Abstract
We have shown previously that stimulation of heterologously expressed P2Y1 nucleotide receptors inhibits M-type K+ currents in sympathetic neurons. We now report that activation of endogenous P2Y1 receptors induces inhibition of the M-current in rat CA1/CA3 hippocampal pyramidal cells in primary neuron cultures. The P2Y1 agonist adenosine 5′-[β-thio]diphosphate trilithium salt (ADPβS) inhibited M-current by up to 52% with an IC50 of 84 nm. The hydrolyzable agonist ADP (10 μm) produced 32% inhibition, whereas the metabotropic glutamate receptor 1/5 agonist DHPG [(S)-3,5-dihydroxyphenylglycine] (10 μm) inhibited M-current by 44%. The M-channel blocker XE991 [10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride] produced 73% inhibition at 3 μm; neither ADPβS nor ADP produced additional inhibition in the presence of XE991. The effect of ADPβS was prevented by a specific P2Y1 antagonist, MRS 2179 (2′-deoxy-N′-methyladenosine-3′,5′-bisphosphate tetra-ammonium salt) (30 μm). Inhibition of the M-current by ADPβS was accompanied by increased neuronal firing in response to injected current pulses. The neurons responding to ADPβS were judged to be pyramidal cells on the basis of (1) morphology, (2) firing characteristics, and (3) their distinctive staining for the pyramidal cell marker neurogranin. Strong immunostaining for P2Y1 receptors was shown in most cells in these cultures: 74% of the cells were positive for both P2Y1 and neurogranin, whereas 16% were only P2Y1 positive. These results show the presence of functional M-current-inhibitory P2Y1 receptors on hippocampal pyramidal neurons, as predicted from their effects when expressed in sympathetic neurons. However, the mechanism of inhibition in the two cell types seems to differ because, unlike nucleotide-mediated M-current inhibition in sympathetic neurons, that in hippocampal neurons did not appear to result from raised intracellular calcium
Keywords: nucleotide receptors, P2Y receptors, hippocampus, pyramidal neurons, potassium channels, M-current
Introduction
We have found previously that activation of the Gq-coupled P2Y1 nucleotide receptor, expressed heterologously in rat sympathetic neurons, potently inhibits the M-type (Kv7.1/Kv7.2) K+ current (Brown et al., 2000). Because P2Y1 receptor mRNA is widely expressed in the brain (Barnard et al., 1997; Webb et al., 1998), it is important to know whether their activation inhibits the M-current in central neurons.
To examine this, we selected rat hippocampal pyramidal neurons. These exhibit prominent M-currents, inhibited via at least two other types of Gq-coupled receptors: muscarinic acetylcholine receptors (Halliwell and Adams, 1982; Madison et al., 1987) and metabotropic glutamate receptors (mGluRs) (Charpak et al., 1990). ATP is known to be constantly available at the pyramidal neurons, being synaptically coreleased onto them (Pankratov et al., 1998), although another important source of ATP there is its release and localized diffusion from associated astrocytes (Koizumi et al., 2003; J. M. Zhang et al., 2003; Bowser and Khakh, 2004). Furthermore, in the human (Moore et al., 2000) and rat (Moran-Jimenez and Matute, 2000) hippocampal pyramidal cell layer and adjacent interneurons, strong P2Y1 receptor immunoreactivity has been reported. Recently, P2Y1 receptor mRNA was shown to be expressed in microdissected glutamatergic pyramidal neurons, with P2Y1 receptor immunoreactivity reported in glutamatergic nerve terminals and postsynaptic active zones of rat hippocampus (Rodrigues et al., 2005).
Despite several reports of P2Y-like receptors modulating membrane currents in this and other brain regions (for review, see Illes and Ribeiro, 2004), there has been much uncertainty regarding the identity of the receptor type and subtype and channel involved in any given case, attributable in part to the enzymatic breakdown of ATP and its derivatives in hippocampal slices and hence action at their adenosine A1 receptors (A1Rs) (O’Kane and Stone, 2000; Masino et al., 2002). The latter action is particularly powerful in brain slices of certain regions. Thus, hippocampal adenosine receptors are mainly concentrated in the densely packed pyramidal cell layer (Ochiishi et al., 1999); on the intact structures in hippocampal slices, the surface ectonucleotidase activity is especially high, with restricted extracellular space there for adenosine diffusion (Dunwiddie et al., 1997; J. M. Zhang et al., 2003). Indeed, in those slices, all of the previously observed actions of ATP on postsynaptic currents in pyramidal cells are abolished by an A1 receptor antagonist (J. M. Zhang et al., 2003; Kukley et al., 2004; Pascual et al., 2005), but this is not so on interneurons in other layers (Koch et al., 1997; Kawamura et al., 2004). In hippocampal cultures, conversely, EPSCs similarly modulated by ATP can be recorded, and a P2Y-type response to exogenous or endogenously released ATP persists during adenosine receptor blockade (J. M. Zhang et al., 2003).
We therefore used such hippocampal cell cultures to investigate the action of the P2Y1 receptors on a defined membrane channel, the M-current K+ channel, located on their principal cells, i.e., identified pyramidal neurons. Hitherto, no identified ion channel has been associated with any P2Y receptor action in brain neurons, nor has any P2Y subtype action in the pyramidal cells been established.
Materials and Methods
Primary cell culture.
Rat pups (3 d old) were killed by cervical dislocation, in accordance with the United Kingdom animal experimentation regulations. A zone of the hippocampus containing the CA1 and CA3 region was dissected. The cells were dissociated and maintained in culture in Neurobasal medium supplemented with 0.5% (w/v) l-glutamine and 2% B27 serum-free supplement but without antibiotics as described previously (Alger et al., 1994; Shah et al., 2002).
Electrophysiology.
