Abstract
With the emergence of epidemic Neisseria meningitidis W135 meningitis in Burkina Faso during early 2002, the public health community was faced with the challenge of providing access to an appropriate and affordable vaccine in time for the upcoming 2003 epidemic season.
Recognizing the implications of the emergent threat, the World Health Organization developed a strategy, established a public–private partnership to provide the needed vaccine, and then ensured that a stockpile was available for future use.
The trivalent N meningitidis ACW135 polysaccharide vaccine that resulted is now one of the primary tools for epidemic response in African meningitis belt countries. It will remain so for the foreseeable future and until appropriate and affordable conjugate vaccines become part of national immunization programs in the region.
DURING 2000 AND 2001, AN unusually high number of Neisseria meningitidis W135 meningitis cases were confirmed among Hajj and Umra pilgrims and their contacts.1–3 This coincided with a high number of N meningitidis W135 meningitis cases being confirmed in Burkina Faso and other African meningitis belt countries (countries located in the semi-arid region of sub-Saharan Africa stretching from Senegal to Ethiopia and subject to seasonal meningitis epidemics between November and June; Figure 1▶) toward the end of the 2000–2001 epidemic season (October 2000 to June 2001).4–10 In response, the World Health Organization (WHO) and its partners reiterated recommendations for the vaccination of pilgrims and chemoprophylaxis for case contacts and worked to reinforce meningococcal disease surveillance, especially in African meningitis belt countries.
FIGURE 1—
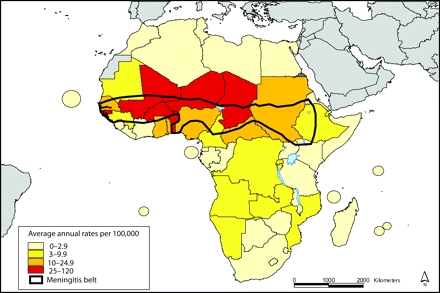
Annual meningitis attack rates by country: 1995 to 2003.
Source. World Health Organization.55
In January 2002, the first districtwide meningitis epidemic of the season was detected in Burkina Faso, an African meningitis belt country. By the end of February, 5 epidemic districts had been identified, and reinforced surveillance confirmed an exceptionally high proportion of N meningitidis W135 cases (87%).11,12 The epidemic later spread to 29 of 53 districts, placing more than 7 million persons at risk of disease. During the 2001–2002 epidemic season, a total of 13 735 meningitis cases and 1640 deaths were reported in Burkina Faso with 73% of cases being reported in just 8 weeks during March and April. At the peak of the epidemic, 2172 meningitis cases and 220 deaths were reported to the Ministry of Health in 1 week (week 14; Figure 2▶).13
FIGURE 2—
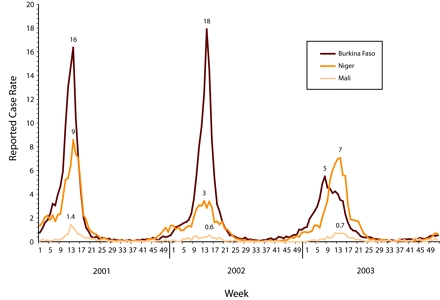
Meningitis surveillance data for weekly reported meningitis cases per 100 000 population from Burkina Faso, Niger, and Mali for 2001–2003.
Source. World Health Organization Multi-disease Surveillance Center, Ouagadougou, Burkina Faso, unpublished data, 2004.
In Africa, the WHO recommends effective surveillance for the early detection and confirmation of meningitis epidemics linked to a response strategy targeted at reducing mortality (provision of antibiotics) and limiting the emergence of disease (mass vaccination, historically with a bivalent polysaccharide vaccine specific for N meningitidis A and C).14,15 Although sporadic cases of N meningitidis W135 meningitis are common in African meningitis belt countries,16–23 the overwhelming predominance of N meningitidis W135 among confirmed cases together with the magnitude of the 2002 meningitis epidemic in Burkina Faso posed a new challenge to epidemic management in the region.
WHO RESPONSE AND STRATEGY
As the N meningitidis W135 meningitis epidemic developed in the first quarter of 2002, the WHO and its partners were quick to recognize the limitations of the existing strategy. In particular, sufficient stocks of an affordable meningococcal vaccine that included an N meningitidis W135 component were not available for mass vaccination campaigns. Their immediate response focused on 3 areas: (1) the early detection and laboratory confirmation of meningitis cases, (2) the provision of antibiotics (oily chloramphenicol) at no cost to patients or their family, and (3) the implementation of mass vaccination campaigns using either bivalent N meningitidis AC polysaccharide vaccine or available stocks of tetravalent N meningitidis ACYW135 polysaccharide vaccine.
