Abstract
Homeobox genes control developmental patterning and are increasingly being found to be deregulated in tumors. The DLX4 homeobox gene maps to the 17q21.3-q22 region that is amplified in some epithelial ovarian cancers. Because amplification of this region correlates with poor prognosis, we investigated whether DLX4 overexpression contributes to aggressive behavior of this disease. DLX4 was not detected in normal ovary and cystadenomas, whereas its expression in ovarian carcinomas was strongly associated with high tumor grade and advanced disease stage. Overexpression of DLX4 in ovarian cancer cells promoted growth in low serum and colony formation. Imaging of mice bearing intraperitoneal tumors revealed that DLX4 overexpression substantially increased tumor burden. Tumors that overexpressed DLX4 were more vascularized than vector-control tumors. Conditioned medium of DLX4-overexpressing tumor cells was more effective than medium conditioned by vector-control cells in stimulating endothelial cell growth. These observations were associated with the ability of DLX4 to induce expression of vascular endothelial growth factor as well as intracellular and secreted isoforms of fibroblast growth factor-2. Moreover, increased levels of these fibroblast growth factor-2 isoforms induced vascular endothelial growth factor expression in tumor cells. This study reveals a novel role for a homeobox gene in ovarian tumorigenicity by its induction of a proangiogenic, growth-stimulatory molecular program.
Homeobox genes constitute an evolutionarily conserved superfamily of genes that play essential roles in controlling cell differentiation and morphogenesis during embryonic development.1 Members of this gene superfamily encode transcription factors and have been increasingly reported to be up- or down-regulated in a variety of cancers.2,3 Although the functional significance of many of these genes to tumor progression has yet to be defined, recent studies indicate that their aberrant expression promotes self-renewal, cell proliferation, and survival.2,3 For example, HOXA5 regulates the p53 promoter and its expression is lost in >60% of breast cancers.4 On the other hand, overexpression of SIX1 in breast cancers induces cyclin A1 and deregulates cell cycle control.5
Various mechanisms give rise to aberrant homeobox gene expression in tumors. NKX3.1 maps to a region that frequently undergoes loss of heterozygosity in prostate intraepithelial neoplasia and prostate cancers, and NKX3.1 loss is thought to be an important initiating event in prostate neoplasia.6,7 Loss of expression of HOXA5 in breast cancers and of HOXA10 in endometrial cancers has been attributed to promoter methylation.4,8 Deregulation of many homeobox genes in leukemias is attributable to chromosomal translocations.3 Very few reports attribute overexpression of homeobox genes in solid tumors to gene amplification. One notable example is amplifications within the 17q21-q22 region that occur in ∼10% of breast and ovarian cancers and are associated with poor prognosis.9,10,11 This region contains the HOXB gene cluster. HOXB7 and HOXB13 are members of this cluster and are overexpressed in breast and ovarian cancers.12,13,14,15 HOXB13 has been reported to promote invasiveness of breast and ovarian epithelial cells.13,14 HOXB7 also promotes invasive behavior of breast cancer cells15 and growth of ovarian epithelial cells.12
DLX4 is a member of the DLX family of mammalian homeobox genes that are related to the Drosophila gene Distal-less. The DLX4 gene (also reported as DLX7, DLX8, and BP1) is not expressed in most normal adult tissues and maps to the 17q21.3-q22 region adjacent to the HOXB gene cluster.16,17,18 Because of the significance of this chromosomal hot-spot to the pathobiology of epithelial ovarian cancer, we investigated whether DLX4 is aberrantly expressed in ovarian cancer and contributes to the aggressive behavior of this disease. Our studies indicate that overexpression of DLX4 in ovarian cancers is strongly associated with high tumor grade and advanced disease stage. Our findings reveal a surprising role of DLX4 in promoting ovarian tumor progression by its control of a proangiogenic molecular program involving fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF).
Materials and Methods
Cell Lines
Sources of ovarian cancer cell lines were as follows: OVCAR-3, SKOV3, and MDAH 2774 (American Type Culture Collection, Rockville, MD); ES-2 (Patrice Morin, National Institute on Aging, Baltimore, MD); A2780, HEY, DOV13, OVCA420, OVCAR-8, and CAOV3 (Gordon Mills, M.D. Anderson Cancer Center, Houston, TX); and 2008 (Zahid Siddik, M.D. Anderson Cancer Center). Nontumorigenic ovarian surface epithelial cell lines IOSE-29 and IOSE-80 were provided by Nelly Auersperg (University of British Columbia, Vancouver, BC, Canada). Immortalized endothelial cells were derived from ovaries of H-2Kb-tsA58 mice as previously described.19
Plasmids
A BP1 cDNA clone20 that contains the coding region of human DLX4 (GenBank BC016145) was provided by Patricia Berg (George Washington University, Washington, DC). We subcloned this cDNA into the pIRES-EGFP2 vector (BD Biosciences Clontech, Palo Alto, CA) to generate the pIRES-EGFP2-DLX4 plasmid. cDNAs encoding wild-type, high-molecular weight (HMW) and low-molecular weight (LMW) isoforms of FGF-221 were provided by Natalina Quarto (Stanford University, Stanford, CA) and subcloned into the pIRES-EGFP2 vector.
Tissue Specimens
Frozen tissue specimens were obtained from the Gynecologic Tumor Bank at the M.D. Anderson Cancer Center. Tissue microarrays were constructed from core biopsies of paraffin-embedded specimens of normal, benign, and malignant ovarian tissues using a Beecher Instruments (Silver Spring, MD) tissue arrayer. Use of specimens was approved by the institutional review board. Cases included normal ovarian tissues (n = 11), benign ovarian cystadenomas (n = 18), borderline ovarian tumors (n = 20), low-grade ovarian carcinomas (n = 13), and high-grade ovarian carcinomas (n = 19). Histopathological analysis of hematoxylin and eosin (H&E)-stained slides from paraffin blocks of each case was performed by two gynecological pathologists (J.L., D.R.). For each case, two separate core biopsies were taken from blocks that were representative of the tumor grade as a whole.
Immunohistochemical Staining and Analysis
Staining with DLX4 antibody (Ab) (A-20; Santa Cruz Biotechnology, Santa Cruz, CA) (1:100) was detected with streptavidin-peroxidase (DAKO, Carpinteria, CA). Percentage of epithelial cells showing nuclear staining was determined in two separate microscopic fields for each tissue core viewed at ×200 magnification. Each field contained a minimum of 500 epithelial cells. An average percentage of epithelial cells with positively stained nuclei was calculated for core biopsies of each case. Histopathological diagnosis of specimens was performed independently of immunohistochemical analysis to eliminate bias in scoring staining. Sections of tumor tissues collected from mice were stained with H&E and with Abs to DLX4, CD31 (Pharmingen, San Diego, CA) (1:50), and CD34 (MEC14.7; Abcam, Cambridge, MA) (1:50). Microvessel density was determined by initially screening sections stained with Abs to CD31 and CD34 for regions within the tumor parenchyma of highest vascularity under low-power magnification. Within these regions, the number of vessels, viewed under high-power magnification, was counted within five separate 0.4-mm2 fields in each section.
Tumor Cell Culture and Transfection
A2780 and ES-2 cells were cultured in RPMI 1640 and McCoy’s 5A medium, respectively (Invitrogen, Carlsbad, CA). Media were supplemented with 10% fetal bovine serum (FBS), 2 mmol/L l-glutamine, and penicillin-streptomycin. A2780 and ES-2 cells were stably transfected with the pIRES-EGFP2-DLX4 plasmid and with empty pIRES-EGFP2 vector using FuGENE6 reagent (Roche, Indianapolis, IN). Stably transfected A2780 and ES-2 clones, derived from single colonies, were selected by G418 (400 μg/ml) (Invitrogen) and assayed for ectopic DLX4 expression by reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot as described below. FGF-2 cDNAs were transfected into A2780 and ES-2 cells using FuGENE6 and transiently expressed for 2 days. In other experiments, cells were treated with recombinant human FGF-2 (rhFGF-2) (R&D Systems, Minneapolis, MN) at final concentrations of 0, 1, 10, and 100 ng/ml and with neutralizing FGF-2 Ab (AB-233-NA; Sigma, St. Louis, MO) at 10 μg/ml for 2 days.
