor-Derived Fibroblasts on Three-Dimensional Growth and Hormonal Responsiveness of Breast Cancer Cells
Three-dimensional cultures were set up by mixing 50 × 103 EIII8, EIII8-TAMR, MCF-7, or MDA-MB-231 cells with an equivalent number of 21T or 38T (ER-α+/PgR+ tumor-derived), or 16T or 17T (ER-α−/PgR− tumor-derived) breast fibroblasts and seeded onto chamber slides coated with Matrigel as described above. Cultures were incubated at 37°C for 6 days, after which cell viability was measured in the three-dimensional cultures. The medium was removed, wells were rinsed with phosphate-buffered saline, and the Matrigel was digested with dispase for 2 hours at 37°C. The digested material was centrifuged at 4000 × g for 10 minutes, and the pellet was treated with trypsin to recover single cells from the three-dimensional structures. The number of viable cells was determined by trypan blue exclusion24 and results expressed as average ± SE from three independent experiments. The effects of 17-β-estradiol (E2; Sigma Chemical Co., St. Louis, MO) and 4-hydroxy tamoxifen (4-OHT; Sigma) on three-dimensional growth of heterotypic cultures were tested as described above except that after seeding of cells, slides were incubated overnight to allow cells to attach to the surface and treated with vehicle [0.01% ethanol (v/v)], 10 nmol/L E2, or a combination of 10 nmol/L E2 and a 100-fold molar excess of 4-OHT.
Morphological Evaluation of Three-Dimensional Cultures
Morphologies of three-dimensional cocultures of EIII8 or EIII8-TamR with 16T or 21T fibroblasts treated with E2 or E2 plus 4-OHT compared with vehicle-treated cultures were analyzed by phase-contrast microscopy. For histological evaluation, the three-dimensional cocultures were fixed in buffered-formalin and embedded in paraffin, and 4-μm sections were stained with hematoxylin and eosin. For immunohistochemical evaluation, sections were incubated with anti-IGF-1 or IGF-2 polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) or with anti-IGF-1 receptor α subunit monoclonal antibody (Calbiochem, La Jolla, CA). The proliferative status of homotypic fibroblast and heterotypic EIII8 fibroblast cultures were assessed by staining with anti-proliferating cell nuclear antigen (PCNA) antibody (DAKO, Carpinteria, CA), and the potential presence of contaminating fibroblast-like cells, ie, cancer cells that have undergone epithelial-mesenchymal transition (EMT), in our fibroblast preparations were assessed in homotypic fibroblast cultures with anti-Snail1 antibody (Abcam Inc., Cambridge, MA). In each case, negative controls were overlaid with the appropriate mouse or rabbit IgG isotype. The slides were overlaid with avidin-biotin-conjugated goat anti-mouse or anti-rabbit IgG and developed with Vectastain ABC kit (Vector Laboratories, Burlingame, CA).
Western Blot Analysis
Heterotypic three-dimensional cocultures of EIII8 or EIII8-TamR with 16T or 21T fibroblasts, or the corresponding homotypic cultures were established on Matrigel-coated chamber slides in SFM. On day 4, three-dimensional cultures were rinsed, replaced with SFM supplemented with 1 nmol/L E2, 1 nmol/L E2 plus 100 nmol/L 4-OHT, 1 nmol/L E2 plus 100 nmol/L ICI 182,780, or vehicle. Cultures were also treated with 1 μmol/L U0126 or 5 μmol/L LY294002 alone or in the presence of 1 nmol/L E2 or a combination of 1 nmol/L E2 and 100 nmol/L 4-OHT. On the following day, the cultures were rinsed, and Matrigel was digested with dispase. The pellets recovered after centrifugation were lysed in lysis buffer [100 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1% Triton X-100, 1 mmol/L phenylmethyl sulfonyl fluoride, 50 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, 1 mmol/L sodium pyrophosphate, and complete Protease inhibitor cocktail (Roche Diagnostics Corp., Indianapolis, IN)]. Aliquots of precleared samples containing 20 μg of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis with appropriate antibodies. To determine the phosphorylation status of IGF-1R, cell lysates precleared with protein A/G agarose (Oncogene Science, Cambridge, MA) were incubated with anti-IGF-1Rα monoclonal antibody and pelleted with protein A/G agarose. Immune complexes were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with anti-phosphotyrosine antibody. Total IGF-1R levels in immunoprecipitates were detected with anti-IGF-1Rα antibody. Steady-state levels of EGFR phosphorylated at tyrosine 1068 were detected by immunoblotting with anti-phosphoY1068 EGFR antibody, and total EGFR levels with anti-EGFR antibody. The levels of phospho-ERK1/2 relative to total ERK1/2, phosphoSer473-Akt relative to total Akt, phosphoSer118ER-α relative to ER-α, phospho-IGF-1R relative to IGF-1Rα, or phospho-EGFR relative to EGFR were quantitated with NIH Imaging software (Bethesda, MD). Loading of protein was monitored by reprobing stripped membranes with anti-β-actin antibody (Sigma). The following primary antibodies were used: anti-pS2 (Novocastra, Newcastle upon Tyne, UK), anti-IGF-1Rα (Calbiochem), anti-phosphotyrosine (PY20; BD Biosciences, San Diego, CA), anti-ER-α antibody (1D5 clone; DAKO); anti-phosphoSer118-ER-α, anti-phosphoSer473-Akt, anti-Akt, anti-phospho-ERK1/2, anti-ERK1/2, anti-EGFR, and anti-phosphoY1068-EGFR antibodies were purchased from Cell Signaling Technology (Beverly, MA). The blots were developed by using the Enhanced Chemiluminescence Plus kit (GE Healthcare, Bio-Sciences Corp., Piscataway, NJ).
ERE- and AP-1 Transcriptional Activity
EIII8 or EIII8-TamR (100 × 103) cells were transfected in suspension with 1 μg of pSV40-ERE-Luc or pCMV-AP1-Luc, or the corresponding empty control vectors using Metafectine transfection reagent (Biontex Laboratories GmbH, Munich, Germany), and seeded on Matrigel-coated chamber slides. pRL-TK-Renilla luciferase plasmid (1 ng/well) was co-transfected to monitor and control for transfection efficiency variations. On the following day, cultures were treated with 1 nmol/L E2, 1 nmol/L E2 plus a 100-fold molar excess of 4-OHT, 1 μmol/L U0126, 1 μmol/L U0126 plus 1 nmol/L E2, 1 μmol/L U0126 plus 1 nmol/L E2/100 nmol/L 4-OHT, 5 μmol/L LY294002, 5 μmol/L LY plus 1 nmol/L E2, 5 μmol/L LY plus 1 nmol/L E2/100 nmol/L 4-OHT, or vehicle [0.01% (v/v) alcohol or dimethyl sulfoxide]. Forty hours after transfection, firefly and Renilla luciferase activities were determined using the dual-luciferase reporter assay system (Promega, Madison, WI). ERE- or AP-1-mediated luciferase activity in treated and untreated samples was expressed relative to the activities of samples transfected with the corresponding empty control vectors.
Statistical Analysis
All tests of statistical significance were determined using Student’s t-test with P < 0.01 considered as statistically significant.
Results
Heterotypic Three-Dimensional Coculture with Breast Tumor Fibroblasts Reveal Differences in Morphogenetic Response of Breast Cancer Cells to Tumor-Derived Fibroblasts
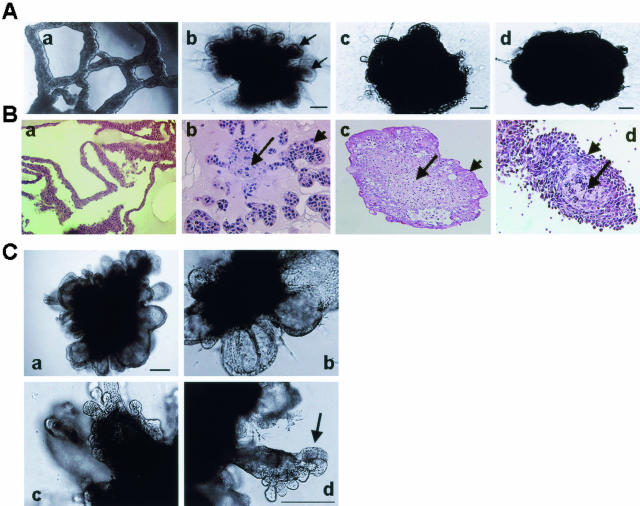
To determine whether breast cancer cells differ in their ability to interact with and/or respond to tumor stroma, we compared the abilities of tumor-derived fibroblasts to evoke morphogenetic effects on premalignant (EIII8), tumorigenic (MCF-7), and metastatic (MDA-MB-231) breast cancer cells. Although in contact-dependent cocultures with tumor-derived fibroblasts, all three breast cancer cell lines produced sealed structures consisting of centrally located fibroblasts with surrounding epithelium, epithelial morphogenesis was most pronounced and dramatic in EIII8 fibroblast cocultures (Figure 1, A and B). The epithelial morphogenetic effects were specifically induced by fibroblasts because a similar culture of homotypic EIII8 cells in Matrigel produces only tubular structures (Figure 1, Aa and Ba).19 In addition, fibroblast-induced epithelial morphogenesis requires intimate cell-cell contact with the epithelial cells because incubation of EIII8 cells in fibroblast culture media failed to evoke epithelial morphogenesis (data not shown). These data suggest that factors synthesized and/or released de novo on heterotypic cell-cell interaction are essential for morphogenetic response. The tumor fibroblast-induced epithelial phenotypic transformation was visible within 2 days of coculture, and mature ductal alveolar structures were conspicuous by day 7 (Figure 1C). These results indicate that although premalignant, tumorigenic, and metastatic breast cancer cells display similar heterotypic interactions with tumor fibroblasts, premalignant breast cancer cells are most receptive and amenable to phenotypic manipulations by the tumor stromal microenvironment.