Looking to the future, the WHO and others recognized that stocks of an affordable N meningitidis W135 containing meningococcal vaccine had to be secured in time for the upcoming 2002–2003 epidemic season, just 6 months away. With this in mind, the WHO launched a review of currently available meningococcal vaccines and those in the development pipeline.
Although several manufacturers were producing meningococcal vaccines, at that time only Glaxo-SmithKline Biologicals (Rixensart, Belgium) and Aventis Pasteur (now Sanofi Pasteur, Lyon, France) had licensed a meningococcal vaccine with an N meningitidis W135 component, a tetravalent N meningitidis ACYW135 polysaccharide vaccine. The Glaxo-SmithKline Biologicals vaccine was targeted at Hajj and Umra pilgrims and others at risk and, as such, was available across Europe, Africa, the Middle East, and parts of Asia.24 The Aventis Pasteur vaccine was targeted at adolescents and adults in the United States but was not registered for use in other countries.
Discussion with these manufacturers revealed that tetravalent polysaccharide vaccine prices were too high to be affordable for mass vaccination campaigns in African meningitis belt countries. Moreover, there were insufficient stocks of tetravalent polysaccharide vaccine for these campaigns, and neither manufacturer reported sufficient capacity at existing facilities to scale up production quickly. Furthermore, without a robust demand forecast backed by advance purchase commitments, there was little incentive for these manufacturers to invest in additional capacity.
As an alternative, the availability of conjugate meningococcal vaccines was investigated.25,26 The findings were disappointing. Although tetravalent N meningitidis ACYW135 conjugate vaccines were under development by at least 2 manufacturers, they were not yet licensed and were expected to be relatively expensive when made available. In addition to these established manufacturers, the Meningitis Vaccine Project, a partnership between WHO and the Program for Appropriate Technology in Health that was developing an N meningitidis A conjugate vaccine for use in sub-Saharan Africa, was consulted. However, the Meningitis Vaccine Project reviewed the situation and concluded that the situation did not justify a change in their vaccine development strategy.
Toward the end of the second quarter of 2002, the WHO reviewed the situation and organized its vaccine procurement strategies into those for the short term (i.e., the upcoming 2002–2003 and the following 2 epidemic seasons) and those for the long term (i.e., 2005–2006 and beyond). These strategies took into account the high likelihood of continuing seasonal N meningitidis A meningitis epidemics, as well as the risk of a continuing or expanding N meningitidis W135 epidemic with the possibility that N meningitidis W135 could gradually replace N meningitidis A in the future.
After considerable consultation, it was decided the short-term vaccine procurement strategy should focus on facilitating access to appropriate vaccines that were already licensed (i.e., the tetravalent polysaccharide vaccines), and the long-term strategy would focus on gaining access to the conjugate N meningitidis W135 containing vaccines that were under development.
As discussions with tetravalent polysaccharide vaccine manufacturers continued, it became increasingly apparent that already licensed N meningitidis W135 containing vaccines could not be made available. Consequently, the WHO began to discuss appropriate alternatives, such as a monovalent N meningitidis W135 or a trivalent N meningitidis ACW135 polysaccharide vaccine.
Discussions covered a range of issues including the implications of excluding vaccine components found in the tetravalent formulation. The conclusion was that epidemiologic evidence from African meningitis belt countries clearly supported the need for N meningitidis A, C, and W135 components but not the Y component. The implications were that either a monovalent W135 vaccine would have to be developed for use with a bivalent AC vaccine as part of a 2-injection strategy or a new trivalent ACW135 vaccine would have to be developed.
Other discussions also favored a trivalent vaccine. National regulatory authorities indicated that because of its similarity to the bivalent and tetravalent vaccines, trivalent vaccine licensure would be less complex than would that for the monovalent vaccine. The monovalent vaccine would likely require prelicensure trials to evaluate performance when administered at the same time as bivalent vaccine. In addition, early feedback from manufacturers was supportive of a trivalent vaccine.
FACILITATING ACCESS TO THE APPROPRIATE VACCINE
As the WHO gained a better understanding of which vaccine would be most appropriate, it also moved to develop a comprehensive strategy for making this vaccine available to African meningitis belt countries. This strategy addressed the following issues: demand forecasting; production capacity; establishment of a guaranteed market for the vaccine through the simultaneous negotiation of purchase precommitment, price, and financing; licensure; WHO pre-qualification for purchase through UNICEF; and registration in the country of use (see the box on this page).