Small Interfering RNA (siRNA) Knockdown
Sequences of DLX4 and negative control siRNA oligomers (synthesized by Qiagen-Xeragon, Germantown, MD) were the same as used by other investigators22 and were as follows: DLX4 siRNA: 5′-GCUCCUGAAGCAGAAUUCUdTdT-3′; negative control siRNA: 5′-CAGUUAGAGGAGAAAAAGGdTdT-3′. One hundred thousand cells were seeded per well in six-well plates and allowed to adhere overnight. Cells were transfected with oligomers at a final concentration of 100 nmol/L using Oligofectamine reagent (Invitrogen). Transfections were repeated at 24 hours thereafter. Lysates were prepared from cells that were collected at 48 hours after the second transfection and analyzed by Western blot. As controls, cells were treated with transfection reagent and no siRNA (mock-transfected).
Proliferation, Colony Formation, and Terminal dUTP Nick-End Labeling (TUNEL) Assays
Tumor cell proliferation was measured by using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche). One thousand cells were seeded per well in 96-well plates. Optical density at 570 nm after MTT addition was measured daily throughout the 3-day time course. For growth factor dependence assays, cells were plated in 10-mm dishes and cultured in media containing 10% or 0.1% FBS. At 3 days thereafter, cells were fixed in 70% ethanol, and the extent of apoptosis was determined by flow cytometric analysis of TUNEL staining using the APO-BRDU kit (Phoenix Flow Systems, San Diego, CA). TUNEL-staining was also performed on cells treated with cisplatin (Bristol-Myers Squibb, Princeton, NJ) at a final concentration of 3 μmol/L. For colony formation assays, 1 × 104 cells were plated in 35-mm dishes containing 0.3% agarose. Number of colonies >100 μm in diameter were counted at 3 weeks after plating. Each assay was performed in triplicate.
Endothelial Cell Growth Assays
To generate tumor cell-conditioned medium, 2 × 105 cells of transfected ovarian cancer lines were seeded per well in 2 ml of medium containing 2.5% FBS in six-well plates. Culture supernatants were collected at 24 hours thereafter, and volumes normalized according to the number of tumor cells counted in each dish at the time of medium collection (ie, 1 ml of medium equivalent to 1 × 105 tumor cells). Mouse ovarian-derived endothelial cells were cultured as previously described.19 Two thousand endothelial cells were seeded per well in 96-well plates in medium containing 5% FBS. At 24 hours thereafter, medium was replaced with tumor cell-conditioned medium. Endothelial cell proliferation was measured daily throughout a 3-day time course by MTT assay.
RT-PCR
Total RNA was isolated from cultured cells and tissues using Trizol reagent (Invitrogen). Reverse transcription of 1 μg of DNase I-treated total RNA was performed using Superscript II reverse transcriptase (Invitrogen) in a reaction volume of 20 μl. One μl of the RT reaction was used for amplification using platinum TaqDNA polymerase (Invitrogen). Primers for detecting RT products of DLX4, VEGF, and actin were as follows: DLX4: 5′-GTA TGGCCACCTCCTGTCTT-3′ (sense), 5′-GAGTAGATGGTCCTCGGCTT-3′ (anti-sense); VEGF: 5′-TCGGGCCTCCGAAACCATGA-3′ (sense); 5′-CCTGGTGAGAGATCTGGT TC-3′ (anti-sense); and actin: 5′-ATGATATCGCCGCGCTCG-3′(sense), 5′-CGCTCGGTGAGGATCTTCA-3′ (anti-sense). Amplification was performed as follows: 2-minute hot-start at 94°C, denaturation at 94°C for 1 minute, annealing at 55°C for 1 minute, and extension at 72°C for 1 minute for 28 cycles for VEGF and 25 cycles for actin. For detecting DLX4 RT products, amplification conditions were as follows: 2-minute hot-start at 94°C, denaturation at 94°C for 1 minute, annealing at 60°C for 1 minute, and extension at 72°C for 1.5 minutes for 28 cycles. Titrations were performed to ensure a linear range of amplification. Southern blot analysis of RT-PCR products was performed by using 32P-labeled actin cDNA (BD Biosciences Clontech) and a fragment containing the first 279 bp of coding sequences that are unique to DLX4.
Western Blot and Enzyme-Linked Immunosorbent Assay (ELISA)
Cell lysates were prepared by using M-PER buffer (Pierce, Rockford, IL). Equal amounts of protein (20 μg per lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Piscataway, NJ). All of the primary Abs were used at 1:1000 dilution: DLX4 (clone 1F11; Abnova Corporation, Taipei, Taiwan), actin (AC-15; Sigma), FGF-2 (FB-8; Sigma). Secondary Abs were obtained from Bio-Rad (Hercules, CA). Levels of human VEGF and FGF-2 in culture supernatants, cell lysates, and ascitic fluid were assayed by ELISA (R&D Systems). For assaying levels of growth factors in cultured cells, 1 × 105 cells were plated per well in 2 ml of culture medium in six-well plates. Culture supernatants were collected at 24, 48, or 72 hours thereafter. Total cellular protein content was determined by Bradford assay. Levels of growth factor present in culture supernatants and in cell lysates as measured by ELISA were normalized according to the total cellular protein content. All assays were performed in triplicate.
Propagation of Tumors in Nude Mice
Animal studies were approved by the institutional animal care and use committee. For subcutaneous inoculation, five 4-week-old female nude mice per group were inoculated at one site on the right flank with 3 × 106 cells of each transfected A2780 line. Volumes of subcutaneous tumors were calculated from two perpendicular measurements of tumor diameters taken using calipers. For intraperitoneal inoculation, five mice per group were inoculated at one site on the lower abdomen with 3 × 106 cells of each A2780 line and with 1 × 106 cells of each ES-2 line. At 18 days thereafter, mice were sacrificed by CO2 asphyxiation. Volume of ascites was measured and ascitic fluid collected by centrifugation. Green fluorescent protein (GFP)-expressing tumors in the abdomen of sacrificed mice were visualized under a MZML III fluorescence stereomicroscope equipped with mercury lamp power supply and GFP filter set (Leica, Bannockburn, IL). Tumor burden was quantified by measuring areas of fluorescence signals within the abdominal cavity in captured images by using Image Pro Plus 5.0 software (Media Cybernetics, Silver Spring, MD).
Statistical Analysis
Data were analyzed using STATISTICA6 software (StatSoft Inc., Tulsa, OK). Statistical significance of differences in percentage of cells with DLX4-positive nuclei between groups of normal, benign, and malignant cases was calculated by the Mann-Whitney U-test. Correlations were determined using Spearman regression analysis. Statistical significance of differences in cell growth, colony formation, tumor volume, and growth factor levels between vector-control and DLX4-transfected cells was calculated by the Student’s t-test. P values >0.05 were considered not significant.