Figure 1.
A: Phase-contrast morphology of homotypic EIII8 (a), and heterotypic three-dimensional cultures of EIII8 (b), MCF-7 (c), or MDA-MB-231 (d) breast cancer cells with 38T breast tumor fibroblasts at 7 days of culture. Note the presence of well-defined epithelial outgrowths (arrows) in EIII8 stromal cultures (b) as opposed to those formed in MCF-7 or MDA-MB-231 stromal cocultures. B: Morphological analysis by H&E staining of homotypic EIII8 (a), and heterotypic cocultures of EIII8 (b), MCF-7 (c), or MDA-MB-231 (d) cells with 38T fibroblasts at 7 days of culture. Long arrow denotes central fibroblast core and short arrow indicates the interacting epithelium. C: Phase-contrast microscopy of ductal-alveolar morphogenesis in heterotypic three-dimensional cultures of EIII8 cells with 38T fibroblasts. a and b: Cultures at day 2; c: cultures at day 4; d: cultures at day 7. Note the presence of well-defined ductal-alveolar unit at day 7 (arrow in d). Scale bars = 40 μm. Original magnifications: ×10 (Ba, Bc); ×20 (Bb, Bd).
Fibroblasts Derived from ER-α+/PgR+ versus ER-α−/PgR− Breast Tumors Differ in Their Ability to Modulate Tamoxifen Sensitivity
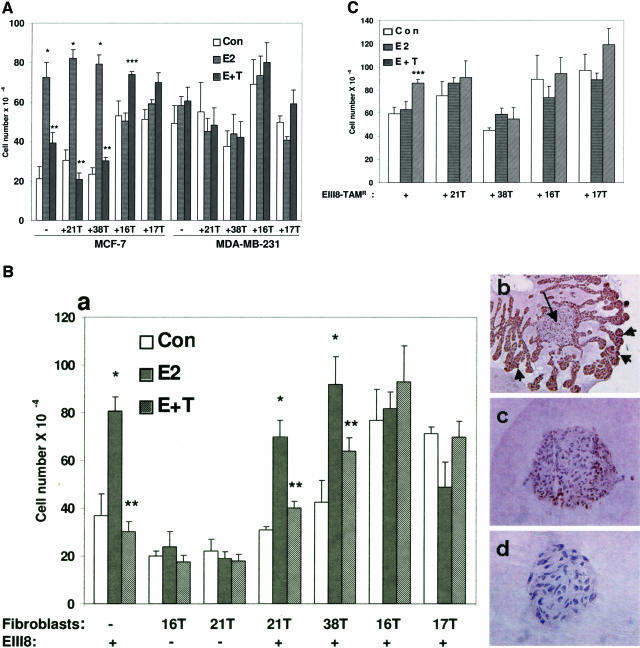
Because the results from Figure 1 revealed dramatic differences in the ability of tumor fibroblasts to induce epithelial morphogenesis of EIII8, MCF-7, or MDA-MB-231 breast cancer cells, we quantitated the effects of tumor fibroblasts on three-dimensional growth of heterotypic cocultures of EIII8, EIII8-TAMR, MCF-7, or MDA-MB-231 breast cancer cells with tumor-derived fibroblasts (16T, 17T, 21T, or 38T), and compared with the corresponding homotypic three-dimensional cultures. We also determined whether fibroblasts derived from ER-α+/PgR+ versus ER-α−/PgR− breast tumors differed in their ability to support/modulate estrogen responsiveness or growth of ER-α+ (EIII8, EIII8-TAMR, MCF-7) or ER-α− (MDA-MB-231) breast cancer cells. Homotypic cultures of EIII8, EIII8-TAMR, MCF-7, or MDA-MB-231 cells or their corresponding heterotypic cocultures with 21T or 38T (ER-α+/PgR+ breast tumor-derived) or with 16T or 17T (ER-α−/PgR− breast tumor-derived) fibroblasts were treated with 10 nmol/L E2 or a combination of 10 nmol/L E2 plus a 100-fold molar excess of 4-OHT, and cell growth was evaluated at 6 days after treatment. Despite variability in cell growth between the individual heterotypic cocultures, MCF-7 or EIII8 cells cocultured with 21T or 38T fibroblasts retained their ability to be growth stimulated or growth inhibited by E2 or 4-OHT, respectively, similar to their corresponding MCF-7 or EIII8 homotypic cultures (Figure 2, A and B). However, similar coculture of MCF-7 or EIII8 cells with 16T or 17T fibroblasts resulted in decreases in tamoxifen-induced growth inhibition (Figure 2, A and B). Although the magnitudes of estrogen-induced growth varied considerably in individual cocultures, three of three fibroblasts derived from ER-α+/PgR+ breast tumors supported tamoxifen-induced growth inhibition of EIII8 or MCF-7 cells. In contrast, placement of otherwise tamoxifen-sensitive EIII8 or MCF-7 cells in intimate contact with fibroblasts derived from ER-α−/PgR− breast tumors impeded their ability to be growth inhibited by tamoxifen in two of three individual fibroblast cultures. A slight increase in tamoxifen-induced growth of heterotypic cultures with 16T or 17T fibroblasts was seen; however, this was statistically significant only in MCF-7–16T cocultures (P < 0.01). It is interesting to note that coculture with ER-α−/PgR− fibroblasts enhanced basal growth and suppressed estrogen-induced growth of both MCF-7 and EIII8 cells (Figure 2, A and B). Regardless of the source of tumor fibroblasts, similar coculture of ER-α+ EIII8-TAMR cells with ER-α+/PgR+ tumor-derived fibroblasts failed to alter the intrinsic tamoxifen resistance of EIII8-TAMR cells (Figure 2C). Likewise, coculture of endocrine nonresponsive metastatic MDA-MB-231 cells with tumor fibroblasts failed to elicit hormone-mediated effects on growth regardless of whether the fibroblasts were derived from ER-α+/PgR+ or ER-α−/PgR− breast tumors (Figure 2A). These data suggest that whereas endocrine responsiveness and tamoxifen sensitivity of ER-α+ breast cancer cells are subject to modulation by the tumor stromal microenvironment, the tumor fibroblasts have little ability to modulate/restore endocrine responsiveness to breast cancer cells that have gone down the path of tamoxifen resistance or endocrine nonresponsiveness (Table 1). The differences in growth of heterotypic cultures do not reflect differences in fibroblast growth because under the three-dimensional culture conditions, <10% of fibroblasts in homotypic (Figure 2, Bc) or heterotypic cocultures (Figure 2, Bb) stained positively for PCNA. In contrast, >95% of the epithelial cells in EIII8- (Figure 2Bb), MCF-7-, or MDA-MB-231 fibroblast cocultures (data not shown) stained positively for PCNA, suggesting that the observed differences in growth is predominantly epithelium-derived. Immunostaining with pan-cytokeratin antibody showed the presence of a small (<5%) cytokeratin-positive fraction in our fibroblast preparations (data not shown). However, these cytokeratin-positive cells remained indolent in both homotypic fibroblast and heterotypic epithelial fibroblast cultures because they lacked the ability to expand or migrate into the surrounding Matrigel. To further rule out the possibility that the fibroblast-induced effects on epithelial cells are not attributable to the presence of contaminating fibroblast-like cells (potentially derived by EMT of cancer cells) in our fibroblast preparations, homotypic fibroblast cultures were immunostained with Snail1 antibody (an EMT marker). No immunoreactivity to Snail1 was detected in the fibroblast cultures (Figure 2, Bd), although intense nuclear reactivity was observed in a positive control cancer cell line (data not shown).