Key Issues Affecting Access to Meningococcal B Vaccines
Appropriate formulation and presentation
Robust demand forecasting
Production capacity
Purchase precommitment, price, financing
Licensure
WHO prequalification for purchase through UNICEF
Registration in the country of use
Given its proven success, WHO’s first step was to work with the International Coordinating Group (ICG) on Vaccine Provision for Epidemic Meningitis Control27 to plan for a regional vaccine stockpile. The ICG had been established in response to the West African meningococcal epidemic of 1995–1997 and used purchase precommitments to establish regional stockpiles of bivalent N meningitidis AC polysaccharide vaccine, oily chloramphenicol, and other materials.
After this, the demand forecasting, price per dose, and financing could be addressed. The price of a meningococcal vaccine for epidemic response had already been considered in WHO consultations during 2001, and the consensus was that countries and international donors would not tolerate a price higher than US $1 per dose.28 These consultations also indicated that a stockpile for use in African meningitis belt countries should be no smaller than 3 million doses, although scenarios for the spread of the epidemic to neighboring countries indicated that 7 million doses would be needed and even more if populations in northern Nigeria or other regions were affected. With this in mind, WHO sought financing for 7 million doses of vaccine at a target price of US $1 per dose.
To secure financing, donors who traditionally supported mass reactive vaccination campaigns in the African meningitis belt were approached first. However, it soon became apparent that their support was targeted at emergency humanitarian assistance and could be made available only after an epidemic had been detected. This setback forced the WHO to look to other funding sources, including governments and organizations with a preventive view and those with specific interest in providing access to vaccines. The result of this effort was an expression of interest from the Bill and Melinda Gates Foundation (www.gatesfoundation.org).
A CALL TO ACTION
By the third quarter of 2002, the results of enhanced surveillance activities in African meningitis belt countries were being used to plan for the upcoming epidemic season. The region’s health officials were increasingly concerned by the threat posed by the emergence of epidemic N meningitidis W135 disease. Country representatives and others called for action at a WHO technical consultation held in Ouagadougou, Burkina Faso, with African meningitis belt countries, the international community, and the ICG in September 2002. Regional health officials wanted to ensure that an affordable W135-containing meningococcal vaccine would be available in time for the 2003 epidemic season.29,30 At the same time, vaccine manufacturers warned that without an immediate purchase precommitment backed by financing, it was increasingly unlikely that sufficient supplies of an appropriate vaccine could be provided.
By October 2002, WHO was in a position to seek recommendations for a short-term strategy. To facilitate this, an expert panel was convened by teleconference that included representatives from WHO’s African regional office, as well as nongovernmental organizations working in African meningitis belt countries, ICG representatives, national regulatory agencies, procurement agencies, and scientists specializing in meningococcal disease and meningococcal vaccines.
Three key questions were addressed. First, recognizing the emergent threat of N meningitidis W135 meningitis in African meningitis belt countries and the issues related to use of a vaccine in mass vaccination campaigns, which vaccine would be most appropriate in the short term: monovalent, trivalent, or tetravalent polysaccharide vaccine? Second, recognizing the humanitarian need to make available an appropriate vaccine for epidemic response as soon as possible, what would be the requirements for licensure and subsequent use of the recommended vaccine during 2003? Third, what plans should be made to ensure the availability of the recommended vaccine until appropriate new generation conjugate meningococcal vaccines can be used for disease prevention in African meningitis belt countries?
After extensive discussion, the panel recommended a short-term strategy of pursuing the development and licensure of a trivalent N meningitidis ACW135 polysaccharide vaccine, because it offered the needed range of protection, was operationally feasible, and was likely to be licensed without additional preclinical data or delay. WHO communicated this recommendation to vaccine manufacturers and informed them that progress had been made toward financing an initial vaccine stockpile.
GlaxoSmithKline Biologicals responded favorably. It offered to produce a trivalent N meningitidis ACW135 polysaccharide vaccine with the stipulation that it be used exclusively for epidemic response in African meningitis belt countries. Because of limitations with production capacity, its proposal focused on releasing 2 million doses by February 2003 to address any immediate need and an additional 3 million doses later in the year for a stockpile.
Additional discussion on the terms of licensure for the trivalent polysaccharide vaccine was promising. Belgian regulatory authorities indicated that full licensure within the short timeframe was feasible, because extensive experience with the already licensed bivalent and tetravalent polysaccharide vaccines from GlaxoSmithKline Biologicals showed them to be safe and effective. With this, full licensure and export authorization in time for the 2002–2003 epidemic season seemed possible.