Electrophysiological recordings were made at room temperature from cells cultured for 14–21 d, on pyramidal cells identified as described in Results. Whole-cell currents and membrane potentials were recorded using the perforated patch-clamp method as described previously (Filippov et al., 1998). Patch pipettes (4–7 MΩ) were filled by dipping the tip into a filtered solution containing 90 mm potassium acetate, 20 mm KCl, 3 mm MgCl2, 40 mm HEPES, and 0.1 mm 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid (BAPTA), adjusted to pH 7.4 by KOH, for 5–10 s. The pipette was then backfilled with the same solution containing 0.125 mg/ml amphotericin B as the permeabilizing agent. Neurons were superfused (20–25 ml/min) with an external modified Krebs’ solution containing 120 mm NaCl, 3 mm KCl, 1.5 mm MgCl2, 2.5 mm CaCl2, 10 mm HEPES, 11.1 mm glucose, adjusted to pH 7.3 with NaOH, and 0.5 μm tetrodotoxin (TTX) when using voltage-clamp recording. In most experiments, CsCl (1 mm) was added to the external solution to block the hyperpolarization-activated Ih current (Halliwell and Adams, 1982; Maccaferri and McBain, 1996). P2Y receptor agonists and antagonists were applied in the same superfusion system; 10 μm 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (DNQX) and 10 μm bicuculline were also present to block excitatory and inhibitory synaptic currents, respectively, plus 8-cyclopentyltheophylline (CPT) at 100 nm to block A1Rs (Fredholm, 1990). Standard voltage protocols designed for M-current recordings (Brown and Adams, 1980) were applied. Neurons were voltage clamped or current clamped using the discontinuous voltage- or current-clamp mode (switching rate, 3–5 kHz) of an Axoclamp 2B amplifier (Molecular Devices, Palo Alto, CA). M-current was preactivated by holding the membrane potential at −20 mV, and the deactivation tail current was recorded by applying 1 s hyperpolarizing steps to −40 mV every 20 s. Current–voltage (I–V) relationships were obtained using incremental voltage steps of 10 mV between −20 and −90 mV; currents were measured at the end of each hyperpolarizing step. For dose–response curves, currents were measured at −30 mV after steady-state I–V relationships were obtained using a ramp voltage command of 20 s from −20 to −90 mV as before (Filippov et al., 1998). The leak component of current was estimated in both cases by extrapolating a linear fit to the I–V relationship negative to −60 mV, at which (in the presence of Cs+) only ohmic currents were observed.
For current-clamp experiments, neurons were first stimulated with a train of ∼20 10-ms pulses of 0.5 nA applied every 100 ms to check for the presence of a slow afterhyperpolarization (sAHP), characteristic of pyramidal neurons (Shah and Haylett, 2000 and references therein). Then the firing pattern of the neuron was examined by applying four to five pulses of incremental amplitude at 10 s intervals, each pulse being 1 s in duration. All commands, recordings, and analysis were made using Digidata 1200 interface and pClamp 8 software (Molecular Devices).
Immunocytochemistry.
Cells from hippocampal primary neuron cultures (14–21 d in vitro) that were used for electrophysiological recordings were marked for identification in the subsequent stainings in their 35 mm culture dishes. At room temperature, cells were fixed in 4% paraformaldehyde for 10 min and then quenched with 50 mm ammonium chloride in PBS for 20 min. They were washed (three times for 5 min) with PBS solution; a PBS-based blocking solution containing 5% normal goat serum and 0.1% Triton X-100 was then applied for 1 h. Then incubations at 4°C were performed in the culture dishes for 24 h with the primary antibodies; i.e., with goat anti-neurogranin antibody (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), or with mouse anti-neurofilament 200 (NF-200) (1:200 dilution; Sigma, St. Louis, MO) in the same blocking solution. The cultures were then washed with PBS (three times for 5 min), and the respective secondary antibody was applied in the blocking solution for 2 h at 25°C. Secondary antibodies were Alexa 555-conjugated anti-goat or Alexa 488-conjugated anti-mouse (all used at 1:1000 dilution; all from Invitrogen, Carlsbad, CA). For cells not used for electrophysiological recording, larger numbers of cells could be analyzed and, with more favorable optical conditions, by growing similar cultures on glass coverslips. For these, the same antibody procedures were used, with the inclusion also of rabbit anti-P2Y1 receptor antibody (APR009; 1:100 dilution; Alomone Labs, Jerusalem, Israel) together with Alexa 488-conjugated anti-rabbit secondary antibody (Invitrogen). These cultures were also incubated with 4′,6′-diamidino-2-phenylindole (DAPI) nuclear stain (0.2 μg/ml; Sigma) when stated. In all cases, the cultures were finally washed again (three times for 5 min) with PBS, rinsed with distilled water, and air dried. They were mounted with fluorescent mounting medium (DakoCytomation, Ely, UK) and examined under a Nikon (Tokyo, Japan) E400 fluorescent phase microscope with the appropriate filters.
Ca2+ imaging.
Cells were incubated with 2 μm fura-2 AM for 30 min at 37°C and then washed for 20 min before starting the experiments. Live hippocampal cell cultures plated on glass coverslips were placed on the stage of a Nikon Diaphot inverted microscope and superfused at 20 ml/min with extracellular solution of the same composition as that used for M-current measurements (with DNQX, bicuculline, TTX, and the adenosine receptor antagonist CPT). Alternate images at 350 and 380 nm excitation wavelengths were recorded at 1 Hz and background subtracted as described previously (Winks et al., 2005). The fluorescence ratio images were used to track changes in intracellular Ca2+ of the designated region of interest inside the cells.
Statistical analysis.
Data are presented as means ± SEM. Student’s test (unpaired) was applied to determine statistical significance (taken as p ≤ 0.05). Dose–response curves were constructed using concentrations added cumulatively, with 1 min exposure times. Curves were fitted (using the Origin version 5 software; Microcal Software, Northampton, MA) to pooled data points according to the following Hill equation: y = ymax × xnH/(xnH + KnH), where y is the observed percentage inhibition, ymax is the extrapolated maximal percentage inhibition, x is the nucleotide concentration (micromolar), K is IC50 (micromolar), and nH is the Hill coefficient.
Chemicals.