Results
DLX4 Expression in Epithelial Ovarian Cancers Is Associated with Aggressive Tumor Behavior
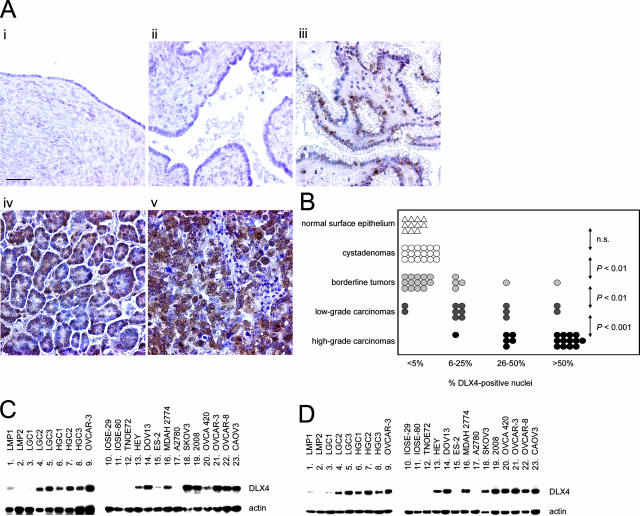
We examined whether DLX4 is aberrantly expressed in ovarian cancers by performing immunohistochemical analysis of tissue microarrays of clinical specimens. Little or no DLX4 staining was detected in normal ovarian surface epithelium and in cystadenomas (Figure 1A). As compared with normal ovarian surface epithelium and benign cysts, a significance increase (P < 0.01) in the percentage of epithelial cells with nuclear staining of DLX4 was observed in borderline tumors (Figure 1B). These tumors are distinguished from ovarian carcinomas by their lack of stromal invasion. All of the borderline tumor cases examined in this study (n = 20) were confined to the ovaries (stage I). Percentage of cells with DLX4-positive nuclei was significantly higher in low-grade carcinomas compared with borderline tumors (P < 0.01), and higher in high-grade carcinomas than in low-grade carcinomas (P < 0.001). In all cases, DLX4 expression in tumors was restricted to the epithelia (Figure 1A). Levels of DLX4 expression in several tissue specimens were confirmed by semiquantitative RT-PCR analysis (Figure 1C). DLX4 expression was not detected by RT-PCR in nontumorigenic ovarian surface epithelial cell lines and was detected at various levels in ovarian cancer cell lines (Figure 1C). Levels of DLX4 expression in tissue specimens and in cell lines were also confirmed by Western blot analysis and were found to be very similar to the levels that were detected by RT-PCR (Figure 1D).
Figure 1.
DLX4 expression in normal ovary and epithelial ovarian tumors. A: Representative examples of immunohistochemical staining of DLX4 in specimens of normal ovary (i), cystadenoma (ii), borderline tumor (iii), and low-grade (iv) and high-grade (v) ovarian carcinomas. B: Summary of DLX4 staining in clinical specimens. An average percentage of epithelial cells with DLX4-positive nuclear staining was determined for tissue cores of each case. Each symbol represents an individual case. Statistical significance of differences in percentage of DLX4-positive cells between the indicated groups of cases was calculated by the Mann-Whitney U-test. P values >0.05 were considered not significant (n.s.). C: Southern blot analysis of DLX4 RT-PCR products in specimens of borderline tumor (lanes 1 and 2), low-grade ovarian carcinoma (lanes 3 to 5), high-grade ovarian carcinoma (lanes 6 to 8), nontumorigenic ovarian surface epithelial cell lines (lanes 10 to 12), and ovarian cancer cell lines (lanes 9 and 13 to 23). D: Detection of DLX4 protein by Western blot in the same tissue specimens and cell lines analyzed by RT-PCR in C. Detection of actin RT-PCR products and protein were included as controls. Scale bar = 50 μm.
DLX4 expression in ovarian carcinoma cases was analyzed in terms of clinical parameters. The percentage of DLX4-positive cells in tumors of patients who developed ascites was significantly higher than for patients without ascites (P < 0.002). The average overall survival time of patients with tumors that had extensive DLX4 expression (>50% of cells with nuclear staining) was markedly shorter than for patients with tumors that had only focal DLX4 expression (Table 1). The poorer survival and higher frequency of ascites in patients with tumors that had extensive DLX4 expression could be related to these patients having more clinically advanced disease (Table 1). Indeed, the percentage of DLX4-positive cells in tumors was found to strongly correlate with the stage of disease (R = −0.72), raising the possibility that DLX4 might play a significant role in promoting ovarian tumor progression.
Table 1.
Association of DLX4 Expression in Ovarian Carcinomas with Clinical Features
| % DLX4+ nuclei | No. of cases | Cases with ascites* | Disease stage†
|
Overall survival (months) | |||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||
| <5 | 2 | 0/2 | 2 | 0 | 0 | 0 | 117 ± 41 |
| 6 to 25 | 7 | 0/7 | 3 | 0 | 4 | 0 | 58 ± 31 |
| 26 to 50 | 8 | 1/8 | 1 | 2 | 4 | 1 | 36 ± 14 |
| >50 | 15 | 10/15 | 1 | 0 | 9 | 5 | 18 ± 16 |
Includes all low- and high-grade carcinoma cases in Figure 1B. Excludes borderline tumors.
Significance of difference in percentage of tumor cells with DLX4+ nuclei between patients with ascites versus patients without ascites as determined by Mann-Whitney U-test; P < 0.002.
Correlation between percentage of tumor cells with DLX4+ nuclei and disease stage R = −0.72, as determined by Spearman regression analysis; P < 0.01.
Growth-Promoting Properties of DLX4 in Ovarian Cancer Cells
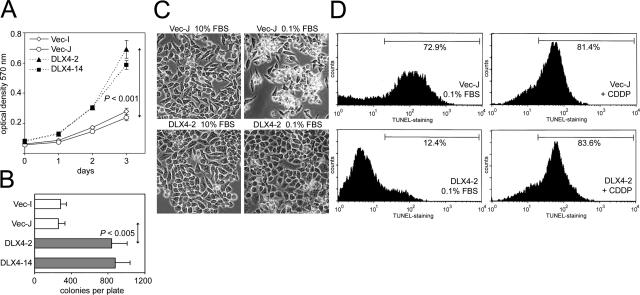
Of the ovarian cancer cell lines screened by RT-PCR and by Western blot, A2780 was only one of two lines that expressed little or no DLX4 (Figure 1, C and D). We therefore used A2780 as a model line to determine the effects of DLX4 on tumor cell growth. Clones were isolated from single colonies of A2780 cells that were stably transfected with DLX4 cDNA cloned into the pIRES-EGFP2 vector and with empty vector. MTT assays revealed that growth of DLX4-transfected A2780 clones was significantly enhanced compared with vector-control cells (P < 0.001) (Figure 2A). Enforced expression of DLX4 also significantly increased colony formation in soft agar (Figure 2B). When cultured under low-serum conditions, vector-control cells underwent extensive cell death as observed by light microscopy and by TUNEL staining (Figure 2, C and D). In contrast, little cell death was observed in cultures of DLX4-transfected cells under low-serum conditions (Figure 2, C and D). However, the susceptibility of DLX4-transfected cells to cisplatin-induced death was very similar to that of vector-control cells (Figure 2D). This indicates that DLX4 promotes survival of tumor cells under conditions of growth factor deprivation but does not protect cells from other forms of cell death.
Figure 2.
Effect of DLX4 on tumor cell growth in vitro. A: Growth rates of A2780 cell lines stably transfected with empty pIRES-EGFP2 vector (Vec-I, Vec-J) and with DLX4 (DLX4-2, DLX4-14) as measured by MTT assays. Shown are the results of triplicate assays. B: Colony-forming ability of stably transfected A2780 cell lines in soft agar. C: A2780 cell lines stably transfected with empty vector (Vec-J) and with DLX4 (DLX4-2) were cultured in media containing 10% FBS and 0.1% FBS for 3 days and viewed under phase-contrast microscopy. D: TUNEL staining of cells of transfected A2780 lines after 3 days of incubation under low-serum conditions (left) and with treatment with cisplatin (CDDP) (right).