Even with this, the WHO expert panel recommended that it would be good public health practice to evaluate the safety and effectiveness of the trivalent vaccine in the context of a mass vaccination campaign, as well as its immunogenicity and reactogenicity. Belgian regulatory authorities agreed. In response, GlaxoSmithKline Biologicals offered the initial trivalent vaccine doses for epidemic response in 2003 and the impact assessment activities at 1 euro per dose at a time when the exchange rate was 1 euro = US $1.10.
FOLLOW THROUGH
With this breakthrough, the WHO established a partnership with GlaxoSmithKline Biologicals focused on the production of a trivalent ACW135 polysaccharide vaccine. Financial support for the purchase of the initial trivalent vaccine doses and the impact assessment activities was provided by the Bill and Melinda Gates Foundation.
From that point, the project moved ahead quickly. In December 2002, the Belgian national regulatory authority began its review of the trivalent vaccine dossier. In mid-January 2003, marketing authorization for the trivalent vaccine was granted by the Belgian national regulatory, conditioned on the postlicensure evaluation of vaccine safety, effectiveness, immunogenicity, and reactogenicity. In late January 2003, the first 3 lots of trivalent vaccine were released by the Belgian national control laboratory. In early February 2003, the national regulatory authorities of meningitis belt countries were contacted, and filing for licensure was initiated. In mid-February 2003, Burkina Faso issued a 5-year license for use of the trivalent vaccine in mass vaccination campaigns, and approvals from other countries followed soon afterward. In September 2003, after an initial commitment of 1 million euro by Médecins Sans Frontières31 and an appeal from WHO and the ICG members,32 a 6-million dose trivalent vaccine stockpile was established.33 In mid-2005, the trivalent vaccine was prequalified by the WHO for purchase through UNICEF.34
THE 2002–2003 EPIDEMIC SEASON
Given the heightened sense of concern, in preparation for the 2002–2003 epidemic season, national surveillance systems in African meningitis belt countries were reinforced with specific attention to the laboratory confirmation of reported meningitis cases. In addition, a regional surveillance team was established to support national activities and to distribute a weekly regional surveillance report that summarized epidemic activity, the responsible pathogens, and the status of response activities.35
As the 2003 epidemic season got under way, surveillance officers in Burkina Faso became increasingly concerned because the initial weekly disease rates were higher than in the 2000–2001 or even 2001–2002 seasons (Figure 2▶). Although the first district to report epidemic meningitis did not confirm any N meningitidis W135 disease, the subsequent emergence of a mixed N meningitidis W135 and N meningitidis A meningitis epidemic in 1 district triggered mass vaccination campaigns using national stocks of tetravalent vaccine. At the same time, the Ministry of Health made an appeal to the ICG for trivalent vaccine in support of additional vaccination campaigns.
By the end of February 2003, 1.5 million doses of trivalent vaccine had been used in mass vaccination campaigns. An additional 500 000 doses would be used starting in March. During the 2003 epidemic season, a total of 2 million doses of trivalent vaccine were used in Burkina Faso for mass vaccination campaigns in 10 epidemic districts with evidence of N meningitidis W135 disease. As recommended by the WHO, bivalent N meningitidis AC polysaccharide vaccine was used in the 1 epidemic district where there was no evidence of N meningitidis W135 disease.
TRIVALENT VACCINE ASSESSMENT ACTIVITY RESULTS
Preparations for the trivalent vaccine postlicensure assessments began in October 2002. These included ensuring safe mass vaccination campaigns and surveillance for adverse events after immunization during the campaigns. Effectiveness was assessed in coordination with the reinforced meningitis surveillance activities and the vaccination campaigns. The immunogenicity assessment was initiated in 2003.
The results of these postlicensure assessments have shown the trivalent vaccine to be safe, effective, and immunogenic. Regarding safety,36 during the trivalent mass vaccination campaigns in Burkina Faso, rates of adverse events after immunization (5.8 total adverse events after immunization cases and 1.5 serious adverse events after immunization cases per 100 000 doses distributed) were shown to be comparable to or less than those of mass vaccination campaigns in other countries where meningococcal polysaccharide vaccines have been used.37,38
Effectiveness39 against N meningitidis A or N meningitidis W135 was 84% (95% confidence interval = 32, 97; P = .01) and against N meningitidis A alone was 94% (95% confidence interval = 59, 99; P < .01). There were insufficient cases to assess effectiveness against N meningitidis W135 alone.
Immunogenicity40–42 and reactogenicity were assessed in northern Ghana among adolescents and adults 15 to 34 years of age in a noninferiority trial. Vaccine response did not differ significantly between the 2 groups through 23 months of follow-up. Results among young children aged 2 to 14 years living in Ethiopia are expected in late 2006.