Drugs were applied to the external solution by bath perfusion (bath exchange rate, ≤5 s). Tetrodotoxin, 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride (XE991), DNQX, (S)-3,5-dihydroxyphenylglycine (DHPG), 2′-deoxy-N′-methyladenosine-3′,5′-bisphosphate tetra-ammonium salt (MRS 2179), and bicuculline were from Tocris Cookson (Ballwin, MO); ADP and adenosine 5′-[β-thio]diphosphate trilithium salt (ADPβS) were from Roche Applied Science (Indianapolis, IN). Triphosphate contamination of ADP compounds was removed by hexokinase/glucose pretreatment as described previously (Simon et al., 2001). BAPTA and amphotericin B were from Sigma, and CsCl was from Aldrich (Milwaukee, WI). Drugs were prepared as stock solutions, kept frozen in batches, and defrosted once only, just before use.
Results
Selection of pyramidal neurons for recording
Primary cell cultures derived from the hippocampus of rats at 3 d were used. Cells within these cultures were initially selected as presumed pyramidal cells by their morphology and by reference to their firing behavior after initial patching, and identification was confirmed by post-experiment immunocytochemistry (see further below).
Cells selected as pyramidal on the basis of morphology showed relatively low-frequency firing (<10 Hz) with varying degrees of spike-frequency adaptation, including (in two cells) periods of missed spiking. Their firing patterns accorded with previous observations on rat hippocampal pyramidal cells in intact brain slices (Madison and Nicoll, 1984; Storm, 1990). The majority (12 of 18 tested) of these neurons showed postburst sAHPs longer than 1 s, as reported previously for pyramidal neurons in culture (Shah and Haylett, 2000 and references therein).
P2Y1 agonists inhibit M-type K+ currents
The identified pyramidal neurons exhibited the characteristic slowly deactivating tail currents associated with M-channel closure (Brown and Adams, 1980) on stepping from a holding potential of −20 to −40 mV (Fig. 1A). In confirmation, addition of the M-channel blocking drug XE991 at 3 μm (Wang et al., 1998) reduced the steady outward current at −20 mV and almost abolished the slow deactivation tails produced by the hyperpolarizing step (Fig. 1B). In four cells, mean inhibition of the deactivation tails by 3 μm XE991 was 73.5 ± 9.15%.
Figure 1.
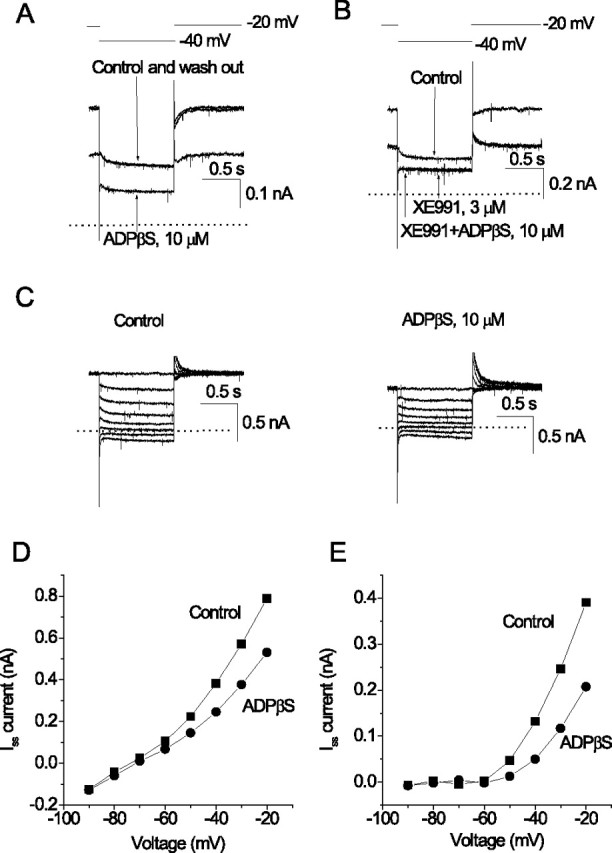
ADPβS inhibits M-current in CA1/CA3 hippocampal neurons. Membrane currents were recorded from presumed pyramidal neurons in hippocampal cell cultures using perforated-patch electrodes. In A and B, the cell was depolarized to −20 mV to preactivate M-current (0 current indicated by dotted line) and then hyperpolarized for 1 s to −40 mV to partially deactivate it (voltage protocol shown on top). A, Currents recorded before (Control), during, and after (washout) application of 10 μm ADPβS. ADPβS reduces the preactivated outward current and the amplitude of the deactivation current tail. B, Currents recorded before (control) and after addition of the M-channel blocking agent XE991(to 3 μm). XE991 abolishes the deactivation current tails. Subsequent addition of 10 μm ADPβS in the presence of XE991 no longer produces an inward current (compare with A). C, Current responses to a series of hyperpolarizing steps between −20 mV (holding potential) and −90 mV in 10 mV increments, recorded before and after adding 10 μm ADPβS. D, Currents in C measured at the end of each hyperpolarizing step plotted against command voltage. E, Currents replotted after subtracting leak currents, by extrapolating the linear component of the current–voltage curve measured negative to −60 mV.
Bath perfusion with the P2Y1 receptor agonist ADPβS (which is relatively resistant to ATPases) also reduced the standing outward current and the amplitude of the M-current deactivation tails (Fig. 1A). It also reduced the outward rectification of the I–V curve positive to −70 mV [an additional feature of M-current inhibition (Brown and Adams, 1980)] but had no significant effect on membrane currents negative to −70 mV (Fig. 1C–E).
Identification of the responding cells
Figure 2A–C illustrates the range of the typical morphology of the selected class of cells. After voltage-clamp recording, several of the cells identified thus and that had responded to the P2Y1 agonist were tested individually in postrecording immunocytochemistry for their content of marker proteins. Examples are shown in Figure 2A: staining using specific antibodies showed that the responding cells are all positive for both NF-200, a marker for neurons, and for neurogranin, which is a marker for principal cells, including specifically the pyramidal cells in the hippocampus (Singec et al., 2004).
Figure 2.