Figure 2.
Regulation of three-dimensional growth of MCF-7, MDA-MB-231 (A), EIII8 (B, a and b), or EIII8-TAMR (C) breast cancer cells by fibroblasts derived from ER-α+/PgR+ (21T, 38T) or ER-α−/PgR− (16T, 17T) breast tumors. B: b and c are representative of typical PCNA-staining patterns observed in heterotypic EIII8-stromal (EIII8–16T shown) and homotypic stromal (16T shown) cultures, respectively. Note the presence of intense PCNA reactivity in >95% of epithelial cells (short arrows) as compared with weak staining (∼10%) in the fibroblast (long arrow). d: Immunostaining of homotypic 16T cultures with Snail1 antibody. Growth was quantitated at 6 days from dispase-treated Matrigel cultures. Control wells received vehicle [0.01% (v/v) ethanol]. Treatments included 10 nmol/L E2 alone or a combination of E2 plus a 100-fold molar excess of 4-OHT. Results obtained from three independent experiments are expressed as mean ± SE. *Significant increase in E2-induced cell growth over corresponding control cultures (P < 0.001); **Significant inhibition of E2-induced cell growth by 4-OHT (P < 0.001); ***4-OHT-mediated increase in cell number as compared with corresponding control or estradiol-treated cultures (P < 0.01). Original magnifications: ×10 (Bb); ×20 (Bc); ×40 (Bd).
Table 1.
Tamoxifen Sensitivity of Breast Cancer Cells in Three-Dimensional Cocultures with Tumor Fibroblast Subsets
| Cells | Homotypic | Heterotypic
|
|
|---|---|---|---|
| ERα+/PgR+ | ERα−/PgR− | ||
| Fibroblasts | NR | 21T, 38T | 16T, 17T |
| EIII8 | S | S | R |
| EIII8-TAMR | R | R | R |
| MCF-7 | S | S | R |
| MDA-MB-231 | NR | NR | NR |
S, sensitive; R, resistant; NR, no response.
Effect of ER-α+/PgR+ Tumor-Derived versus ER-α−/PgR− Tumor-Derived Fibroblasts on Morphology of EIII8 or EIII8-TAMR Premalignant Breast Cancer Cells
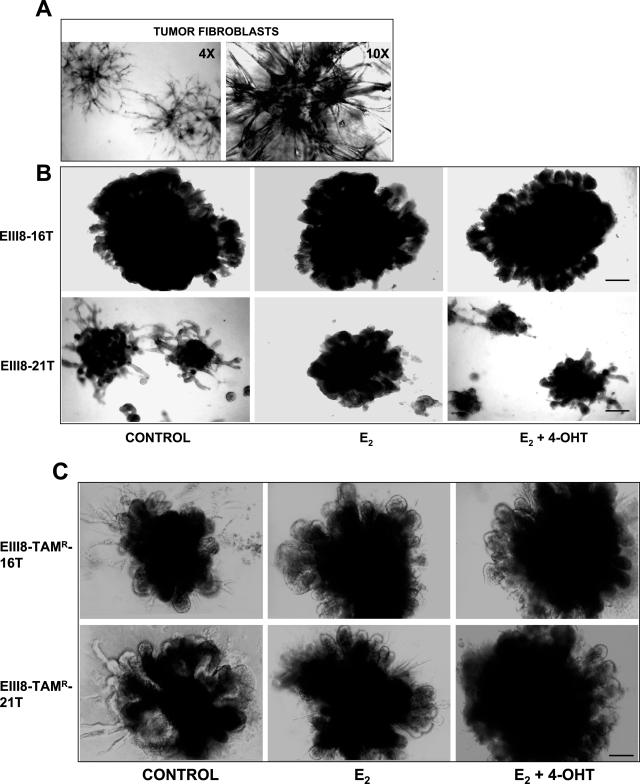
Because the results of Figure 2 showed that tumor fibroblasts exert distinct effects on tamoxifen sensitivity of EIII8 cells but not EIII8-TAMR cells, we examined whether this disparity arose from direct effects on growth or on epithelial morphogenesis of EIII8 or EIII8-TAMR cells. Cocultures of EIII8 or EIII8-TAMR cells with 16T or 21T were established as described above and treated with E2 or a combination of E2 and 4-OHT, or vehicle. Phase-contrast microscopy of cocultures at day 10 showed that EIII8 cells cocultured with 16T fibroblasts showed approximately more than a fivefold increase in overall size of three-dimensional structures as compared with those with 21T fibroblasts (Figure 3B). Consistent with the results of quantitative assessment of three-dimensional growth, EIII8–21T cocultures responded to E2 or 4-OHT with increase or decrease in size, respectively. In contrast, EIII8–16T cocultures were uninfluenced by the presence of E2 or 4-OHT (Figure 3B). The fibroblast-induced differences in three-dimensional growth and hormonal sensitivity of EIII8 cells are not a result of gross changes in epithelial morphogenesis; however, a decrease in the overall density and thickening (hyperplastic growth) of ductal outgrowths were observed in EIII8–21T (or EIII8–38T) cocultures as compared with EIII8–16T (or EIII8–17T) (Figure 3B). Estrogen or tamoxifen treatment does not influence the growth of homotypic fibroblast cultures. The increase or decrease in sizes of the central fibroblast cores observed in estrogen- or tamoxifen-treated cocultures, respectively, seem to correlate with the proliferative status of the epithelium (Figure 3B). It is possible that the fibroblasts are better sustained by a robust and proliferative epithelium. Interestingly, EIII8-TAMR cells also responded to 16T or 21T fibroblasts with pronounced epithelial morphogenesis and produced organized structures with size comparable with those of EIII8–16T (or EIII8–17T) cocultures; however, the tumor fibroblasts failed to evoke visible fibroblast-specific effects on hormonal sensitivity and three-dimensional growth of EIII8-TAMR cells as observed in EIII8 cocultures (Figure 3C).
Figure 3.
Phase-contrast morphology of homotypic three-dimensional cultures of breast tumor fibroblasts (A), heterotypic EIII8–16T and EIII8–21T three-dimensional cocultures (B), and heterotypic EIII8-TAMR-16T and EIII8-TAMR-21T cocultures (C) at day 10 of culture. EIII8 fibroblast cocultures were treated with vehicle [0.01% (v/v) ethanol], 10 nmol/L E2, or a combination of 10 nmol/L E2 plus a 100-fold molar excess of 4-OHT. Scale bars = 40 μm.