FUTURE REGULATORY ISSUES
Soon after the trivalent vaccine was granted a 5-year marketing authorization by the Belgian regulatory authorities on January 22, 2003, the European regulatory environment changed dramatically. In May 2004, new European pharmaceutical legislation was published that categorically excluded licensure of vaccines and medicinal products used exclusively outside the European Union community.43 The new legislation generated considerable concern, because product licensure would then become the responsibility of receiving country national regulatory authorities, and in many cases, these national regulatory authorities are heavily dependent on licensure in the country of origin to ensure quality.
To ensure that there was no disruption in the supply of vaccines and medicinal products important for developing countries and that there is no disincentive for the timely discovery and development of these products during the development of the new policy, the WHO consulted with the European Commission, the European Medicines Agency, and the European Federation of Pharmaceutical Industries and Associations. The result was article 58 of regulation (European Commission) No. 726/2004, which came into effect in May 2005.
Article 58 establishes a mechanism whereby the European Medicines Agency may give a scientific opinion in the context of cooperation with the WHO for the evaluation of certain medicinal products for human use intended exclusively for markets outside the European Union. Article 58 of the regulation responds to the need to protect public health and give scientific assistance to nonmember countries in the context of cooperation with the WHO while at the same time allowing rapid access of those countries to important new medicinal products.44
Article 58 and the scientific opinion procedure apply to vaccines and medicinal products meeting defined criteria. Because the trivalent vaccine is used exclusively outside the European Union and is part of a WHO-managed stockpile for emergency response, it qualifies for the scientific opinion procedure (article 58) and will be submitted for review before January 2008 when the current Belgian marketing authorization expires. It is expected that the trivalent vaccine postlicensure impact assessment data will be included in the application.
CONCLUSIONS
The emergence of epidemic N meningitidis W135 meningitis in Burkina Faso during 2002 posed a new challenge to epidemic management in African meningitis belt countries. Recognizing the implications of the emergent threat, the WHO reviewed the available vaccine options and then worked to make an appropriate and affordable vaccine available within 6 months, in time for the 2003 epidemic season.
The successful response to this challenge resulted from an early and focused commitment from the WHO, as well as its ability to coordinate strategy across diverse public and private organizations and at the international, regional, and national levels. Other key elements that allowed for this unprecedented response to an emerging vaccine need in developing countries included the following: (1) pressing demand from several African countries for a vaccine that would reduce the spread and impact of the highly feared meningitis epidemic, (2) prompt financing from the Bill and Melinda Gates Foundation in support of an initial vaccine stockpile and impact assessment activities, and (3) rapid agreement from GlaxoSmithKline Biologicals to produce an affordable vaccine formulation that provided the range of protection and ease of use that was needed for epidemic response in African meningitis belt countries. It was this environment that resulted in the production of an appropriate and affordable vaccine in record time and that ensured that a stockpile was available for use in the future.
Public–private partnerships have received increasing attention as a mechanism for providing developing countries with access to necessary drugs and vaccines.45–49 These partnerships typically rely on a pharmaceutical partner for drug and vaccine discovery, development, and production and a public sector partner(s) for issues related to access. The Global Alliance for Vaccines and Immunization accelerated development and introduction programs for pneumococcal and rotavirus vaccines, as well as other recent vaccine initiatives (Haemophilus influenzae type B, meningococcal, Japanese encephalitis, and yellow fever) together with guaranteed financing from the Vaccine Fund and others are recent examples of public sector contributions to public–private partnerships.
Through June 2006, 3 million doses of the trivalent vaccine have been used for epidemic response in meningitis belt countries: Burkina Faso (2003), Chad (2005),50 Guinea (2006), and Sudan (200551 and 200652). An additional 576 000 doses of trivalent vaccine were administered during 2006 when epidemic N meningitidis W135 disease emerged among displaced persons in Gulu district, Uganda,53 and across the border in Kenya.54 A stockpile of 3.5 million trivalent doses remains.
Trivalent vaccine is now one of the primary tools for epidemic response in African meningitis belt countries. It will continue to play an important role during the foreseeable future and until affordable and appropriate conjugate vaccines become part of national immunization programs in the region.
Acknowledgments
This work was wholly supported by a grant received by the World Health Organization (grant 26547) from the Bill and Melinda Gates Foundation (www.gatesfoundation.org) in relation to epidemic meningococcal disease surveillance.
One representative from Glaxo-SmithKline Biologicals Biologicals participated on the Trivalent Vaccine Impact Assessment Clinical Task Force (Dominique Boutriau), and another participated as a technical adviser (Thomas Verstraeten).