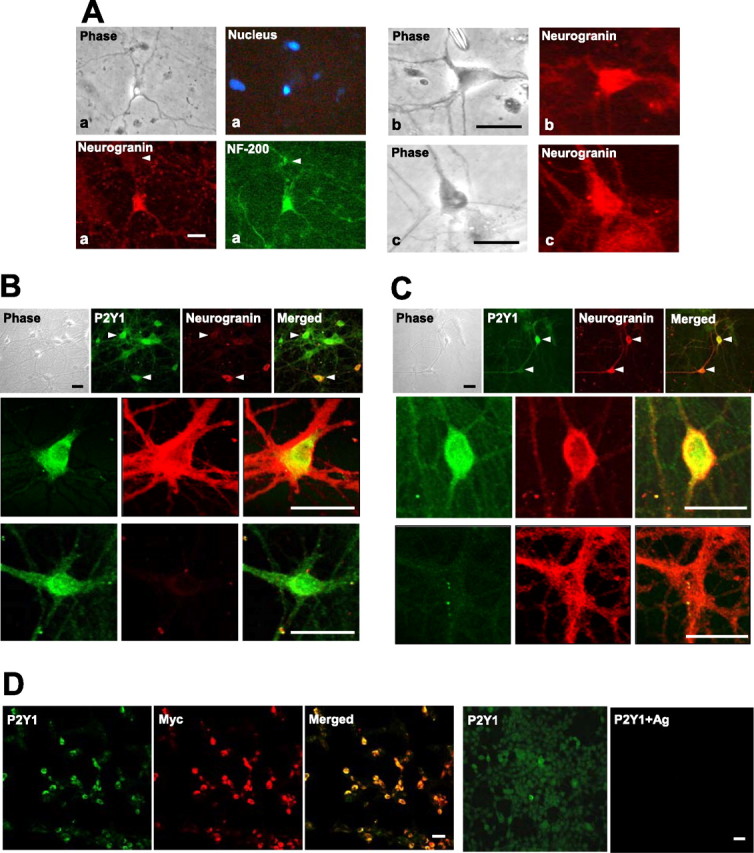
Immunoreactivities in hippocampal cells responsive to P2Y1 agonists. A, Three representative cells are illustrated (a, b, c) that had given responses to ADPβS with clear inhibition of the M-current (as in Fig. 1), in cultures that were then immunostained. They exhibit typical morphologies of pyramidal cells and reactivity for NF-200, a marker for neurons, and for neurogranin, a marker for pyramidal neurons. Also note that, in Aa, there is a smaller interneuron (arrowhead and seen in phase-contrast view) that is positive for NF-200 but not for neurogranin (blue, nuclear stain, DAPI). B, Similar hippocampal cultures tested for overlapping immunoreactivity for the P2Y1 receptor (green). Top row, A group of cells containing much P2Y1; arrowheads compare two such cells with only one of them positive (red) for neurogranin. Two other cells are shown below at high power, with cell bodies and proximal regions of some of their processes either positive for both P2Y1 and neurogranin (row 2) or only for P2Y1 (row 3) (apparently an interneuron). C, Another of these cultures, showing that, conversely, some of the neurons (row 3, and the bottom marked cell in row 1) are positive for neurogranin but not for P2Y1, presumed to be a subclass of pyramidal cells. D, Antigen peptide block of the anti-P2Y1 antibody reaction. HEK293 cells stably expressing Myc-tagged P2Y1 receptors were coreacted with anti-Myc antibody (red) and by the same anti-P2Y1 antibody sample used in B and C (green). Panels 1–3, Showing the same field: the great majority of the cells show coincident strong dual staining; a few cells with a low content of P2Y1 receptor protein (detected by its tag) likewise show a correspondingly weak reaction of the anti-PY1 antibody. Panels 4, 5, A parallel sample, showing complete blockade of the anti-P2Y1 antibody reaction by its antigen peptide. Scale bars, 30 μm.
A larger set of these CA1/CA3-derived cell cultures, not taken for recording, could be examined more extensively by immunocytochemistry. This included staining using an antibody directed against the rat P2Y1 receptor (at an intracellular epitope), in conditions in which it was shown (Tung et al., 2004) to give neither nonspecific staining nor any cross-reactivity to closely related P2Y receptors. Strong immunostaining for P2Y1 receptors was found thus to be present in these primary cultures (Fig. 2B,C), in some cells corresponding in morphology and marker-protein content to those (Fig. 2A) that responded to ADPβS.
The distributions of the staining shown within the P2Y1-positive cells in Figure 2, B and C, are representative of the forms seen in these cultures, with the content of receptors heaviest around the neuronal cell membrane. That stain is also seen, at a lower intensity apparently, in the cytosol, but part of this is attributable to the superimposed cell membrane in these intact cells; the remainder there is presumed to be attributable to the synthesis and cycling of P2Y1 receptors in these growing cultures.
To confirm the specificity of the anti-P2Y1 antibody staining seen on these cultures, first, the reaction was demonstrated to be absent in all cells in these cultures when the peptide antigen (5 μg/μg antibody) was present (data not shown). That is not wholly conclusive, however, because some of the cells here are P2Y1 negative without the peptide presence. Therefore, in a parallel reaction performed on Myc-tagged recombinant P2Y1 receptors expressed in a host cell line, the distribution of the staining seen with the anti-P2Y1 antibody was shown to be identical to that of the simultaneous direct staining of the P2Y1 receptor protein by a fluorescent anti-Myc antibody (Fig. 2D). The variation between cells strongly expressing and weakly expressing the P2Y1 receptor was registered identically by these two types of probe (analysis not shown). Here, the inclusion of the relevant peptide antigen abolished the antibody reaction in all of the cells known to be expressing the P2Y1 receptor (Fig. 2D).
Such immunocytochemical staining performed across a set of the cultures derived from four rats showed that most (74%) of the cells that stained with either antibody there are P2Y1 positive and neurogranin positive (Fig. 2B,C, second rows), and 16% are only P2Y1 positive [neurogranin negative (Fig. 2B, third row)]. That latter 16% appeared morphologically to be interneurons. The remaining 10% are P2Y1 negative, mostly pyramidal like and neurogranin positive (Fig. 2C, third row).
Analysis of the M-current responses
The mean inhibition produced by 10 μm ADPβS was calculated as the fractional inhibition of outward current at −30 mV (from extrapolated leak-subtracted I–V curves, as in Fig. 1E) or as the fractional inhibition of the deactivation tail currents (measured by extrapolation to the onset of the hyperpolarizing step). The two methods gave similar results and yielded a mean value of 46.6 ± 3.0% (n = 18 cells) (Fig. 3E). A more hydrolyzable P2Y1 agonist, ADP (10 μm), produced somewhat less inhibition (31.9 ± 2.9%; n = 7). Neither ADP nor ADPβS produced any additional inward current in the presence of XE991, indicating that their effect was solely the result of M-current inhibition.