The Differential Effects of ER-α+/PgR+ versus ER-α−/PgR− Breast Tumor-Derived Fibroblasts on Tamoxifen Sensitivity Correlate with MAPK- and Akt-Induced ER-α Phosphorylation in the AF-1 Domain
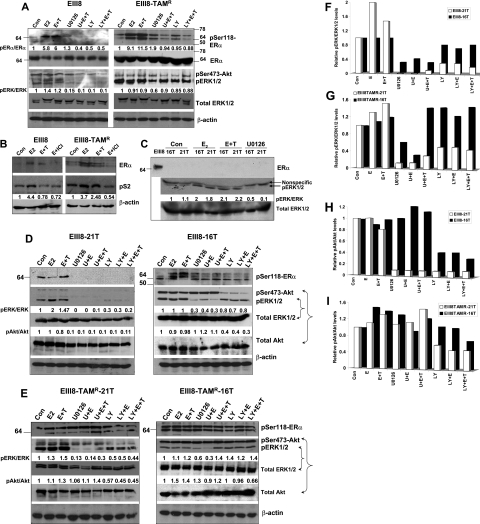
To verify our data from cell growth assays of Figure 2, we determined whether the differences in fibroblast-induced effects on hormonal sensitivity resulted from corresponding alterations in MAPK or Akt activation that emanated from homotypic cell-cell or cell-matrix interactions, or from heterotypic epithelial-fibroblast interactions. Our data show that treatment of EIII8 cultures with U0126 (the noncompetitive inhibitor of MEK1/2) or LY294002 (PI3K/Akt inhibitor) suppressed MAPK and Akt activation, respectively, even in the presence of E2 plus 4-OHT (Figure 4A). Similar treatment of EIII8-TAMR homotypic cultures with U0126 caused a 40% decrease in ERK1/2 activation as compared with control cultures, and limited suppression of ERK1/2 activation in cultures treated with U0126 in the presence of E2 plus 4-OHT (Figure 4A). The PI3K/Akt inhibitor had no effect on Akt phosphorylation (on Ser473) in EIII8-TAMR cultures (Figure 4A). To confirm whether activated MAPK or Akt can crosstalk and phosphorylate ER-α, we analyzed phosphorylation at Ser118 and Ser167, major MAPK- and Akt-mediated phosphorylation sites in the AF-1 domain of ER-α, respectively. Because the antibody to phosphoSer167 ER-α exhibited nonspecific immunoreactivity, the results were not informative. However, pronounced qualitative and quantitative differences in the levels of Ser118-phosphorylated ER-α were observed between EIII8 and EIII8-TAMR homotypic cultures (Figure 4A). Whereas Ser118-phosphorylated ER-α was detected in control EIII8 cultures as a single ∼66-kd band, phosphorylated ER-α in EIII8-TAMR cultures was detectable as a doublet. Treatment with ICI 182,780 caused a marked reduction in E2-induced ER-α reactive doublet, suggesting the presence of ER-α in both bands of the doublet (Figure 4B). Treatment with E2 or a combination of E2 plus 4-OHT stimulated ER-α phosphorylation at Ser118 ∼5.8- to 6-fold or 9.1- to 11.5-fold relative to total ER-α in EIII8 or EIII8-TAMR cultures, respectively. Treatment with U0126 or LY294002 alone, or in the presence of E2 plus 4-OHT blocked MAPK and Akt activation as well as Ser118 phosphorylation of ER-α to levels less than control EIII8 cultures (Figure 4A). Treatment of EIII8-TAMR cultures with U0126 or LY294002 also caused reduction in E2 or E2 plus 4-OHT induced Ser118 ER-α phosphorylation; however, unlike in EIII8 cultures, ER-α phosphorylation levels did not decrease below control levels (Figure 4A). This inability to abrogate ER-α phosphorylation in EIII8-TAMR cultures correlates with the inadequate suppression of MAPK or Akt activities (Figure 4A) or may be indicative of ER-α phosphorylation occurring from crosstalk with other pathways. Immunoblot analysis of activated MAPK in homotypic three-dimensional cultures of 16T or 21T fibroblasts showed the presence of similar levels of MAPK activities in both fibroblast cultures. Although ER-α (Figure 4C) or ER-β (data not shown) is not detectable in homotypic fibroblast cultures, treatment with E2 or E2 plus 4-OHT caused a twofold increase in MAPK activity that was eliminated by treatment with U0126 (Figure 4C).
Figure 4.
Phosphorylation status of MAPK, Akt, and ER-α in homotypic EIII8 and EIII8-TAMR (A), breast tumor fibroblasts (C), heterotypic EIII8–21T and EIII8–16T (D), and heterotypic EIII8-TAMR-21T and EIII8-TAMR-16T (E) three-dimensional cultures. B: Steady-state levels of pS2. Cultures were treated overnight with E2 (1 nmol/L), a combination of E2 and a 100-fold molar excess of 4-OHT (E+T), or E2 and a 100-fold molar excess of ICI 182,780 (E+ICI). Cultures were also treated with 1 μmol/L U0126 (U) or 5 μmol/L LY294002 (LY) alone or in the presence of E2 or E+T. Levels of phospho-ERK1/2 or phospho-Akt relative to total ERK1/2 or Akt, respectively, in EIII8 and EIII8-TAMR stromal cultures from D and E are shown graphically in F–I.
To determine whether the fibroblast-induced alterations in hormonal sensitivity of EIII8 cells observed in heterotypic cell growth assays (Figure 2) resulted from coincident alterations in ER-α phosphorylation and MAPK/Akt activities, we compared profiles of Ser118-phosphorylated ER-α, and ratios of activated ERK1/2 to total ERK1/2, and phospho-Akt (Ser473 phosphorylation) to total Akt between EIII8–16T and EIII8–21T, or EIII8-TAMR-16T and EIII8-TAMR-21T cocultures. Placement of EIII8 cells in contact with 16T fibroblasts induced dramatic qualitative alterations in Ser118 ER-α phosphorylation profiles and decreased sensitivity to inhibition by U0126 and LY294002 compared with EIII8–21T cocultures (Figure 4, D, F, and H). Ser118-phosphorylated ER-α was observed as a single 66-kd band in EIII8–21T cocultures, and the addition of U0126 or LY294002 alone or in the presence of E2 or E2 plus 4-OHT abrogated MAPK and Akt activation, respectively, as well as Ser118 ER-α phosphorylation (Figure 4, D, F, and H). These results resemble the data from EIII8 homotypic cultures and suggest that fibroblasts derived from ER-α+/PgR+ breast tumors do not overpower, but rather comply with moderate levels of growth factor receptor-ER crosstalk occurring in homotypic EIII8 cultures. In contrast, placement of EIII8 cells in contact with 16T fibroblasts resulted in a decrease in inhibition of MAPK and Akt activation by U0126 and LY294002, respectively, as well as dramatic qualitative and quantitative changes in anti-phospho-Ser118 ER-α immunoreactive species (Figure 4, D, F, and H). Treatment with E2 or E2 plus 4-OHT induced hyperphosphorylation of ER-α compared with control (Figure 4D) and suggests that loss of E2-induced growth in EIII8–16T cocultures (Figure 2, Ba) is not a result of decreased uptake of estrogen. The ER-α hyperphosphorylation in EIII8–16T cocultures is mediated via MAPK and Akt activities because the addition of U0126 or LY294002 inhibited MAPK or Akt activation, respectively, and resulted in concomitant decreases (∼60%) in levels of phosphorylated ER-α; however, unlike in EIII8–21T cocultures, a significant amount of Ser118-phosphorylated ER-α persisted in EIII8–16T cocultures (Figure 4D). Treatment with U0126 or LY294002 also resulted in (nonspecific) inhibition of Akt or MAPK activation, respectively, in EIII8–21T cultures; however, interestingly, this cross-inhibitory effect was lost or diminished when cocultured with 16T fibroblasts (Figure 4, D, F, and H). These data suggest that fibroblast-derived factor(s) synthesized or released de novo on establishment of novel epithelial-fibroblast or epithelial-fibroblast-matrix interactions contribute to hyperactivation of MAPK and Akt with consequential effects on ER-α hyperphosphorylation in its AF-1 domain and acquisition of tamoxifen resistance.
We next examined whether the activities of MAPK or Akt, and ER-α phosphorylation status in EIII8-TAMR cells are similarly modified by tumor fibroblasts as in EIII8 cultures. Consistent with the results from homotypic EIII8-TAMR cultures (Figure 4A), two distinct anti-phospho-Ser118 ER-α-immunoreactive bands were observed in both EIII8-TAMR-21T and EIII8-TAMR-16T cocultures (Figure 4D); however, unlike in homotypic EIII8-TAMR cultures, the ER-α phosphorylation status is uninfluenced by fibroblasts and U0126 or LY294002 (Figure 4E). EIII8-TAMR-16T cocultures displayed decreased sensitivity to inhibition of MAPK activation by U0126 as compared with EIII8-TAMR-21T cocultures (Figure 4, E and G). Akt activation was unaffected by LY294002 in EIII8-TAMR-16T cocultures and moderately inhibited in EIII8-TAMR-21T cocultures (Figure 4, E and I). Treatment of EIII8-TAMR-21T cocultures with U0126 or LY294002 also resulted in (nonspecific) inhibition of Akt or MAPK activation, respectively, as in EIII8–21T cultures, and this cross-inhibitory effect was lost when EIII8-TAMR cells were cocultured with 16T fibroblasts (Figure 4, E, G, and I). These data suggest that the net activities (and sensitivities to inhibitors) of MAPK and Akt in EIII8 and EIII8-TAMR cells are subject to differential modulation by the tumor stromal microenvironment.