The authors acknowledge Dominique Boutriau, Trivalent Vaccine Clinical Task Force member and Glaxo-SmithKline Biologicals, Rixensart, Belgium; L. Chocarro, World Health Organization, Geneva, Switzerland; Roland Dobbelaer, Belgian Scientific Institute of Public Health (National Regulatory Authority), Brussels, Belgium; M. M. Hacen, World Health Organization representative, Ouagadougou, Burkina Faso; F. Marc LaForce, Meningitis Vaccine Project/Programme for Appropriate Technology in Health, Ferney-Voltaire, France; Rosamund Lewis, World Health Organization, Kampala, Uganda; Julie Milstein, retired from World Health Organization, Geneva, Switzerland; Guenael Rodier, World Health Organization, Geneva, Switzerland; Mike Ryan, World Health Organization, Geneva, Switzerland; Thomas Verstraeten, GlaxoSmithKline Biologicals, Rixensart, Belgium; and Sosthene Zombre, Director General of Public Health Services, Burkina Faso.
This paper is dedicated to the memory of our friend and colleague Nicolas Nathan who passed away unexpectedly in May 2004.
Note. The funding agency played no role in protocol development; in the collection, analysis, and interpretation of data; in the writing of reports; or in the decision to submit articles for presentation or publication.
Human Participant Protection No protocol approval was needed for this activity.
Peer Reviewed
Contributors C.B. Nelson acted as the World Health Organization Department of Immunizations, Vaccines and Biologicals focal point for meningitis surveillance and response and as the technical coordinator of the Trivalent Vaccine Impact Assessment activities (effectiveness, adverse events after immunization surveillance, and immunogenicity and reactogenicity in adults and children). M. Birmingham is a Trivalent Vaccine Clinical Task Force member. A. Costa is the focal point for supply issues. J. Daviaud is the focal point for regulatory issues and vaccine prequalification for purchase by United Nation agencies. W. Perea is the World Health Organization Department of Communicable Disease Surveillance and Response focal point for meningitis surveillance and response and a Trivalent Vaccine Clinical Task Force member. M.-P. Kieny and D. Tarantola are Trivalent Vaccine Clinical Task Force members.
Trivalent Vaccine Clinical Task Force members and the technical coordinator contributed to the conception, design, and implementation of the project and specific technical activities; contributed to critical revision of technical protocols and subsequent presentations and publications for important intellectual content; and provided administrative, technical, and material support and supervision to the project and obtained funding.
References
- 1.World Health Organization. Meningococcal disease serogroup W135. Wkly Epidemiol Rec. 2001;76:141–142. [PubMed] [Google Scholar]
- 2.Nicolas P, M’Barek N Ait, Al-Awaidy S, et al. Pharyngeal carriage of serogroup W135 Neisseria meningitidis in Hajjees and their family contacts in Morocco, Oman and Sudan. APMIS. 2005;113: 182–186. [DOI] [PubMed] [Google Scholar]
- 3.Lingappa JR, Al-Rabeah AM, Hajjeh R, et al. Serogroup W135 meningococcal disease during the Hajj, 2000. Emerg Infect Dis. 2003;9:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouedraogo-Traore R, Hoiby EA, Sanou I, et al. Molecular characteristics of Neisseria meningitidis strains isolated in Burkina Faso in 2001. Scand J Infect Dis. 2002;34:804–807. [PubMed] [Google Scholar]
- 5.Taha M-k, Parent du Chatelet I, Schlumberger M, et al. Neisseria meningitidis serogroups W135 and A were equally prevalent among meningitis cases occurring at the end of the 2001 epidemics in Burkina Faso and Niger. J Clin Microbiol. 2002;40:1083–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapeyssonie L. La méningite cérébrospinale en Afrique. Bull World Health Organ. 1963;28(suppl):3–114. [Google Scholar]
- 7.Greenwood BM. The epidemiology of acute bacterial meningitis in tropical Africa. In: Williams JD, Burnie J, eds. Bacterial Meningitis. London, England: Academic Press; 1987:61–91.
- 8.Greenwood B. Manson Lecture: meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;93: 341–353. [DOI] [PubMed] [Google Scholar]
- 9.Molesworth AM, Thomson MC, Connor SJ, et al. Where is the meningitis belt? Defining an area at risk of epidemic meningitis in Africa. Trans R Soc Trop Med Hyg. 2002;96:242–249. [DOI] [PubMed] [Google Scholar]
- 10.Cuevas LE, Jeanne I, Molesworth A, et al. Risk mapping and early warning systems for the control of meningitis in Africa. Vaccine. In press. [DOI] [PubMed]
- 11.World Health Organization. Meningococcal disease, serogroup W135, Burkina Faso Preliminary report. Wkly Epidemiol Rec. 2002;77:152–156. [PubMed] [Google Scholar]
- 12.Koumare B, Ouedraogo-Traore R, Sanou I, et al. The first large epidemic of meningococcal disease caused by serogroup W135, Burkina Faso, 2002. Vaccine. In press. [DOI] [PubMed]
- 13.Surveillance data, 2002 [database]. Burkina Faso Ministry of Health. Ouagadougou, Burkina Faso, 2002.