Figure 3.
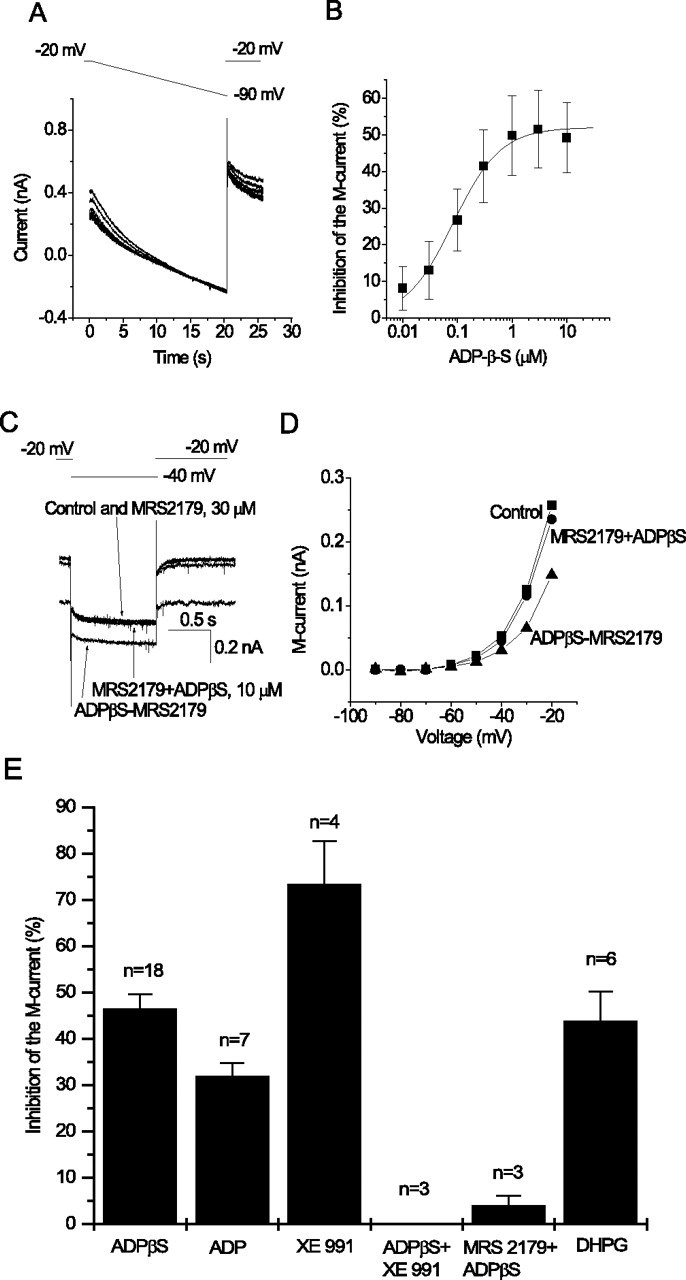
Characteristics of the M-current inhibition mediated by P2Y1 receptors on hippocampal pyramidal neurons. A, Currents recorded in response to voltage ramps (20 s) from −20 to −90 mV (voltage protocol above) applied in the presence of increasing concentrations of ADPβS. Note the progressive reduction in outward rectification in the evoked current. B, Concentration dependence of M-current inhibition, measured as percentage reduction of ramp-evoked current at −30 mV, after leak subtraction by extrapolation of the linear part of the ramp I–V relationship negative to −60 mV. Points show mean ± SEM from three cells. Increasing concentrations of ADPβS were added cumulatively using 1 min exposure times. Curves were fitted to pooled data points using Origin 5 software to the following Hill equation: y = ymax × xnH/(xnH + KnH), where y is the observed percentage inhibition, ymax is the extrapolated maximal percentage inhibition, x is the nucleotide concentration (micromolar), K is the IC50 (micromolar), and nH is the Hill coefficient. Mean values of constants were ymax of 52.1 ± 1.8%, K of 84.0 ± 12.7 nm, and nH of 1.0 ± 0.1. C, The specific P2Y1 receptor antagonist MRS 2179 prevents inhibition of M-current by ADPβS. M-current deactivations recorded as in Figure 1A. Currents were recorded before and after adding MRS 2179 (30 μm) alone, then after adding ADPβS (10 μm) in the presence of MRS 2179, and then on adding ADPβS after washing out MRS 2179. D, Current–voltage relationships (determined as in Fig. 1) before adding drugs (squares) or after adding ADPβS in the presence of MRS 2179 (circles) and then on adding ADPβS after washing out MRS 2179 (triangles). E, Mean percentage inhibition of M-current by the P2Y1 agonists ADPβS and ADP (10 μm each), the M-channel blocker XE991 (3 μm), ADPβS (10 μm) in the presence of XE991 (3 μm), ADPβS (10 μm) in the presence of the P2Y1 antagonist MRS 2179 (30 μm), and the group 1 metabotropic glutamate receptor agonist DHPG (10 μm). Error bars show SEM of inhibition of the M-current by the drugs indicated measured at −30 mV from steady-state current–voltage relationship or from deactivation tail amplitude (n indicates the number of cells tested).
The absence of any response to ADP or ADPβS in the presence of XE991 (Fig. 3) or at potentials negative to −70 mV (Fig. 1) implies that these agonists did not activate P2X receptors. This does not necessarily mean that the cells were devoid of such receptors because these nucleotides are relatively weak P2X agonists.
M-currents recorded from pyramidal neurons in rat hippocampal slices are inhibited by stimulating Gq/11-coupled metabotropic glutamate receptors (Charpak et al., 1990). We therefore tested whether M-currents in the cultured neurons that we selected for the above experiments were also inhibited by the selective group 1 mGluR agonist DHPG (10 μm) (Schoepp et al., 1994). This compound inhibited the M-current by 43.8 ± 6.4% (n = 6), i.e., a similar level to that produced by ADPβS (Fig. 3E). Five of these neurons had been tested initially also with a P2Y1 agonist. Three of them responded to ADPβS or ADP by inhibition of the M-current. However, in two of those neurons in which DHPG inhibited the M-current by 43 and 31%, ADPβS had no clear effect. Hence, of a total of 20 pyramidal cells tested with ADPβS, in 18 the M-type K+ channel coupled to the P2Y1 receptor and in two it did not but still coupled to mGluR1.