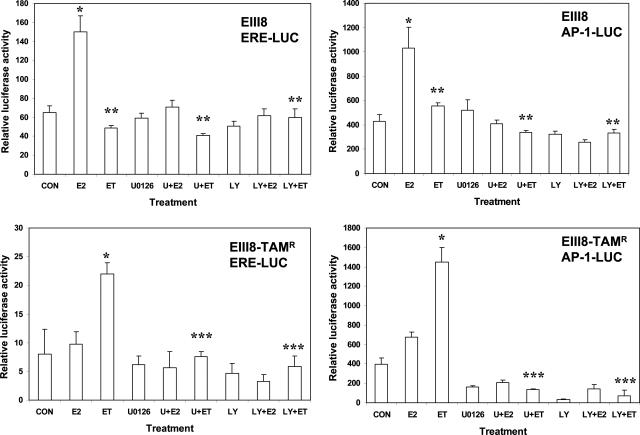
Transcriptional Activity of ER in EIII8 and EIII8-TAMR Cells
To further determine whether Akt- and MAPK-induced phosphorylation of the AF-1 domain in ER-α influences the transcriptional activity of ER and its response to 4-OHT, we compared the transcriptional responses of luciferase reporter constructs containing ERE or AP-1 elements relative to pRLTK internal control in homotypic EIII8 or EIII8-TAMR three-dimensional cultures. Because of considerable variability in transfection efficiencies between the individual epithelial fibroblast heterotypic cocultures, a comparison of ER transcriptional activity between heterotypic cocultures of EIII8 or EIII8-TAMR cells with tumor fibroblasts could not be made. In homotypic EIII8 cultures, the addition of 1 nmol/L E2 enhanced both AP-1- and ERE-mediated luciferase transcription by approximately twofold to threefold, respectively, above control cultures (Figure 5), and addition of 4-OHT blocked E2-induced ERE- and AP-1-mediated luciferase expression (P < 0.001). In contrast, similar analysis of ER transcriptional activity in homotypic EIII8-TAMR cultures showed that the addition of 4-OHT stimulated ERE- or AP-1-mediated luciferase transcription ∼3- to 3.5-fold above control, and treatment with E2 failed to enhance ERE-mediated or weakly enhanced (∼1.5-fold) AP-1-mediated luciferase expression over untreated control cultures (Figure 5). Because these data contradict with E2-induced activation profiles of MAPK and Akt in EIII8-TAMR cells (Figure 4A), the effects of estrogen on expression levels of pS2 (an estrogen-regulated transcriptional target) was determined. Steady-state levels of pS2 protein were enhanced ∼3.5-fold in E2-treated EIII8-TAMR cells. Interestingly, whereas tamoxifen suppressed E2-induced pS2 expression in EIII8 cells, it had only marginal effects on pS2 levels in EIII8-TAMR cells (Figure 4B). Treatment with ICI 182,780 inhibited pS2 expression in both cell lines (Figure 4B). The reasons for the inability of estrogen to induce reporter expression from ERE- and AP-1 contexts in EIII8-TAMR cells are not known; however, it does point to the potential shortcomings of transient reporter assays under certain circumstances. To determine whether estrogen- or tamoxifen-mediated ER transcriptional activity resulted from cross talk with MAPK or Akt activity, EIII8 or EIII8-TAMR cultures were treated with U0126 or LY294002. Treatment with U0126 or LY294002 eliminated both E2- and 4-OHT-induced ERE- and AP-1-mediated luciferase expression in EIII8 and EIII8-TAMR cultures, respectively (Figure 5). These data are consistent with the inhibitory effects of U0126 or LY294002 on ER-α phosphorylation observed in EIII8 and EIII8-TAMR cultures (Figure 4A). Because a good correlation between ER transcriptional activity and ER-α phosphorylation status and sensitivity to U0126 or LY294002 was observed in EIII8 homotypic three-dimensional cultures, our findings suggest that the observed differences in ER-α phosphorylation and MAPK/Akt activities between EIII8–21T versus EIII8–16T cocultures are indeed a result of tumor fibroblast-induced effect(s) on the premalignant epithelium (heterotypic fibroblast-epithelial interaction) rather than from homotypic cell-cell or cell-extracellular matrix (Matrigel) interactions.
Figure 5.
Inhibition of MAPK or Akt reverses tamoxifen-stimulated increase in reporter gene expression from ERE- and AP-1-mediated luciferase reporter constructs. EIII8 or EIII8-TAMR cells were transiently transfected with ERE or AP-1 luciferase constructs or corresponding control vectors along with Renilla luciferase plasmid. Cultures were treated with vehicle [0.01% (v/v) ethanol; CON], E2 (1 nmol/L), a combination of E2 plus a 100-fold molar excess of 4-OHT (ET), and 1 μmol/L U0126 (U) or 5 μmol/L LY294002 (LY) alone or in the presence of 10 nmol/L E2 or a combination of E2 plus 4-OHT. Luciferase activity was normalized against Renilla luciferase, and activity in treated samples was expressed relative to the activities of samples transfected with the corresponding empty control vectors. Results obtained from three independent experiments are expressed as mean ± SE. *E2- or tamoxifen-induced increase in reporter gene expression relative to Renilla luciferase as compared with the corresponding controls (P < 0.001). **Inhibition of E2-induced increase in luciferase expression by 4-OHT, MAPK, or PI3K/Akt inhibitors in EIII8 cultures (P < 0.005); ***Inhibition of tamoxifen-stimulated increase in reporter expression by MAPK or PI3K/Akt inhibitors in EIII8-TAMR cultures (P < 0.001).
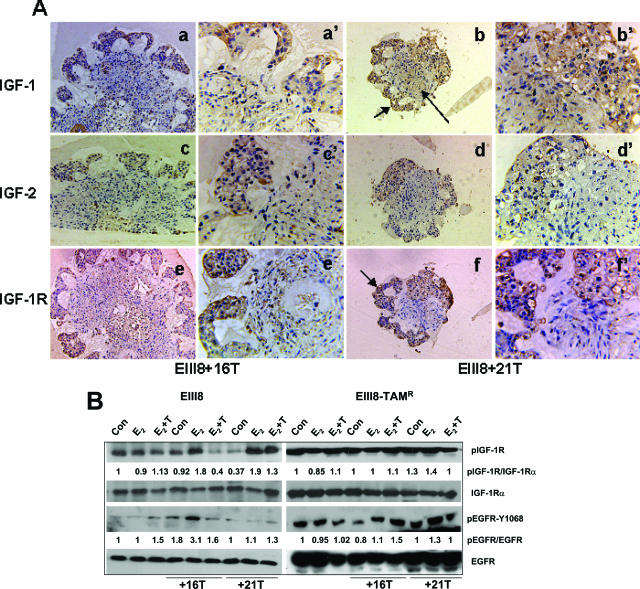
Fibroblast-Induced Tamoxifen Resistance of EIII8 Cells Is Not Contributed by Differences in Activation of IGF-1R or EGFR Pathways
Cross talk between ER-α signaling pathways and IGFs at the level of nuclear ER-α/ERE-mediated transcription is regarded to play a pivotal role in modulating the cell’s sensitivity to estrogen.26 Paraffin sections of EIII8–16T or EIII8–21T cocultures were stained with anti-IGF-1, IGF-2, or IGF-1Rα antibodies. Strong staining for IGF-1 and moderate expression of IGF-2 were observed in the fibroblastic core and epithelial outgrowths of EIII8–16T and EIII8–21T cocultures (Figure 6A). Immunostaining with anti-IGFR-1α antibody showed similar levels of expression in the epithelial compartments of both cocultures (Figure 6A). To determine whether the cocultures differed in levels of activated IGF-1R, cell lysates were subjected to immunoprecipitation with anti-IGF-1Rα antibody and immunocomplexes analyzed by Western blotting with anti-phosphotyrosine antibody. Interestingly, there was no correlation between IGF-1R phosphorylation status and tamoxifen resistance because tamoxifen-sensitive EIII8–21T cocultures displayed ∼5- and 3.5-fold increase in phosphorylated IGF-1R levels in E2- and E2 plus 4-OHT-treated cultures, respectively, as compared with corresponding control cultures treated with vehicle (Figure 6B). Furthermore, in tamoxifen-resistant EIII8–16T cocultures, unlike in EIII8–21T cultures, the E2-induced increase in phosphorylated IGF-1R was abrogated by tamoxifen (Figure 6B). However, our findings provide support for a role for stroma-derived IGFs in activation of IGF-1R because approximately twofold higher levels of phosphorylated IGF-1R relative to total IGF-1R were observed in E2-treated EIII8 fibroblast cocultures as compared with E2-treated EIII8 homotypic cultures (Figure 6B). These results suggest that augmented signaling through the IGF/IGF-1R axis is not a major contributor to hyperactivation of MAPK or Akt, hyperphosphorylation of ER-α, and loss of hormonal sensitivity of EIII8–16T cocultures. Low levels of Y1068-phosphorylated EGFR were observed in both tamoxifen-sensitive (EIII8 and EIII8–21T) and -resistant (EIII8–16T) cultures; however, approximately threefold higher levels of phosphorylated EGFR observed in E2-treated EIII8–16T cocultures as compared with control were suppressed by treatment with tamoxifen (Figure 6B), indicating a lack of correlation between EGFR activation and tamoxifen sensitivity of EIII8 fibroblast cultures. Similar analysis of phosphorylated IGF-1R and EGFR in EIII8-TAMR cultures showed presence of activated IGF-1R and EGFR with no significant regulation by the stroma (Figure 6B). These results suggest that signaling through the EGFR and IGF-1R cascades may contribute to constitutive hyperactivation of MAPK and Akt, ER-α phosphorylation, and intrinsic tamoxifen resistance of EIII8-TAMR cells.
Figure 6.