- 14.World Health Organization. Control of Epidemic Meningococcal Disease: WHO Practical Guidelines, 2nd Edit. World Health Organization; Geneva, Switzerland; 1998. WHO/EMC/BAC/98.3.
- 15.World Health Organization. Detecting Meningococcal meningitis epidemics in highly-endemic African countries. Wkly Epidemiol Rec. 2000;75: 306–309. [PubMed] [Google Scholar]
- 16.Denis F, Rey JL, Amadou A, et al. Emergence of meningococcal meningitis caused by W135 serogroup in Africa. Lancet. 1982;2:1335–1336. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood BM, Smith AW, Hassan-King M, et al. The efficacy of meningococcal polysaccharide vaccine in preventing group A meningococcal disease in the Gambia, West Africa. Trans R Soc Trop Med Hyg. 1986;80: 1006–1007. [DOI] [PubMed] [Google Scholar]
- 18.Odugbemi T, Ademidun O, Agbabiaka A, Banjo T. Nasopharyngeal carriage of Neisseria meningitidis among school children at Ijede, Lagos State, Nigeria. Ethiop Med J. 1992;30: 33–36. [PubMed] [Google Scholar]
- 19.World Health Organization. Neisseria meningitidis strains identified in Africa. Wkly Epidemiol Rec. 1993;68: 311–312. [PubMed] [Google Scholar]
- 20.Guibourdenche M, Hoiby EA, Riou JY, et al. Epidemics of serogroup A Neisseria meningitidis of subgroup III in Africa, 1989–94. Epidemiol Infect. 1996;116:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwara A, Adegbola RA, Corrah PT, et al. Meningitis caused by a serogroup W135 clone of the ET-37 complex of Neisseria meningitidis in West Africa. Trop Med Intl Health. 1998;3:742–746. [DOI] [PubMed] [Google Scholar]
- 22.Fonkoua MC, Taha MK, Nicolas P, et al. Recent increase in meningitis caused by Neisseria meningitidis serogroups A and W135, Yaoundé, Cameroon. Emerg Infect Dis. 2002;8: 327–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fourn L, Makoutode M, Ouendo M, and Tounkara B. Meningococcal meningitis epidemics in Benin [in French]. Sante. 2004;14:153–159. [PubMed] [Google Scholar]
- 24.World Health Organization. Health conditions for travelers to Saudi Arabia for the pilgrimage to Mecca (Hajj). Wkly Epidemiol Rec. 2005;80: 431–432. [PubMed] [Google Scholar]
- 25.Jodar L, Feavers IM, Salisbury D, Granoff DM. Development of vaccines against meningococcal disease. Lancet. 2002;359:1499–1508. [DOI] [PubMed] [Google Scholar]
- 26.Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev. 2006;19:142–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. International Coordinating Group (ICG) on Vaccine Provision for Epidemic Meningitis Control. Available at: http://www.who.int/csr/disease/meningococcal/icg/en. Accessed May 25, 2006.
- 28.World Health Organization. Emergence of W135 meningococcal disease: report of a WHO consultation, September 17–18, 2001, Geneva, 9–10. World Health Organization: Geneva, Switzerland; 2002. WHO/CDS/CSR/GAR/2002.1.
- 29.World Health Organization. Prevention and control of epidemic meningococcal disease in Africa. Report of a WHO technical consultation meeting. Ouagadougou, Burkina Faso, September 23–24, 2002. World Health Organization: Geneva, Switzerland; 2003. WHO/CDS/CSR/GAR/2003.10.
- 30.Decosas J, Koama JBT. Chronicle of an outbreak foretold: meningococcal meningitis W135 in Burkina Faso. Lancet Infect Dis. 2002;2:763–765. [DOI] [PubMed] [Google Scholar]
- 31.Médecins Sans Frontiéres. Press release: Opportunity to fight meningitis in Africa thwarted by funding gag. Available at: http://www.msf.org. Accessed May 25, 2006.
- 32.World Health Organization. Press release: New meningitis threat being contained by web of partnerships. Available at: http://www.who.int/mediacentre/news/releases/2004/pr25/en/print.html. Accessed May 25, 2006.