Full concentration–inhibition curves for ADPβS were obtained in three neurons using voltage ramps to determine I–V curves in the presence of incremental concentrations of agonist (Fig. 3A). Inhibition was measured as the percentage reduction of leak-subtracted outward current at −30 mV. The mean curve (Fig. 3B) yielded an IC50 value of 84.0 ± 12.7 nm and maximal inhibition of 52.1 ± 1.8%. The latter does not differ significantly from the mean inhibition produced by a single application of 10 μm ADPβS (see above), indicating that cumulative addition of agonist did not produce desensitization.
Effect of a P2Y1 antagonist
To verify that the responses to ADPβS were mediated by P2Y1 receptors, we tested the effect of a specific P2Y1 receptor antagonist, MRS 2179 (30 μm) (Nandaran et al., 2000). This compound did not itself produce any effect, indicating that there was no tonic activation of endogenous P2Y1 receptors. However, MRS 2179 prevented inhibition of the M-current by ADPβS (10 μm, a maximally effective concentration when applied alone) (Fig. 3C,D). As indicated in the protocol used for these experiments (see legend to Fig. 3C,D), inhibition of the M-current by ADPβS was immediately restored (within <20 s) after MRS 2179 was removed from the perfusing medium. In three cells, ADPβS inhibited M-current negligibly (4.0 ± 2.1%) in the presence of MRS 2179 (Fig. 3C–E). These results demonstrate that it is the endogenous neuronal P2Y1 receptor that is responsible for inhibition of the M-current recorded in our experiments.
M-current inhibition by a P2Y1 receptor agonist increases neuronal firing
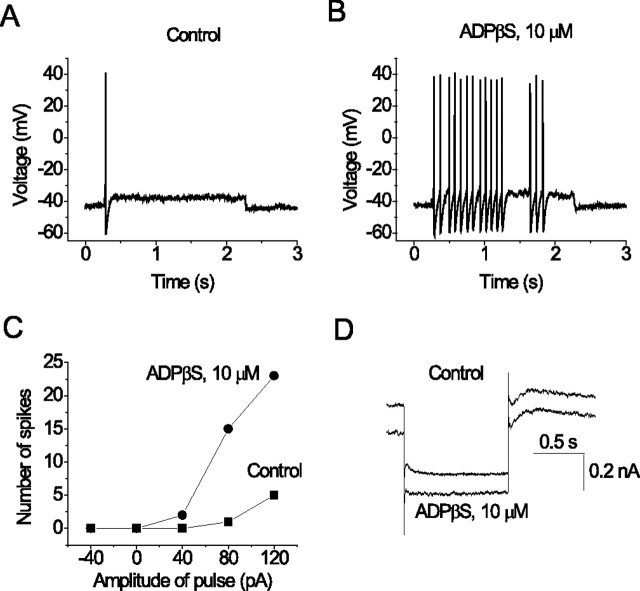
Inhibition of M-current in sympathetic neurons by a heterologously expressed Gq-coupled P2Y receptor (P2Y2) increases cell excitability (Filippov et al., 1998). Activation of endogenous P2Y1 receptors had a similar effect in hippocampal pyramidal neurons. Thus, application of ADPβS significantly increased the firing of pyramidal neurons in response to injected current pulses (Fig. 4). In three experiments, number of spikes initiated by a 2 s. 80 pA superthreshold pulse was 1.3 ± 0.9 before application of ADPβS and increased to 13.0 ± 1.5 after application. This effect was reversible and was accompanied by an increased input resistance as measured by the voltage responses to hyperpolarizing current injections, as expected (data not shown).
Figure 4.
Activation of P2Y1 receptor induces a parallel increase of repetitive firing and inhibition of the M-current in the pyramidal neurons. Records in the top panel show firing patterns of a pyramidal neuron stimulated with a depolarizing pulse (2 s, 80 pA) from the same initial membrane potential in control (A) and after application of ADPβS (B). The graph in C shows the number of action potentials induced by a range of current pulses (each 2 s) in control and with ADPβS. Records in D show M-current deactivations recorded from the same cell. Currents were recorded as in Figure 1A.
Probing the intracellular mechanism of P2Y1-mediated M-current inhibition
The mechanisms whereby G-protein-coupled receptors inhibit M-currents have been the subject of much experimentation and discussion (Delmas and Brown, 2005). In sympathetic neurons, M-current inhibition by expressed P2Y1 receptors is insensitive to pertussis toxin (Brown et al., 2000) and is therefore likely to result from activation of a member of the Gq/11 family of G-proteins. Two major mechanisms for Gq/11-mediated M-current inhibition have been described previously in these neurons, both dependent on accelerated hydrolysis of phosphatidyl-4,5-bisphosphate (PIP2): release of Ca2+ from intracellular stores and subsequent activation of channel-bound calmodulin (Cruzblanca et al., 1998; Gamper and Shapiro, 2003) and depletion of membrane PIP2, which is required to maintain channel opening (Suh and Hille, 2002; H. Zhang et al., 2003; Winks et al., 2005). Previous experiments on rat sympathetic neurons have suggested that M-current inhibition by endogenous UTP-sensitive (i.e., non-P2Y1) receptors was mediated by the former (Ca2+ release) mechanism, in that it was prevented by intracellular Ca2+ chelation, Ca2+ store depletion, or inositol-4,5-trisphosphate receptor blockade (Bofill-Cardona et al., 2000). We therefore tested whether this might be responsible for P2Y1-mediated inhibition of hippocampal pyramidal cell M-currents by the following three approaches.