Differences in tamoxifen sensitivities of EIII8 stromal cocultures are not contributed by activities of EGFR or IGF-1R. A: Paraffin-embedded sections of EIII8–16T (a, c, e) or EIII8–21T (b, d, f) three-dimensional cultures were stained with antibodies to IGF-1 (a, a′, b, b′), IGF-2 (c, c′, d, d′), or IGF-1Rα (e, e′, f, f′). Note the presence of IGFs in the central stromal core (long arrow, b) as well as in the epithelial outgrowths (small arrow, b). In contrast, IGF-1Rα expression is restricted to the epithelial outgrowths (arrow in f). B: Western blot analysis of activation status of EGFR and IGF-1R in homotypic and heterotypic EIII8 or EIII8-TAMR cultures. Three-dimensional cultures were treated with vehicle [0.01% (v/v) ethanol; Con], E2 (1 nmol/L), or a combination of E2 plus a 100-fold molar excess of 4-OHT (E+T). Phosphorylated IGF-1R levels were determined in IGF-1Rα-immunoprecipitated proteins with anti-phosphotyrosine antibody. Total levels of IGF-1R were determined with anti-IGF-1Rα antibody. Phosphorylated EGFR and total EGFR levels were determined with anti-phospho Y1068-specific EGFR or EGFR antibody, respectively. Original magnifications: ×4 (a–f); ×20 (a′–f′).
Discussion
A substantial proportion of tumors in patients presenting with localized breast disease and all tumors in patients with metastatic disease become resistant to endocrine therapies whether or not they retain ER-α. However, the mechanisms for intrinsic and acquired endocrine resistance are poorly understood although there is general agreement that anti-estrogen resistance is accompanied by enhanced activation by paracrine and/or autocrine activation pathways. Evidence that pathological alterations in the tumor microenvironment facilitate tumorigenesis has been derived from experimental model systems and clinical settings.27,28 Previous data from our laboratory have shown that breast tumor fibroblasts play an active role as a morphogenetic and mitogenic inducer of premalignant breast epithelial cells.19 However, the direct role of tumor microenvironment in loss of sensitivity to tamoxifen or acquisition of tamoxifen resistance has not been previously investigated. In this study, using contact-dependent three-dimensional coculture systems and serum-free conditions, we compared the ability/receptivity of ER-α+ tamoxifen-sensitive premalignant (EIII8) or tumorigenic (MCF-7), ER-α+ tamoxifen-resistant (EIII8-TAMR), or ER-α− endocrine nonresponsive MDA-MB-231 breast cancer cells to interact and undergo phenotypic alterations (epithelial morphogenesis) on association with subsets of breast tumor fibroblasts derived from ER-α+/PgR+ versus ER-α−/PgR− breast tumors, and the molecular impact of tumor fibroblast subsets on tamoxifen sensitivity of EIII8 cells and their intrinsically tamoxifen-resistant counterpart EIII8-TAMR cells. Our data show that although all breast cancer cell lines, whether premalignant, tumorigenic, or metastatic, have the ability to establish heterotypic interactions with tumor fibroblasts, the premalignant EIII8 and EIII8-TAMR breast cancer cells are the most receptive and responsive to tumor fibroblasts, responding to the fibroblast microenvironment with pronounced and dramatic, albeit aberrant, epithelial morphogenesis. This difference in fibroblast responsiveness and phenotypic plasticity may be attributed to the stem cell property of EIII8/EIII8-TAMR cells because they form lesions in vivo containing simple ducts that progress to ductal hyperplasia, ductal carcinoma in situ, and frank carcinoma, precursors of breast cancer,23,25,29 as opposed to MCF-7 and MDA-MB-231 breast cancer cells that represent progressed cancer cells that lack the ability to form tumors with similar phenotypic heterogeneity. Despite this difference in fibroblast-induced morphogenetic response, our data from growth assays show that tumor fibroblast subsets exert similar effects on hormonal sensitivities of both ER-α+ tamoxifen-sensitive EIII8 and MCF-7 breast cancer cells; ie, retention of tamoxifen sensitivity when placed in contact with fibroblasts derived from ER-α+/PgR+ tumors, and loss of tamoxifen sensitivity or acquisition of tamoxifen resistance when placed in contact with fibroblasts from ER-α−/PgR− breast tumors. Interestingly, coculture of EIII8 or MCF-7 cells with ER-α−/PgR− tumor-derived fibroblasts also suppressed estrogen-induced growth, but enhanced basal growth. Thus, whereas the tumor stroma can collaborate or comply with the autocrine- or paracrine-mediated growth factor/ER cross talk pathways of breast cancer cells, under certain conditions such as those observed in cocultures with ER-α−/PgR− tumor-derived fibroblasts, they can exaggerate these growth regulatory pathways and actively participate in loss of hormone sensitivity and acquisition of tamoxifen resistance. These data suggest that distinct fibroblast-derived factor(s) modulate epithelial morphogenesis and hormonal sensitivity of breast cancer cells. Because similar placement of intrinsically tamoxifen-resistant EIII8-TAMR or endocrine nonresponsive MDA-MB-231 breast cancer cells in contact with fibroblast subsets failed to induce alterations in hormonal sensitivity/responsiveness, our findings suggest that autocrine and/or paracrine activation mechanisms originating from the tumor cells rather than from the tumor fibroblasts play a dominant role in regulation of growth of these cells. These data not only highlight the defined heterogeneity of breast tumor fibroblasts but also emphasize the need to take this stromal complexity into account when designing strategies for overcoming hormonal resistance.
Alterations in fibroblasts immediately adjacent to transformed epithelial cells have been documented in several tumor systems.30,31 The origin of these fibroblasts remains to be clarified. It has been suggested that these fibroblast-like cells may be derived from epithelial tumor cells via EMT.32,33 However, a fibroblast-like cell line derived from breast cancer cells that have undergone EMT fails to form tumors in nude mice.34 Our results suggest that the tumor-derived fibroblast preparations used in our study are not derived from cancer cells that have undergone EMT because these cells stain negatively for the EMT marker Snail1.35 Furthermore, the tumor-derived fibroblast preparations used in our study promote aberrant epithelial morphogenesis and growth in vitro.19 These data are in agreement with a tumor-promoting role for tumor-derived fibroblasts in vivo.36 Our data show that establishment of intimate epithelial fibroblast cell-cell interactions are necessary for induction of fibroblast-mediated epithelial morphogenesis. Thus, differences in the ability of tumor fibroblast subsets derived from ER-α+/PgR+ versus ER-α−/PgR− breast tumors to influence epithelial morphogenesis and hormonal response may result from variations in reciprocal communications between the epithelial and fibroblast compartments, that consequently influence the de novo synthesis/release of molecules elaborated during such heterotypic interactions. A number of soluble paracrine factors such as EGF, FGF(s),37 transforming growth factor-β,38 hepatocyte growth factor,39 and IGFs31 have been implicated. Tamoxifen-stimulated up-regulation of transforming growth factor-β has been implicated in emergence of tumors with greater invasiveness and tumor histologies correlative with poor prognosis.40,41,42,43 Because loss of tamoxifen sensitivity of EIII8 cells is promoted by fibroblasts derived from ER-α− breast tumors rather than from ER-α+ tumors, it remains to be determined whether the observed differences are contributed by differences in transforming growth factor-β levels/activity. Antibody-mediated depletion assays have identified EGF and IGF-1 as major growth-modulating factors of breast cancer cells expressed by lymph node stromal cells.44 Activation of the IGF-1R by IGFs results in autophosphorylation and subsequent activation of downstream signaling pathways, including PI3K/Akt and ras/Raf/MAPK. Increased expression of IGF-1R and its ligands IGF-1 or IGF-2, and its associated downstream signaling components have been reported in clinical breast tumor tissues and have been associated with breast cancer progression and recurrence.45,46 Recent evidence suggests a role for IGF-1R signaling in tamoxifen resistance. Increased sensitivity to the proliferative effects of IGF1/2 has been reported in tamoxifen-resistant MCF-7 cell lines after treatment with estrogen or tamoxifen.47,48 However, activation of IGF-1R signaling is not sufficient to explain the fibroblast-induced tamoxifen resistance of EIII8 cells because similar levels of IGF-1 and -2, and IGF-1R are expressed in tamoxifen-sensitive (EIII8–21T, EIII8–38T) and tamoxifen-resistant (EIII8–16T, EIII8–17T) cocultures. Furthermore, this acquired tamoxifen resistance is not mediated by increased activation of IGF/IGF-1R axis and priming of downstream signaling kinases that induce ER-α phosphorylation because higher levels of phosphorylated IGF-1R relative to total IGF-1R levels are observed in tamoxifen-treated EIII8–21T (tamoxifen-sensitive) cocultures as compared with tamoxifen-resistant EIII8–16T cocultures. Embryonic mammary buds transplanted from IGF-1R knockout mice into syngeneic recipients have been reported to show decreased proliferation of cap cells in terminal end buds and decreased branching and extension of ducts into the mammary fat pad.49 Because pronounced ductal morphogenesis was observed in both tamoxifen-sensitive and -resistant EIII8 fibroblast cocultures, the IGF/IGF-1R axis may contribute to epithelial morphogenesis and growth of these cocultures.