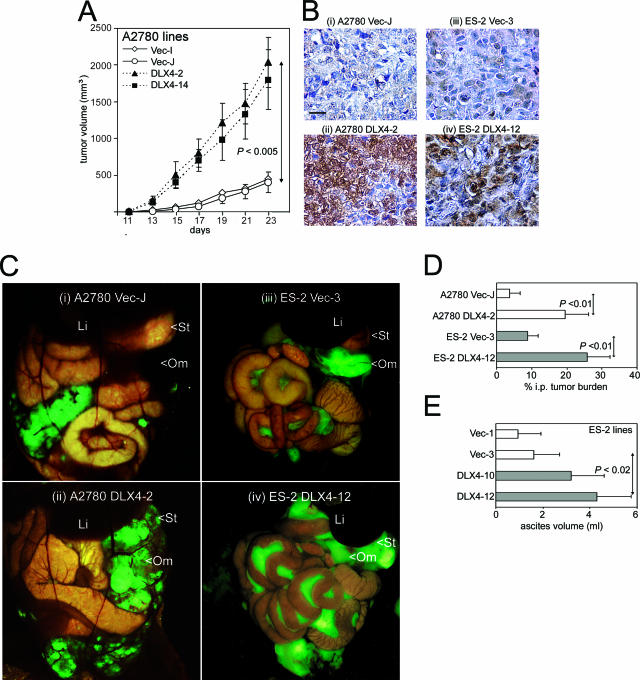
The ability of DLX4 to promote tumor growth was confirmed in mouse xenograft models. As shown in Figure 3A, growth rates of tumors that formed in female nude mice inoculated subcutaneously with cells of DLX4-transfected A2780 lines were significantly higher than growth rates of subcutaneous tumors that formed after inoculation with equivalent numbers of vector-control cells (P < 0.005). Sections of tumor tissues were stained with DLX4 Ab to confirm that enforced DLX4 expression was maintained in tumors (Figure 3B). We also determined the ability of DLX4 to promote progression of tumors propagated intraperitoneally in mice by visualizing GFP-expressing tumors under a fluorescence stereoscope (Figure 3C) and quantifying tumor burden by measuring areas of fluorescence signals within the abdominal cavity (Figure 3D). The total tumor burden in mice inoculated intraperitoneally with DLX4-expressing A2780 cells was markedly greater than in mice inoculated with equivalent numbers of vector-control cells (Figure 3C, compare ii with i). In particular, tumor involvement of the omentum was more extensive in mice with DLX4-expressing tumors than in mice with vector-control tumors (Figure 3Cii). The ability of DLX4 to promote tumor progression was confirmed by using additional models that were generated by stably transfecting the ES-2 ovarian cancer cell line with DLX4 and with empty vector. Fluorescence imaging revealed substantially greater tumor burden, particularly throughout the mesentery, in mice inoculated with DLX4-transfected ES-2 cells than in mice inoculated with equivalent numbers of vector-control cells (Figure 3C, compare iv with iii). In addition, ascites formation was greater in mice inoculated with DLX4-transfected ES-2 cells than in mice inoculated with vector-control cells (P < 0.02) (Figure 3E).
Figure 3.
Effect of DLX4 on tumor progression in mice. A: Growth rates of subcutaneous tumors in female nude mice inoculated with equivalent numbers (3 × 106) of cells of stably transfected A2780 lines. B: Immunohistochemical staining of DLX4 in tumors collected from mice inoculated with vector-control (Vec-J) (i), DLX4-transfected (DLX4-2) A2780 (ii), vector-control (Vec-3) (iii), and DLX4-transfected (DLX4-12) ES-2 (iv) cells. C: Visualization under a fluorescence stereoscope of GFP-expressing intraperitoneal tumors in the abdominal cavities of mice inoculated with A2780 Vec-J (i), A2780 DLX4-2 (ii), ES-2 Vec-3 (iii), and ES-2 DLX4-12 (iv) cells. Indicated are the liver (Li), stomach (St), and omentum (Om). D: Quantification of intraperitoneal tumor burden in mice expressed as the percentage of area of fluorescence within the abdominal cavity in captured images. E: Volume of ascites collected from mice at 18 days after inoculation with equivalent numbers (1 × 106) of cells of vector-control ES-2 lines (Vec-1, Vec-3) and DLX4-transfected ES-2 lines (DLX4-10, DLX4-12). Scale bar = 20 μm.
DLX4 Expression in Ovarian Cancer Cells Leads to Increased Endothelial Cell Growth
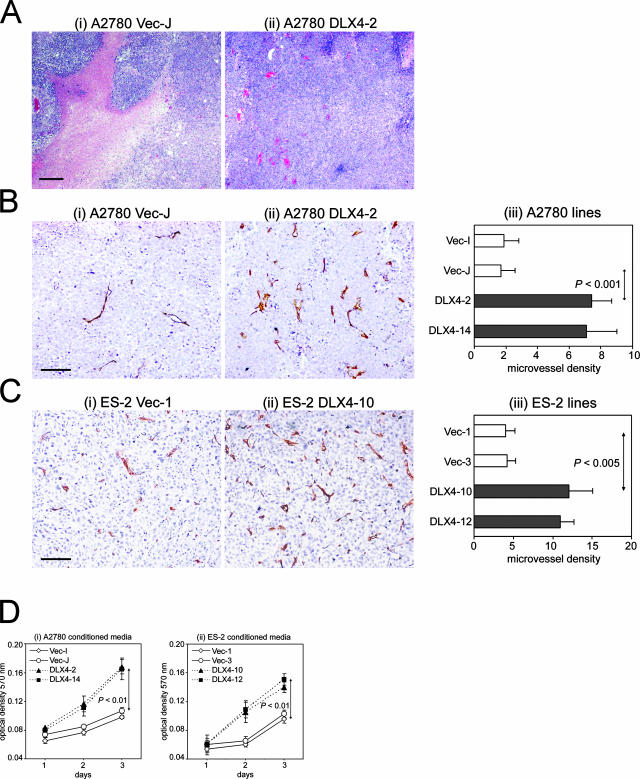
Histopathological examination of tissues collected from host mice revealed that tumors that formed from vector-control A2780 cells were poorly vascularized and contained extensive necrosis (Figure 4Ai). In contrast, tumors that formed from A2780 cells that stably expressed DLX4 were well vascularized (Figure 4Aii). We confirmed these findings by determining microvessel density in A2780 cell line-derived tumors stained with Abs to CD31 and CD34 (Figure 4B). In addition, microvessel density was determined in tumors that derived from vector-control and DLX4-overexpressing ES-2 cell lines (Figure 4C). Microvessel density was determined within subcutaneous tumors and nodules attached to the peritoneal lining of the abdominal cavity wall because vessels within these tumors would be more likely to represent tumor-associated vessels, whereas tumors attached to the broad ligament, mesentery, and omentum might also involve normal vessels of these tissues. Microvessel density in tumors that derived from A2780 and ES-2 lines that overexpressed DLX4 was approximately threefold higher than in tumors that derived from the respective vector-control lines (Figure 4, B and C).
Figure 4.
Effect of DLX4 expression in tumors on microvessel density and endothelial cell growth. A: H&E-stained sections of tumors in mice inoculated with vector-control (Vec-J) (i) and DLX4-transfected (DLX4-2) (ii) A2780 cells. B: CD34 staining of subcutaneous tumors derived from A2780 cell lines (i, ii). In iii, microvessel density was determined by counting the number of vessels in five separate 0.4-mm2 fields in stained sections of tumors. C: CD34 staining of peritoneal implants derived from ES-2 cell lines (i, ii) and quantification of microvessel density (iii). D: Growth rates of mouse endothelial cells stimulated with conditioned media of vector-control and DLX4-transfected A2780 (i) and ES-2 (ii) cell lines, as measured by MTT assay. Scale bars: 200 μm (A); 100 μm (B, C).