- 33.News: Vaccination halts meningitis outbreak in Burkina Faso. Lancet. 2004; 363:1290. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. United Nations Prequalified Vaccines. Available at: http://www.who.int/vaccines-access/quality/un_prequalified/prequalvaccinesproducers.html. Accessed May 25, 2006.
- 35.World Health Organization. Detecting Meningococcal meningitis epidemics in highly-endemic African countries: WHO recommendation. Wkly Epidemiol Rec 2000;75:306–309. [PubMed] [Google Scholar]
- 36.Bentsi-Enchill AD, Zongo I, Khamassi S, et al. Monitoring of adverse events during a mass vaccination campaign with a trivalent ACW135 polysaccharide meningitis vaccine in Burkina Faso, 2003. Vaccine. In press. [DOI] [PubMed]
- 37.Yergeau A, Alain L, Pless R, Robert Y. Adverse events temporally associated with meningococcal vaccines. Can Med Assoc J. 1996;154:503–507. [PMC free article] [PubMed] [Google Scholar]
- 38.US Centers for Disease Control and Prevention. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49:1–10. [Google Scholar]
- 39.Soriano-Gabarró M, Toé L, Tiendrebeogo SRM, et al. Effectiveness of a trivalent serogroup A/C/W-135 Meningococcal polysaccharide vaccine. 2003; Burkina Faso. Vaccine. In press. [DOI] [PubMed]
- 40.Chandramohan D, Hodgson A, Coleman P, et al. An evaluation of the immunogenicity and safety of a new trivalent meningococcal polysaccharide vaccine in adolescents and adults. Vaccine. In Press. [DOI] [PubMed]
- 41.Chandramohan D, Coleman P, Nelson CB et al. Review of immunogenicity endpoints for the evaluation of meningococcal vaccines in affected populations. Vaccine. In press.
- 42.Aseffa A, Bedru A, Yamuah L, et al. Immunogenicity of Mencevax ACW135 vaccine among young children. Vaccine. In press.
- 43.The European Agency for the Evaluation of Medicinal Products (EMEA). Press release: New pharmaceutical legislation enters into choice on 20 May 2004. Available at: http://www.emea.eu.int/pdfs/general/direct/pr/1233204en.pdf. Accessed May 25, 2006. EMEA/12332/04.
- 44.The European Medicines Agency (EMEA) Committee for Medicinal Products for Human Use (CHMP). Guidelines on procedural aspects regarding a CHMP scientific opinion in the context of cooperation with the World Health Organisation (WHO) for the evaluation of medicinal products intended for exclusively for markets outside the community. Available at: http://www.emea.eu.int/pdfs/human/euleg/557904en.pdf. Accessed May 25, 2006. EMEA/CHMP/5579/04.
- 45.Webber D, Kremer M. Perspectives on stimulating industrial research and development for neglected infectious diseases. Bull World Health Organ. 2001;79:735–741. [PMC free article] [PubMed] [Google Scholar]
- 46.Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N. Drug development for neglected disease: a deficient market and public-health policy failure. Lancet 2002;359:2188–2194. [DOI] [PubMed] [Google Scholar]
- 47.Widdus R. Public-private partnerships for health: their main targets, their diversity, and their future directions. Bull World Health Organ. 2001;79: 713–720. [PMC free article] [PubMed] [Google Scholar]
- 48.Drugs for Neglected Diseases initiative. Available at: http://www.dndi.org. Accessed May 25, 2006.
- 49.Towse A, Kettler H. Advance price or purchase commitments to create markets for treatments for diseases of poverty: lessons from three policies. Bull World Health Organ. 2005;83: 301–307. [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. Meningococcal disease in Chad–update 2. Disease Outbreak News. Available at: http://www.who.int/csr/don/2005_01_28/en. Accessed May 25, 2006.
- 51.World Health Organization. Meningococcal disease in Sudan—update. Disease Outbreak News. Available at: http://www.who.int/csr/don/2005_04_06a/en. Accessed May 25, 2006.
- 52.World Health Organization. Meningococcal disease in the African Meningitis Belt, epidemic season 2006. Disease Outbreak News. Available at: http://www.who.int/csr/don/2006_03_21/en. Accessed May 25, 2006.
- 53.World Health Organization. Meningococcal disease in the African Meningitis Belt, epidemic season 2006. Disease Outbreak News. Available at: http://www.who.int/csr/don/2006_03_21/en. Accessed May 25, 2006.
- 54.World Health Organization. Meningococcal disease in Kenya. Disease Outbreak News. Available at: http://www.who.int/csr/don/2006_03_03/en. Accessed May 25, 2006.
- 55.World Health Organization Department of Communicable Disease Surveillance and Response (CDS). Geneva, Switzerland: World Health Organization; 2005.