Overexpression of EGFR has been implicated in acquisition of tamoxifen resistance,50 but its role in fibroblast-epithelial interactions is unclear. A role for EGFR in progression of prostate cancers from androgen-responsive to androgen-independent or refractory has been demonstrated.51 However, drugs targeting the EGFR/Her-2/neu pathway have failed to elicit a significant therapeutic response in many solid tumors, including breast cancer.52 Our results rule out the possibility of EGF/EGFR as a significant contributor to acquired tamoxifen resistance of EIII8 cells because all cocultures were maintained in serum-free media supplemented with EGF, thus eliminating their dependence on EGF expression by epithelial or fibroblast cells, and similar levels of total EGFR and phosphorylated EGFR were observed under conditions of tamoxifen exposure in both tamoxifen-sensitive and tamoxifen-resistant EIII8 fibroblast cocultures. In homotypic EIII8-TAMR and heterotypic EIII8-TAMR fibroblast cultures, an overall increase in levels of total and activated EGFR and IGF-1R that is independent of stroma along with hyperactivation of MAPK, Akt, and phosphorylation of ER-α are observed. It is possible that direct and indirect interactions between IGF and EGF pathways demonstrated in several cell types including breast cancer cells53,54 might play an important role in the intrinsic tamoxifen resistance of EIII8-TAMR cells.
Because hyperactivation of MAPK and Akt (decreased sensitivity to inhibitors) along with hyperphosphorylation of ER-α in the AF-1 domain occur in EIII8–16T as opposed to EIII8–21T cocultures, we posit that fibroblast-induced tamoxifen resistance involves cross talk between growth factor signaling pathway(s), other than EGF/EGFR and IGF/IGF-1R pathways, and ER-α. This hypothesis is further supported by our data that show that whereas treatment with MAPK or PI3K/Akt inhibitors abrogate ER-α phosphorylation in tamoxifen-sensitive EIII8 fibroblast cocultures, similar treatment only partially abrogates ER-α hyperphosphorylation in tamoxifen-resistant EIII8 fibroblast cocultures. Thus, it is likely that fibroblast subsets differ in expression of growth regulatory molecule(s) that mediate activation of specific signaling kinases with resultant effects on ER-α phosphorylation and transcription activation function.
Serine residues at 104, 106, 118, and 167 clustered within the AF-1 domain of ER are potential sites for cyclin A/CDK2-, MAPK-, and Akt-induced phosphorylation.55,56,57 The consensus Akt phosphorylation site is not present in ER-β; however, recent data suggest that both AF-1 and AF-2 domains of ER-β are targets for kinase signaling by Akt.58 This raises the possibility that the fibroblasts induced effects on loss of tamoxifen sensitivity of EIII8 cells, and the intrinsic tamoxifen resistance of EIII8-TAMR cells may be mediated through ER-α and ER-β. The appearance of multiple phospho-Ser118 ER-α-immunoreactive bands is indicative of ER-α molecules phosphorylated at multiple sites or the presence of ER-α isoforms. The latter could be ascribed to expression of ER-α splice variants59; alternatively, the multiple bands could arise from proteolysis of the native ER-α protein. However, because the sizes and relative proportions of these bands are not significantly altered, it is unlikely that these bands are products of proteolysis. Because treatment with MEK or PI3K/Akt inhibitors only partially abrogated Ser118-ER-α phosphorylation in intrinsically tamoxifen-resistant EIII8-TAMR homotypic cultures, but abolished tamoxifen-induced ER transcriptional activity, these data suggest that ER-α is subjected to further phosphorylation by additional kinases; however, only those modified by Akt and MAPK play an important role in regulating ER transcriptional activity in EIII8-TAMR cells.
In summary, this is the first report that demonstrates in vitro the direct participation of breast tumor fibroblasts in loss of hormone sensitivity and acquisition of endocrine resistance. Although the number of primary fibroblast cultures was few, using defined serum-free conditions and cocultures on a reconstituted basement membrane matrix, we have shown that subsets of tumor fibroblasts functionally separated based on their differential effects on MAPK/Akt activation and sensitivity to their inhibitors, and ER-α modification, can exert different outcomes on tamoxifen sensitivity of ER-α+ breast cancer cells. Fibroblast modulation of breast epithelial morphogenesis requires intimate cell-cell contact, thus, factors produced/released de novo during such interactions are necessary. Proteomics-based approaches are being used to identify the factor(s) elaborated in these epithelial fibroblast cocultures.
Acknowledgments
We thank the Tissue Core Facility of the Karmanos Cancer Institute for providing breast tumor tissues for isolation of fibroblast cells and Dr. Gloria Heppner for critical review of the manuscript.
Footnotes
Address reprint requests to Malathy P.V. Shekhar, Ph.D., Breast Cancer Program, Karmanos Cancer Institute, 110 East Warren Ave., Detroit, MI 48201. E-mail: shekharm@karmanos.org.
Supported by the US Army Medical Research and Materiel Command (grants DAMD17-99-I-9443 and DAMD-17-02-1-0618 to M.P.V.S).
References
- Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SR. Acquired tamoxifen resistance in human breast cancer—potential mechanisms and clinical implications. Anticancer Drugs. 1997;8:911–930. doi: 10.1097/00001813-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubin M, Nicholson RI. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signaling and estrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12:S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- Lahooti H, White R, Danielian PS, Parker MG. Characterization of ligand-dependent phosphorylation of the estrogen receptor. Mol Endocrinol. 1994;8:182–188. doi: 10.1210/mend.8.2.8170474. [DOI] [PubMed] [Google Scholar]
- Le Goff P, Montano MM, Schodin DJ, Katzenellenbogen BS. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J Biol Chem. 1994;269:4458–4466. [PubMed] [Google Scholar]
- Katzenellenbogen BS, Montano MM, Ekena K, Herman ME, McInerney EM. William L. McGuire Memorial Lecture. Antiestrogens: mechanisms of action and resistance in breast cancer. Breast Cancer Res Treat. 1997;44:23–38. doi: 10.1023/a:1005835428423. [DOI] [PubMed] [Google Scholar]
- Perez-Tenorio G, Stal O, Southeast Sweden Breast Cancer Group Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86:540–545. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, Bartlett JM. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol. 2005;207:139–146. doi: 10.1002/path.1829. [DOI] [PubMed] [Google Scholar]
- Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- Sivaraman VS, Wang H, Nuovo GJ, Malbon CC. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest. 2000;99:1032–1037. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R, Massarweh S, Shou J, Osborne CK. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res. 2003;9:447S–454S. [PubMed] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR. Stromal regulation of epithelial function. Cancer Treat Res. 1991;53:335–364. doi: 10.1007/978-1-4615-3940-7_16. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Bigsby RM, Cooke PS, Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ. 1985;17:137–148. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- Camps JL, Chang S, Hsu TC, Freeman MR, Hong S, Zhau HE, von Eschenbach AC, Chung LWK. Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci USA. 1990;87:75–79. doi: 10.1073/pnas.87.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard O, Rolland Y, Poupon MF. Fibroblast-dependent tumorigenicity of cells in nude mice. Cancer Res. 1986;46:3290–3294. [PubMed] [Google Scholar]
- Grey AM, Schor AM, Rushton G, Ellis I, Schor SL. Purification of the migration-stimulating factor produced by fetal and breast cancer patient fibroblasts. Proc Natl Acad Sci USA. 1989;86:2438–2442. doi: 10.1073/pnas.86.7.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor growth and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- Basolo F, Elliott J, Tait L, Chen XQ, Maloney T, Russo IH, Pauley R, Momiki S, Caamano J, Klein-Szanto AJ, Russo J. Transformation of human breast epithelial cells by c-Ha-ras oncogene. Mol Carcinog. 1991;4:25–35. doi: 10.1002/mc.2940040106. [DOI] [PubMed] [Google Scholar]