To determine whether DLX4-expressing tumor cells release factors that stimulate endothelial cell growth, we compared the ability of conditioned media collected from empty vector- and DLX4-transfected ovarian cancer cell lines to stimulate growth of endothelial cells. To minimize the effect of DLX4 on tumor cell proliferation, conditioned medium was collected from transfected cells at 1 day after seeding when little difference in tumor cell proliferation was observed and volumes were normalized to reflect equivalent numbers of tumor cells at the time of medium collection (ie, 1 ml of medium equivalent to 1 × 105 tumor cells). Conditioned medium of DLX4-transfected A2780 cells was more effective than conditioned medium of vector-control A2780 cells in stimulating proliferation of mouse ovarian-derived endothelial cells (Figure 4Di). The stimulatory effect on endothelial cell growth of conditioned medium of DLX4-transfected tumor cells was confirmed using the ES-2 model cell lines (Figure 4Dii).
DLX4 Induces Expression of VEGF
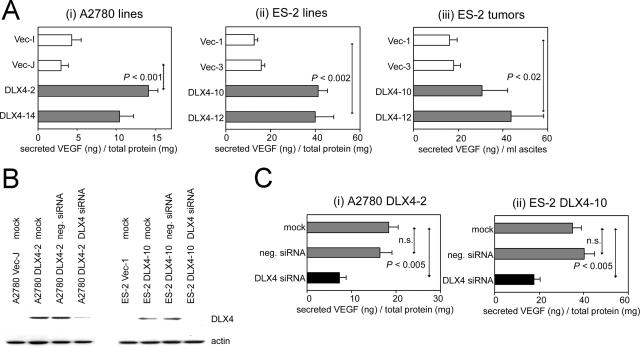
Because overexpression of DLX4 in ovarian cancer cells led to increases in endothelial cell growth and microvessel density within tumors, we investigated whether DLX4 induces expression of VEGF. Levels of VEGF in culture supernatants of DLX4-expressing A2780 cells were threefold higher than that of vector-control cells (Figure 5Ai). A similar induction in VEGF levels by DLX4 was observed in ES-2 cells (Figure 5Aii). Because VEGF levels in culture supernatants were normalized to the total cellular protein content, we can exclude the possibility that the increased VEGF level in culture supernatants of DLX4-transfected cells was attributable to an increase in cell number. Levels of human tumor-derived VEGF in ascites collected from mice inoculated with DLX4-transfected ES-2 cells was also markedly higher than that of mice with vector-control ES-2 tumors (Figure 5Aiii). To confirm our findings, we determined the effect on VEGF levels of inhibiting DLX4 expression in the A2780 and ES-2 cell lines using an siRNA oligomer that targets DLX4 (Figure 5B). VEGF levels were markedly reduced in culture supernatants of cells in which DLX4 was effectively knocked down by DLX4-specific siRNA but not in cells treated with negative control siRNA (Figure 5C).
Figure 5.
Induction of VEGF expression by DLX4. A: Levels of VEGF in culture supernatants of A2780 (i) and ES-2 (ii) cell lines at 24 hours after seeding, as measured by ELISA and normalized to the total cellular protein content, and in ascites (iii) collected from mice at 18 days after inoculation with the indicated stably transfected ES-2 cell lines. B: Western blot of DLX4 expression in A2780 DLX4-2 cells and in ES-2 DLX4-10 cells, after treatment with DLX4-specific siRNA, negative control siRNA (neg.) and with no siRNA (mock). C: Levels of VEGF in supernatants of A2780 DLX4-2 (i) and ES-2 DLX4-10 (ii) cells after mock and siRNA treatment.
Induction of FGF-2 Expression by DLX4 and Its Effect on VEGF Levels
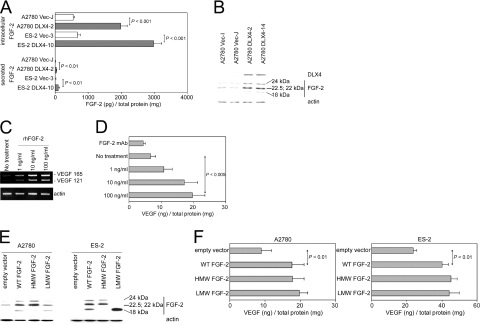
In addition to VEGF, FGF-2 is a major angiogenic factor produced by ovarian cancers that also acts as a growth factor for tumor cells.23,24,25 Because we observed increased growth of DLX4-expressing tumor cells in vitro and in mice as well as increased microvessel density, we investigated whether DLX4 induces expression of FGF-2. Levels of FGF-2 in culture supernatants of DLX4-transfected cells and intracellular FGF-2 levels were threefold to fourfold higher than those of vector-control cells (Figure 6A). Nearly 95% of the total FGF-2 produced by vector-control and DLX4-transfected cells was intracellular (Figure 6A). Western blot analysis revealed that enforced expression of DLX4 induced levels of several isoforms of FGF-2 (Figure 6B). These included the LMW 18-kd isoform that is cytosolic and also secreted and several HMW isoforms (22, 22.5, 24 kd) that are generated from alternative initiation of translation at CUG codons and are predominantly localized in the nucleus.21,26
Figure 6.
Induction of FGF-2 expression by DLX4 and effect of FGF-2 on VEGF levels. A: FGF-2 levels in cell lysates and culture supernatants of stably transfected A2780 and ES-2 cell lines at 48 hours after seeding, as measured by ELISA and normalized to the total cellular protein content. B: Western blot analysis of DLX4 and different isoforms of FGF-2 in lysates of vector-control and DLX4-transfected A2780 cell lines. C: RT-PCR analysis of VEGF in parental A2780 cells after stimulation for 2 days with 0, 1, 10, and 100 ng/ml rhFGF-2. D: VEGF levels in culture supernatants of parental A2780 cells left unstimulated, stimulated with rhFGF-2, and treated with neutralizing FGF-2 Ab (10 μg/ml) for 2 days. E: Western blot analysis of FGF-2 in lysates of parental A2780 and ES-2 cells that were transfected with empty pIRES-EGFP2 vector, wild-type (WT) FGF-2 cDNA, and cDNAs encoding the HMW and LMW isoforms of FGF-2. F: VEGF levels in culture supernatants collected from A2780 and ES-2 cells at 2 days after transfection with cDNAs encoding the different FGF-2 isoforms.
Addition of exogenous FGF-2 has been reported to increase VEGF levels in several cell types including endothelial cells and breast cancer cells.27,28 This prompted us to investigate whether FGF-2 could induce VEGF expression in ovarian cancer cells. Stimulation of parental A2780 cells with rhFGF-2 induced dose-dependent increases in VEGF transcripts (Figure 6C) and in levels of VEGF protein (Figure 6D). Treatment of cells with FGF-2-neutralizing antibody modestly decreased VEGF levels (Figure 6D). This modest inhibition could be attributable to the low levels of FGF-2 that are normally secreted by A2780 cells (Figure 6A). Because the LMW and HMW isoforms of FGF-2 can induce different cellular phenotypes,29,30,31 we determined whether VEGF expression was solely induced by the LMW isoform. To address this question, we transfected A2780 and ES-2 cells with cDNAs encoding the LMW isoform, the three HMW isoforms, and both the LMW and HMW isoforms (WT). Expression of these isoforms was confirmed by Western blot (Figure 6E). As shown in Figure 6F, VEGF levels in A2780 and in ES-2 cells were increased when the LMW isoform of FGF-2 was overexpressed. Expression of the HMW FGF-2 isoforms also induced VEGF levels (Figure 6F). These results together indicate that VEGF expression can be induced by pathways activated by both the LMW and HMW isoforms of FGF-2 and that DLX4 could induce VEGF levels in ovarian cancer cells, at least in part, via its induction of LMW and HMW FGF-2 isoforms.