Intracellular mobilization of Ca2+
It has been reported previously that the P2Y1 receptor agonist 2-methylthio ATP (2-MeSATP) raises intracellular Ca2+ in interneurons and astrocytes (but not in pyramidal neurons) of hippocampal slices (Kawamura et al., 2004). However, the lack of effect on pyramidal neurons could be explained by high activity of ATPases in the slice and fast breakdown of the agonist (see Introduction). Thus, we tested whether the more stable P2Y1 agonist ADPβS raised intracellular Ca2+ in hippocampal pyramidal neurons in culture as measured by single-cell fura-2 fluorescence (see Materials and Methods). No significant change in the fluorescence ratio in any of the pyramidal-like neurons could be detected in any of six experiments (on six dishes) using ADPβS concentrations up to the maximally effective M-current inhibitory concentration of 3 μm. As a control, application of 30–60 mm KCl after washing out ADPβS produced a robust increase of intracellular Ca2+ in all of the same cells tested (Fig. 5A).
Figure 5.
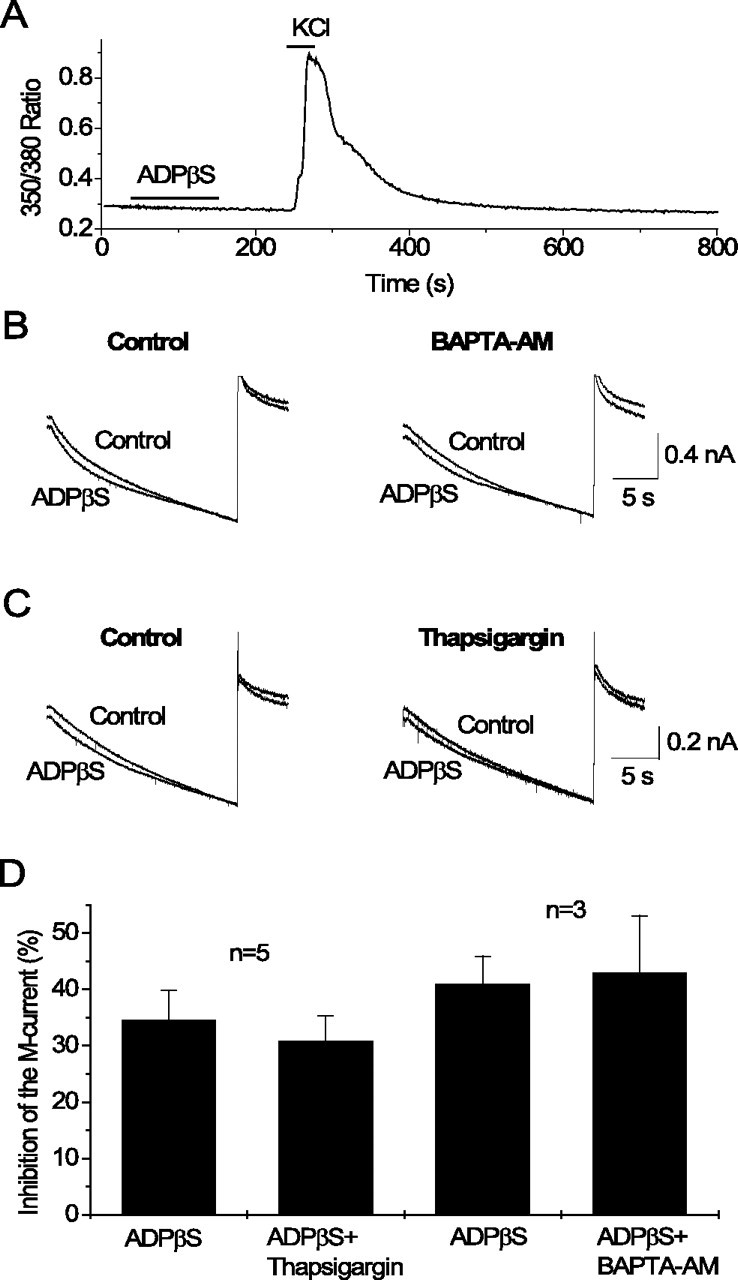
Release of intracellular Ca2+ is not involved in M-current inhibition by P2Y1 receptors in hippocampal pyramidal cells. A, Changes of intracellular Ca2+ measured as the 350/380 nm fluorescence ratio recorded at 1 Hz from a pyramidal-like neuron loaded with fura-2. Bars indicate time of drug application. Note that ADPβS at 3 μm does not induce any changes of intracellular Ca2+, whereas subsequent application of KCl at 60 mm induces a substantial increase. B, C, Currents recorded in response to voltage ramps (20 s) from −20 to −90 mV (as in Fig. 3A) in control and after application of ADPβS at 3 μm before (Control) and after 15 min superfusion of the same cell with BAPTA-AM at 10 μm (B) or with thapsigargin at 1 μm (C). D, Mean percentage inhibition of M-current by ADPβS before and after 15 min superfusion with BAPTA-AM or thapsigargin. Error bars show SEM of inhibition of the M-current by ADPβS measured at −30 mV from steady-state current–voltage relationships. n indicates the number of experiments.
Intracellular Ca2+ chelation
We then tested whether chelation of intracellular Ca2+ by BAPTA-AM affected M-current inhibition by ADPβS. We applied 3 μm ADPβS before and after 15 min perfusion with 10 μm BAPTA-AM (3 μm having produced complete suppression of the response to UTP in the experiments by Bofill-Cardona et al., 2000). In three separate experiments, ADPβS at 3 μm inhibited the M-current (measured at −30 mV from ramp current–voltage relationships) by 41.0 ± 4.9%, whereas after superfusing the same cells with BAPTA-AM, ADPβS inhibited the M-current by 43.0 ± 10.1%, the difference being statistically insignificant (Fig. 5B,D).
Ca2+ store depletion
We further tested whether the Ca2+-ATPase inhibitor thapsigargin affected M-current inhibition by ADPβS. We applied 3 μm ADPβS before and after perfusion for 15 min with 1 μm thapsigargin (as in the experiments by Bofill-Cardona et al., 2000). In five separate experiments, ADPβS at 3 μm inhibited the M-current (measured at −30 mV from current–voltage relationships) by 34.7 ± 5.1%, whereas after superfusing the same cells with thapsigargin, ADPβS inhibited the M-current by 30.9 ± 4.4%, the difference being statistically insignificant (Fig. 5C,D).
In summary, our data indicate that the release of intracellular Ca2+ is not involved in M-current inhibition by P2Y1 receptors in hippocampal pyramidal cells.