- Miller FR, Soule HD, Tait L, Pauley RJ, Wolman SR, Dawson PJ, Heppner GH. Xenograft model of human proliferative breast disease. J Natl Cancer Inst. 1993;85:1725–1732. doi: 10.1093/jnci/85.21.1725. [DOI] [PubMed] [Google Scholar]
- Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR. MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol. 1996;148:313–319. [PMC free article] [PubMed] [Google Scholar]
- Shekhar MP, Nangia-Makker P, Wolman SR, Tait L, Heppner GH, Visscher DW. Direct action of estrogen on sequence of progression of human preneoplastic breast disease. Am J Pathol. 1998;152:1129–1132. [PMC free article] [PubMed] [Google Scholar]
- Shekhar PVM, Werdell J, Tait L. Interaction with endothelial cells is a prerequisite for branching ductal-alveolar morphogenesis and hyperplasia of preneoplastic human breast epithelial cells. Cancer Res. 2000;60:439–449. [PubMed] [Google Scholar]
- Visscher DW, Nangia-Makker P, Heppner G, Shekhar PV. Tamoxifen suppresses histologic progression to atypia and DCIS in MCF10AT xenografts, a model of early breast cancer. Breast Cancer Res Treat. 2001;65:41–47. doi: 10.1023/a:1006490000659. [DOI] [PubMed] [Google Scholar]
- Hamelers IH, Steenbergh P. Interaction between estrogen and insulin-like growth factor signaling pathways in human breast tumor cells. Endocr Relat Cancer. 2003;10:331–345. doi: 10.1677/erc.0.0100331. [DOI] [PubMed] [Google Scholar]
- Noël A, Hajitou A, L’Hoir C, Maquoi E, Baramova E, Lewalle JM, Remacle A, Kebers F, Brown P, Calberg-Bacq CM, Foidart JM. Inhibition of stromal matrix metalloproteases: effects on breast-tumor promotion by fibroblasts. Int J Cancer. 1998;76:267–273. doi: 10.1002/(sici)1097-0215(19980413)76:2<267::aid-ijc15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- van Roozendaal CEP, Klijn JGM, van Ooijen B, Claassen C, Eggermont AMM, Henzen-Logmans SC, Fockens JA. Differential regulation of breast tumor cell proliferation by stromal fibroblasts of various breast tissue sources. Int J Cancer. 1996;65:120–125. doi: 10.1002/(SICI)1097-0215(19960103)65:1<120::AID-IJC20>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Tait L, Dawson PJ, Wolman SR, Galea K, Miller FR. Multipotent human breast stem cell line MCF10AT. Int J Oncol. 1996;9:263–267. doi: 10.3892/ijo.9.2.263. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986;47:131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Singer C, Rasmussen A, Smith HS, Lippman ME, Lynch HT, Cullen KJ. Malignant breast epithelium selects for insulin-like growth factor II expression in breast stroma: evidence for paracrine function. Cancer Res. 1995;55:2448–2454. [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Prindull G, Zipori D. Environmental guidance of normal and tumor cell plasticity: epithelial mesenchymal transitions as a paradigm. Blood. 2004;103:2892–2899. doi: 10.1182/blood-2003-08-2807. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, Bissell MJ, Ronnov-Jessen L. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiró S, Escriva M, Puig I, Barbera MJ, Dave N, Herranz N, Larriba MJ, Takkunen M, Franci C, Munoz A, Virtanen I, Baulida J, Garcia de Herreros A. Snail1 transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res. 2006;34:2077–2084. doi: 10.1093/nar/gkl141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res. 1999;5:1063–1071. [PubMed] [Google Scholar]
- Akhurst RJ, Balmain A. Genetic events and the role of TGF-β in epithelial tumor progression. J Pathol. 1999;187:82–90. doi: 10.1002/(SICI)1096-9896(199901)187:1<82::AID-PATH248>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kasai S, Sugimura K, Matsumoto K, Nishi N, Kishimoto T, Nakamura T. Hepatocyte growth factor is a paracrine regulator of rat prostate epithelial growth. Biochem Biophys Res Commun. 1996;228:646–652. doi: 10.1006/bbrc.1996.1710. [DOI] [PubMed] [Google Scholar]
- Knabbe C, Lippman ME, Wakefield LM, Flanders KC, Kasid A, Derynck R, Dickson RB. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987;48:417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Kerr DJ, Steel CM. Transforming growth factor beta 1 is implicated in the failure of tamoxifen therapy in human breast cancer. Br J Cancer. 1991;63:609–614. doi: 10.1038/bjc.1991.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JR, Baum M, Colletta AA. Role of TGF beta in the anti-estrogen response/resistance of human breast cancer. J Mammary Gland Biol Neoplasia. 1996;1:381–389. doi: 10.1007/BF02017394. [DOI] [PubMed] [Google Scholar]
- Arteaga CL, Koli KM, Dugger TC, Clarke R. Reversal of tamoxifen resistance of human breast carcinomas in vivo by neutralizing antibodies to transforming growth factor-beta. J Natl Cancer Inst. 1999;91:46–53. doi: 10.1093/jnci/91.1.46. [DOI] [PubMed] [Google Scholar]
- LeBedis C, Chen K, Fallavollita L, Boutros T, Brodt P. Peripheral lymph node stromal cells can promote growth and tumorigenicity of breast carcinoma cells through the release of IGF-1 and EGF. Int J Cancer. 2002;100:2–8. doi: 10.1002/ijc.10481. [DOI] [PubMed] [Google Scholar]
- Rocha RL, Hilsenbeck SG, Jackson JG, Van DenBerg CL, Weng C, Lee AV, Yee D. Insulin-like-growth factor binding protein-3 and insulin receptor substrate-1 in breast cancer: correlation with clinical parameters and disease-free survival. Clin Cancer Res. 1997;3:103–109. [PubMed] [Google Scholar]
- Turner BC, Haffty BG, Narayanan L, Yuan J, Havre PA, Gumbs AA, Kaplan L, Burgaud JL, Carter D, Baserga R, Glazer PM. Insulin-like growth factor-I receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res. 1997;57:3079–3083. [PubMed] [Google Scholar]
- Parisot JP, Hu XF, DeLuise M, Zalcberg JR. Altered expression of the IGF-1 receptor in a tamoxifen-resistant human breast cancer cell line. Br J Cancer. 1999;79:693–700. doi: 10.1038/sj.bjc.6690112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman LR, Johnson MD, Wakeling AE, Lykkesfeldt AE, May FE, Westley BR. Type I IGF receptor and acquired tamoxifen resistance in oestrogen-responsive human breast cancer cells. Eur J Cancer. 1993;29A:2256–2264. doi: 10.1016/0959-8049(93)90218-5. [DOI] [PubMed] [Google Scholar]
- Bonnette SG, Hadsell DL. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology. 2001;142:4937–4945. doi: 10.1210/endo.142.11.8500. [DOI] [PubMed] [Google Scholar]
- McClelland RA, Barrow D, Madden TA, Dutkowski CM, Pamment J, Knowlden JM, Gee JM, Nicholson RI. Enhanced epidermal growth factor receptor signaling in MCF-7 breast cancer cells after long term culture in the presence of the pure antiestrogen ICI 182, 780 (Faslodex). Endocrinology. 2001;142:2776–2788. doi: 10.1210/endo.142.7.8259. [DOI] [PubMed] [Google Scholar]
- Djakiew D. Dysregulated expression of growth factors and their receptors in the development of prostate cancer. Prostate. 2000;42:150–160. doi: 10.1002/(sici)1097-0045(20000201)42:2<150::aid-pros10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, Tortora G, D’Agostino D, Caputo F, Cancello G, Montagna E, Malorni L, Zinno L, Lauria R, Bianco AR, De Placido S. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- Balañá ME, Labriola L, Salatino M, Movsichoff F, Peters G, Charreau EH, Elizalde PV. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene. 2001;20:34–47. doi: 10.1038/sj.onc.1204050. [DOI] [PubMed] [Google Scholar]
- Coppola D, Ferber A, Miura M, Sell C, D’Ambrosio C, Rubin R, Baserga R. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol. 1994;14:4588–4595. doi: 10.1128/mcb.14.7.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Trowbridge JM, Garabedian MJ. Potentiation of human estrogen receptor a transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-cdk2 complex. J Biol Chem. 1999;274:22296–22302. doi: 10.1074/jbc.274.32.22296. [DOI] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- Duong BN, Elliott S, Frigo DE, Melnik LI, Vanhoy L, Tomchuck S, Lebeau HP, David O, Beckman BS, Alam J, Bratton MR, McLachlan JA, Burow ME. Akt regulation of estrogen receptor β transcriptional activity in breast cancer. Cancer Res. 2006;66:8373–8381. doi: 10.1158/0008-5472.CAN-05-3845. [DOI] [PubMed] [Google Scholar]
- Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. Identification of a new isoform of the human estrogen receptor alpha (hERα) that is encoded by distinct transcripts and that is able to repress hERα activation function-1. EMBO J. 2000;19:4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]