Discussion
Although 5-year survival rates can approach 90% for women diagnosed with tumor confined to the ovaries, 70% of patients present with advanced-stage disease. Despite advances in platinum-taxol regimens, the average 5-year survival rate for women with advanced-stage ovarian cancer is 30%.32 The most widely recognized behavior of ovarian cancer is the formation of ascites and seeding of the abdominal cavity with nests of tumor cells. The degree of tumor vascularization is a strong predictor of patient outcome.33 VEGF and FGF-2 exert distinct effects on vessel functionality and maturation and stimulate vascularization synergistically.34 In this study, we found DLX4 expression in ovarian carcinomas to be strongly associated with high tumor grade and to correlate with disease stage. The association of DLX4 overexpression with these indicators of poor prognosis is highly consistent with our findings that overexpression of DLX4 in ovarian cancer cells induces levels of VEGF and FGF-2, stimulates endothelial cell growth, and leads to increased microvessel density in tumors.
In addition to its potent angiogenic property, VEGF is a causative factor of ascites formation through its induction of vascular permeability.35,36 The ability of DLX4 to induce expression of VEGF might explain the higher frequency of ascites in patients with tumors that highly express DLX4 and also the increased formation of ascites in mice with DLX4-expressing ES-2 tumors. However, because DLX4 is able to stimulate growth of ovarian cancer cells independently of its effects on endothelial cells (Figure 2), it is possible that increased ascites accumulation might not be a direct effect of DLX4 but rather is associated with the advanced disease in patients and in mice with DLX4-overexpressing tumors. It is interesting to note that mice inoculated intraperitoneally with parental A2780 cells do not typically develop ascites and that overexpression of DLX4 in these cells, although able to induce VEGF levels, did not result in ascites. Manenti and colleagues37 reported formation of ascites in mice inoculated intraperitoneally with A2780 cells that were stably transfected with VEGF. It should be noted that the levels of VEGF released by transfected A2780 cells in that study were very high (averaging 12,790 pg/ml), whereas levels released by our DLX4-transfected A2780 cell lines under the same culture conditions as the previous study were 200 to 250 pg/ml. It is possible that the VEGF level induced by DLX4 in our A2780 xenograft models is insufficient to induce ascites and that VEGF levels required to induce ascites might vary depending on the individual tumor. Indeed, although elevated VEGF levels often correlate with the presence of ascites, low VEGF levels have been detected in some patients with ascites and high levels in others without ascites.38 Although a direct effect of DLX4 on ascites is not supported by our findings, our study strongly supports a role for DLX4 in promoting tumor progression by stimulating endothelial cell growth and vascularization that are mediated at least in part by its ability to induce VEGF. The induction of VEGF by DLX4 might also promote tumor dissemination. VEGF has been reported to promote invasive behavior of ovarian cancer cells by stimulating secretion of matrix metalloproteinases (MMPs), in particular the activated form of MMP-2.39 In preliminary studies, we have observed higher levels of the 62-kd activated form of MMP-2 in conditioned medium of DLX4-overexpressing ES-2 cell lines as compared with medium conditioned by equivalent numbers of vector-control ES-2 cells (R.R.L., F.H., and H.N., unpublished data). However, activation of MMP-2 by DLX4 was not observed in A2780 cells, a cell line that normally does not express VEGF receptors.40
Our results indicate that one mechanism by which DLX4 could induce VEGF expression in ovarian cancer cells is indirectly through the induction by DLX4 of FGF-2 expression. FGF-2 also induces mesothelial cells of the omentum, a primary site of ovarian cancer cell implantation, to express VEGF.41 In addition, FGF-2 induces VEGF expression in endothelial cells.27 Activation of the mitogen-activated protein kinase pathway by FGF-2 leads to activation of HIF-1α, a major transcriptional regulator of the VEGF gene.28 However, the possibility cannot be excluded that DLX4 could induce VEGF expression by mechanisms independent of its induction of FGF-2. The induction of FGF-2 by DLX4 could also significantly affect several other important processes. Gene expression profiling of advanced-stage ovarian cancers has revealed a distinct molecular profile enriched for genes encoding components of the extracellular matrix and the FGF-2 signaling pathway.25 This molecular profile was found to overlap extensively with a mesenchymal-like signature and to correspond with the ability of FGF-2 to promote cell migration.25 The ability of DLX4 to up-regulate FGF-2 could explain at least in part the increased dissemination of intraperitoneal tumors that overexpress DLX4. This induction of FGF-2 could also explain the enhanced growth of DLX4-transfected cells, and the protective effect of DLX4 on cells under growth factor-deprived conditions (Figure 2). Considerable evidence indicates that FGF-2-mediated growth stimulation and cell survival occurs in a wide variety of cell types via an intracrine mechanism involving the HMW FGF-2 isoforms, whereas the 18-kd isoform primarily promotes cell migration.29,30,31 These studies imply that the LMW and HMW isoforms regulate distinct pathways. However, the observed ability of both LMW and HMW isoforms to induce VEGF expression in our study suggests the existence of at least some common mechanisms. Although DLX4 induced expression of both the LMW and HMW isoforms, the high abundance of intracellular FGF-2 suggests that the induction of FGF-2 by DLX4 might affect tumor cell growth more greatly than cell migration.
Most of the aberrantly expressed homeobox genes studied in tumors to date have been found to enhance self-renewal, cell proliferation, and survival.2,3 Several homeobox genes control normal endothelial cell growth and maturation.42 The only other homeobox gene that has been linked to enhanced vascularity in tumors is HOXB7.43 The expression and function of DLX4 is intriguing in its similarity to HOXB7 and might reflect the evolutionary relationship of linked HOX and DLX gene clusters.44 Both DLX4 and HOXB7 are widely overexpressed in ovarian cancers and promote cell proliferation.12 HOXB7 is also overexpressed in breast cancers and has been found in breast cancer cells to up-regulate levels of VEGF and FGF-2.15,43 HOXB7 has been demonstrated in melanoma cells to activate transcription of the FGF-2 gene.45 Our preliminary studies indicate that DLX4 activates transcription of the FGF-2 gene in ovarian cancer cells (F.H. and H.N., unpublished data). Although homeodomain proteins are widely regarded to function as transcription factors, only few direct downstream targets have been identified. This stems from the promiscuous DNA-binding specificities of homeodomain proteins. Given this promiscuity, DLX4 might also activate FGF-2 gene transcription through the same regulatory element as HOXB7, but this remains to be determined.
In this study, 15 of the 32 ovarian carcinoma cases (47%) were found to exhibit extensive DLX4 expression (ie, >50% of cells with nuclear staining), whereas amplification of the chromosomal hot-spot to which DLX4 maps is less prevalent.10,11 This indicates that gene amplification is not the sole mechanism underlying the overexpression of DLX4. Various fibroblast growth factors including FGF-2 can induce or maintain expression of DLX genes in specific tissues during embryonic development.46,47 Because we found that DLX4 induces FGF-2 expression in ovarian cancer cells, one intriguing possibility might be that DLX4 represents a component of an FGF-2 autocrine loop. However, it remains to be determined whether physiological levels of FGF-2 expressed by ovarian tumors are capable of inducing DLX4. Another question that remains to be resolved is whether DLX4 might promote progression of other types of cancer by similar mechanisms. DLX4 is also overexpressed in choriocarcinomas, and inhibition of DLX4 expression in choriocarcinoma cells has been reported to induce apoptosis.48 As we observed a protective effect of DLX4 in ovarian cancer cells under growth factor-deprived conditions, DLX4 might promote cell survival in various cell types. It is difficult to speculate how the role of DLX4 in promoting tumor progression is related to its normal embryonic function, because the latter is not known. DLX genes control many aspects of embryonic morphogenesis, including patterning of the branchial arch skeleton; bone and cartilage formation; and development of the brain, sensory organs, and limbs.44 DLX4 expression is absent from most normal adult tissues but is expressed in the endometrium and placenta.49,50 Because these two tissues are particularly well vascularized, it is possible that DLX4 plays an important role in the functional development of these tissues by inducing an angiogenic molecular program.
Homeobox genes, like the Hedgehog and Wnt signaling pathways, have emerged as important regulators of embryonic patterning that, when aberrantly expressed, significantly contribute to tumor progression. In addition to studies that indicate roles of various aberrantly expressed homeobox genes in self-renewal, cell proliferation, and survival,2,3 several recent studies implicate deregulation of other homeobox genes in promoting tumor cell invasiveness.8,13,14,15 Furthermore, we have found that aberrant activation of HOX genes that normally regulate developmental patterning of the reproductive tract gives rise to the unique histological differentiation patterns of the major subtypes of ovarian cancer.51 The present study supports increasing evidence that homeobox genes can contribute to tumor progression by deregulating a spectrum of other processes—including induction of an angiogenic molecular program. Further studies of the pathways controlled by this intriguing class of developmental regulators will provide important insights into the molecular control of tumor behavior.
Acknowledgments
We thank Kimberly Head, Sabine Thonard, and Jie Feng for technical assistance.
Footnotes
Address reprint requests to Honami Naora, Ph.D., University of Texas M.D. Anderson Cancer Center, Department of Molecular Therapeutics, Unit 950, 7435 Fannin St., South Campus Research Building II, Rm 3.2028, Houston, TX 77054. E-mail: hnaora@mdanderson.org.
Supported by the US Department of Defense (grant W81XWH-06-1-0259 to H.N.).
References
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- Samuel S, Naora H. Homeobox gene expression in cancer: insights from developmental regulation and deregulation. Eur J Cancer. 2005;41:2428–2437. doi: 10.1016/j.ejca.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Raman V, Martensen SA, Reisman D, Evron E, Odenwald WF, Jaffee E, Marks J, Sukumar S. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature. 2000;405:974–978. doi: 10.1038/35016125. [DOI] [PubMed] [Google Scholar]
- Coletta RD, Christensen K, Reichenberger KJ, Lamb J, Micomonaco D, Huang L, Wolf DM, Muller-Tidow C, Golub TR, Kawakami K, Ford HL. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci USA. 2004;101:6478–6483. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert-Buck MR, Vocke CD, Pozzatti RO, Duray PH, Jennings SB, Florence CD, Zhuang Z, Bostwick DG, Liotta LA, Linehan WM. Allelic loss on chromosome 8p12-21 in microdissected prostatic intraepithelial neoplasia. Cancer Res. 1995;55:2959–2962. [PubMed] [Google Scholar]
- He WW, Sciavolino PJ, Wing J, Augustus M, Hudson P, Meissner PS, Curtis RT, Shell BK, Bostwick DG, Tindall DJ, Gelmann EP, Abate-Shen C, Carter KC. A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer. Genomics. 1997;43:69–77. doi: 10.1006/geno.1997.4715. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Broaddus R, Cheng W, Xie S, Naora H. Deregulation of the HOXA10 homeobox gene in endometrial carcinoma: role in epithelial-mesenchymal transition. Cancer Res. 2006;66:889–897. doi: 10.1158/0008-5472.CAN-05-2828. [DOI] [PubMed] [Google Scholar]
- Hyman E, Kauraniemi P, Hautaniemi S, Wolf M, Mousses S, Rozenblum E, Ringner M, Sauter G, Monni O, Elkahloun A, Kallioniemi OP, Kallioniemi A. Impact of DNA amplification on gene expression patterns in breast cancer. Cancer Res. 2002;62:6240–6245. [PubMed] [Google Scholar]
- Watanabe T, Imoto I, Kosugi Y, Ishiwata I, Inoue S, Takayama M, Sato A, Inazawa J. A novel amplification at 17q21-23 in ovarian cancer cell lines detected by comparative genomic hybridization. Gynecol Oncol. 2001;81:172–177. doi: 10.1006/gyno.2001.6132. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Saito-Ohara F, Inoue J, Aoki D, Susumu N, Yokoyama T, Nozawa S, Inazawa J, Imoto I. Association of 17q21–q24 gain in ovarian clear cell adenocarcinomas with poor prognosis and identification of PPM1D and APPBP2 as likely amplification targets. Clin Cancer Res. 2003;9:1995–2004. [PubMed] [Google Scholar]
- Naora H, Yang YQ, Montz FJ, Seidman JD, Kurman RJ, Roden RB. A serologically identified tumor antigen encoded by a homeobox gene promotes growth of ovarian epithelial cells. Proc Natl Acad Sci USA. 2001;98:4060–4065. doi: 10.1073/pnas.071594398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XJ, Wang Z, Ryan PD, Isakoff SJ, Barmettler A, Fuller A, Muir B, Mohapatra G, Salunga R, Tuggle JT, Tran Y, Tran D, Tassin A, Amon P, Wang W, Wang W, Enright E, Stecker K, Estepa-Sabal E, Smith B, Younger J, Balis U, Michaelson J, Bhan A, Habin K, Baer TM, Brugge J, Haber DA, Erlander MG, Sgroi DC. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5:607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tazawa S, Yawei Z, Katayama H, Kato Y, Nishiwaki K, Yokohama Y, Ishikawa M. Suppression of invasive characteristics by antisense introduction of overexpressed HOX genes in ovarian cancer cells. Int J Oncol. 2006;28:931–938. [PubMed] [Google Scholar]
- Wu X, Chen H, Parker B, Rubin E, Zhu T, Lee JS, Argani P, Sukumar S. HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res. 2006;66:9527–9534. doi: 10.1158/0008-5472.CAN-05-4470. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Stock DW, Wydner KL, Bollekens JA, Takeshita K, Nagai BM, Chiba S, Kitamura T, Freeland TM, Zhao Z, Minowada J, Lawrence JB, Weiss KM, Ruddle FH. Genomic analysis of a new mammalian distal-less gene: Dlx7. Genomics. 1996;38:314–324. doi: 10.1006/geno.1996.0634. [DOI] [PubMed] [Google Scholar]
- Morasso MI, Yonescu R, Griffin CA, Sargent TD. Localization of human DLX8 to chromosome 17q21.3-q22 by fluorescence in situ hybridization. Mamm Genome. 1997;8:302–303. doi: 10.1007/s003359900427. [DOI] [PubMed] [Google Scholar]
- Chase MB, Fu S, Haga SB, Davenport G, Stevenson H, Do K, Morgan D, Mah AL, Berg PE. BP1: a homeodomain-containing isoform of DLX4, represses the beta-globin gene. Mol Cell Biol. 2002;22:2505–2514. doi: 10.1128/MCB.22.8.2505-2514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2Kb-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63:2971–2976. [PubMed] [Google Scholar]
- Haga SB, Fu S, Karp JE, Ross DD, Williams DM, Hankins WD, Behm F, Ruscetti FW, Chang M, Smith BD, Becton D, Raimondi SC, Berg PE. BP1, a new homeobox gene, is frequently expressed in acute leukemias. Leukemia. 2000;14:1867–1875. doi: 10.1038/sj.leu.2401912. [DOI] [PubMed] [Google Scholar]
- Quarto N, Talarico D, Sommer A, Florkiewicz R, Basilico C, Rifkin DB. Transformation by basic fibroblast growth factor requires high levels of expression: comparison with transformation by hst/K-fgf. Oncogene Res. 1989;5:101–110. [PubMed] [Google Scholar]
- Zoueva OP, Rodgers GP. Inhibition of beta protein 1 expression enhances beta-globin promoter activity and beta-globin mRNA levels in the human erythroleukemia (K562) cell line. Exp Hematol. 2004;32:700–708. doi: 10.1016/j.exphem.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Barton DP, Cai A, Wendt K, Young M, Gamero A, De Cesare S. Angiogenic protein expression in advanced epithelial ovarian cancer. Clin Cancer Res. 1997;3:1579–1586. [PubMed] [Google Scholar]
- Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- De Cecco L, Marchionni L, Gariboldi M, Reid JF, Lagonigro MS, Caramuta S, Ferrario C, Bussani E, Mezzanzanica D, Turatti F, Delia D, Daidone MG, Oggionni M, Bertuletti N, Ditto A, Raspagliesi F, Pilotti S, Pierotti MA, Canevari S, Schneider C. Gene expression profiling of advanced ovarian cancer: characterization of a molecular signature involving fibroblast growth factor 2. Oncogene. 2004;23:8171–8183. doi: 10.1038/sj.onc.1207979. [DOI] [PubMed] [Google Scholar]
- Bugler B, Amalric F, Prats H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol Cell Biol. 1991;11:573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YH, Wang YX, Bingle L, Gong LH, Heng WJ, Li Y, Fang WG. In vitro study of HIF-1 activation and VEGF release by bFGF in the T47D breast cancer cell line under normoxic conditions: involvement of PI-3K/Akt and MEK1/ERK pathways. J Pathol. 2005;205:530–536. doi: 10.1002/path.1734. [DOI] [PubMed] [Google Scholar]
- Bikfalvi A, Klein S, Pintucci G, Quarto N, Mignatti P, Rifkin DB. Differential modulation of cell phenotype by different molecular weight forms of basic fibroblast growth factor: possible intracellular signaling by the high molecular weight forms. J Cell Biol. 1995;129:233–243. doi: 10.1083/jcb.129.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner S, Touriol C, Galy B, Audigier S, Gensac MC, Amalric F, Bayard F, Prats H, Prats AC. Translation of CUG- but not AUG-initiated forms of fibroblast growth factor 2 is activated in transformed and stressed cells. J Cell Biol. 1996;135:1391–1402. doi: 10.1083/jcb.135.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Bikfalvi A, Birkenmeier TM, Giancotti FG, Rifkin DB. Integrin regulation by endogenous expression of 18-kDa fibroblast growth factor-2. J Biol Chem. 1996;271:22583–22590. doi: 10.1074/jbc.271.37.22583. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Hollingsworth HC, Kohn EC, Steinberg SM, Rothenberg ML, Merino MJ. Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol. 1995;147:33–41. [PMC free article] [PubMed] [Google Scholar]
- Giavazzi R, Sennino B, Coltrini D, Garofalo A, Dossi R, Ronca R, Tosatti MP, Presta M. Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis. Am J Pathol. 2003;162:1913–1926. doi: 10.1016/S0002-9440(10)64325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang N, Garcia JR, Mohamed A, Benencia F, Rubin SC, Allman D, Coukos G. Generation of a syngeneic mouse model to study the effects of vascular endothelial growth factor in ovarian carcinoma. Am J Pathol. 2002;161:2295–2309. doi: 10.1016/s0002-9440(10)64505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AT, Ross L, Holash J, Nakanishi M, Hu L, Hofmann JI, Yancopoulos GD, Jaffe RB. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res. 2003;9:5721–5728. [PubMed] [Google Scholar]
- Manenti L, Riccardi E, Marchini S, Naumova E, Floriani I, Garofalo A, Dossi R, Marrazzo E, Ribatti D, Scanziani E, Bani M, Belotti D, Broggini M, Giavazzi R. Circulating plasma vascular endothelial growth factor in mice bearing human ovarian carcinoma xenograft correlates with tumor progression and response to therapy. Mol Cancer Ther. 2005;4:715–725. doi: 10.1158/1535-7163.MCT-04-0305. [DOI] [PubMed] [Google Scholar]
- Cooper BC, Ritchie JM, Broghammer CL, Coffin J, Sorosky JI, Buller RE, Hendrix MJ, Sood AK. Preoperative serum vascular endothelial growth factor levels: significance in ovarian cancer. Clin Cancer Res. 2002;8:3193–3197. [PubMed] [Google Scholar]
- Wang FQ, So J, Reierstad S, Fishman DA. Vascular endothelial growth factor-regulated ovarian cancer invasion and migration involves expression and activation of matrix metalloproteinases. Int J Cancer. 2006;118:879–888. doi: 10.1002/ijc.21421. [DOI] [PubMed] [Google Scholar]
- Vikhanskaya F, Bani MR, Borsotti P, Ghilardi C, Ceruti R, Ghisleni G, Marabese M, Giavazzi R, Broggini M, Taraboletti G. p73 overexpression increases VEGF and reduces thrombospondin-1 production: implications for tumor angiogenesis. Oncogene. 2001;20:7293–7300. doi: 10.1038/sj.onc.1204896. [DOI] [PubMed] [Google Scholar]
- Sako A, Kitayama J, Yamaguchi H, Kaisaki S, Suzuki H, Fukatsu K, Fujii S, Nagawa H. Vascular endothelial growth factor synthesis by human omental mesothelial cells is augmented by fibroblast growth factor-2: possible role of mesothelial cells on the development of peritoneal metastasis. J Surg Res. 2003;115:113–120. doi: 10.1016/s0022-4804(03)00307-x. [DOI] [PubMed] [Google Scholar]
- Gorski DH, Walsh K. The role of homeobox genes in vascular remodeling and angiogenesis. Circ Res. 2000;87:865–872. doi: 10.1161/01.res.87.10.865. [DOI] [PubMed] [Google Scholar]
- Carè A, Felicetti F, Meccia E, Bottero L, Parenza M, Stoppacciaro A, Peschle C, Colombo MP. HOXB7: a key factor for tumor-associated angiogenic switch. Cancer Res. 2001;61:6532–6539. [PubMed] [Google Scholar]
- Panganiban G, Rubenstein JL. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002;129:4371–4386. doi: 10.1242/dev.129.19.4371. [DOI] [PubMed] [Google Scholar]
- Carè A, Silvani A, Meccia E, Mattia G, Stoppacciaro A, Parmiani G, Peschle C, Colombo MP. HOXB7 constitutively activates basic fibroblast growth factor in melanomas. Mol Cell Biol. 1996;16:4842–4851. doi: 10.1128/mcb.16.9.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen LM, Bryant SV, Torok MA, Blumberg B, Gardiner DM. Nerve dependency of regeneration: the role of Distal-less and FGF signaling in amphibian limb regeneration. Development. 1996;122:3487–3497. doi: 10.1242/dev.122.11.3487. [DOI] [PubMed] [Google Scholar]
- Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development. 1998;125:4325–4333. doi: 10.1242/dev.125.21.4325. [DOI] [PubMed] [Google Scholar]
- Sun Y, Lu X, Yin L, Zhao F, Feng Y. Inhibition of DLX4 promotes apoptosis in choriocarcinoma cell lines. Placenta. 2006;27:375–383. doi: 10.1016/j.placenta.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Quinn LM, Latham SE, Kalionis B. A distal-less class homeobox gene, DLX4, is a candidate for regulating epithelial-mesenchymal cell interactions in the human placenta. Placenta. 1998;19:87–93. doi: 10.1016/s0143-4004(98)90103-5. [DOI] [PubMed] [Google Scholar]
- Quinn LM, Kilpatrick LM, Latham SE, Kalionis B. Homeobox genes DLX4 and HB24 are expressed in regions of epithelial-mesenchymal cell interaction in the adult endometrium. Mol Hum Reprod. 1998;4:497–501. doi: 10.1093/molehr/4.5.497. [DOI] [PubMed] [Google Scholar]
- Cheng W, Liu J, Yoshida H, Rosen D, Naora H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11:531–537. doi: 10.1038/nm1230. [DOI] [PubMed] [Google Scholar]