Discussion
In these experiments, we have shown that functional endogenous P2Y1 receptors are present in pyramidal neurons from the CA1/CA3 area of hippocampus and that activation of these P2Y1 receptors potently inhibits the M-current.
For comparison, two studies have recently detected functional P2Y1 receptors in some of the interneurons in recordings made on mouse (Bowser and Khakh, 2004) and rat (Kawamura et al., 2004) hippocampal slices. Activation by ATP of these receptors excited IPSCs in those neurons (as recorded from the pyramidal cells to which they projected) through an action on ion channels as yet unidentified. Kawamura et al. (2004) concluded that P2Y1 receptors were not present on pyramidal neurons because 2-MeSATP did not raise intracellular Ca2+ in the pyramidal cell layer. However, as indicated by the results of the present experiments, P2Y1 receptor activation would not be expected to have such an effect in these neurons (see further below). Bowser and Khakh (2004) did not test effects of ADP or ADPβS on pyramidal cells. They reported an inward current in interneurons but did not specifically record M-currents.
Independent evidence is available to confirm the presence of P2Y1 receptors on pyramidal neurons, because it has been established recently by single-cell reverse transcription (RT)-PCR analysis on laser-dissected individual neurons that pyramidal cells in the young rat hippocampus strongly express the P2Y1 receptor mRNA (Rodrigues et al., 2005). The existing electrophysiological evidence discussed above on P2Y1 receptors in the CA1 and CA3 regions (Bowser and Khakh, 2004; Kawamura et al., 2004) indicated that some of them reside at sites on interneurons presynaptic to pyramidal cells. We indeed found that some of the interneurons contain P2Y1 receptor immunoreactivity. We conclude, however, that the great majority of the P2Y1 receptors on pyramidal cells must be postsynaptic to afferent neurons, because of the following: (1) in analyses recently reported of rat hippocampal synaptosomes, the P2Y1 receptor protein is abundant and primarily segregates with postsynaptic density protein (Rodrigues et al., 2005); (2) P2Y1 receptor mRNA is clearly expressed in the pyramidal cells, as noted above, confirming that the protein originates within them; and (3) this likewise holds for the M-current channel KCNQ (Kv7) subunits, in which single-cell RT-PCR again showed that their mRNA is also well expressed in the pyramidal cells, and their protein is seen on their somatic membrane and dendrites (Shah et al., 2002) (see also Roche et al., 2002). Furthermore, the M-current inhibition that we recorded cannot have resulted indirectly from the release of a transmitter from interneurons because it was recorded in the presence of TTX, DNQX, and bicuculline.
There is strong evidence that most, if not all, of the cultured neurons in which nucleotides can inhibit the M-current are pyramidal neurons. First, when neurons that had produced a membrane current response to the P2Y1 nucleotides were subsequently stained with antibodies, they were positive for neurogranin (Fig. 2A). Second, the same neurons, plus the others not post-stained but giving an equivalent membrane current response, also showed the firing behavior expected for pyramidal cells. Finally, although some hippocampal interneurons have been reported to express Kv7 (KCNQ) channel subunits (Cooper et al., 2000) and can express M-currents (Lawrence et al., 2006), not all interneurons do so, whereas pyramidal cells consistently exhibit robust M-currents, both in situ (Storm, 1990; Peters et al., 2005) and in culture (Shah et al., 2002).
The inhibition of the M-current in hippocampal neurons through activation of their endogenous P2Y1 receptors was predicted from previous experiments on sympathetic neurons, in which the native M-current could be robustly and potently inhibited by adenosine nucleotides when P2Y1 receptors were expressed therein (Brown et al., 2000) (for a comparable effect in PC12 cells, see Moskvina et al., 2003). In these experiments, activation of expressed receptors with ADP also inhibited the N-type Ca2+ current in sympathetic neurons with equal potency (Brown et al., 2000). That shared function appears to extend also to hippocampal neurons, because J. M. Zhang et al. (2003) reported that ATP inhibited both somatic Ca2+ currents and glutamatergic excitatory transmission through a P2Y receptor in hippocampal cell cultures.
Activation of endogenous P2Y receptors (including P2Y1 receptors) increases intracellular Ca2+ in mouse sympathetic neurons (Calvert et al., 2004), and previous experiments had suggested that the release of intracellular Ca2+ was responsible for M-current inhibition by UTP-sensitive P2Y receptors in rat sympathetic neurons (Bofill-Cardona et al., 2000). However, this appears not to apply to the effect of P2Y1 receptors in hippocampal neurons, because ADPβS did not produce a detectable increase in intracellular Ca2+, and M-current inhibition was unaffected by chelating intracellular Ca2+ with BAPTA-AM or by the Ca2+-ATPase inhibitor thapsigargin, which would be expected to deplete intracellular Ca2+ stores. As noted above, Kawamura et al. (2004) also found that P2Y1 receptor activation failed to release intracellular Ca2+ in pyramidal neurons in fresh hippocampal slices. Thus, our findings agree with previous conclusions regarding the inhibition of M-currents in frog sympathetic ganglia by their (unidentified) ATP-sensitive P2Y receptor (Stemkowski et al., 2002). Instead, in the latter, P2Y-mediated inhibition probably results from depletion of membrane PIP2 after PIP2 hydrolysis (Ford et al., 2003). We therefore suspect this to be the most likely mechanism for P2Y1-mediated M-current inhibition in hippocampal neurons.
What might the physiological significance of our observations be? We should first recall that the M-current is now known to play a key role in regulating neuronal firing frequency and excitability in brain neurons (Aiken et al., 1995; Shah et al., 2002 and references therein; Yue and Yaari, 2004; Peters et al., 2005; Shen et al., 2005). Its control there by a neurotransmitter system widely available in the brain, ATP or ADP, for which this is the first demonstration, can be expected to be functionally significant. Thus, in the present experiments, we observed that stimulation of the endogenous P2Y1 nucleotide receptors in CA1/CA3 pyramidal neurons significantly increases depolarization-induced neuronal firing, in the manner observed previously after heterologous expression of P2Y2 receptors in sympathetic neurons (Filippov et al., 1998) A second and related point to note is that adenosine nucleotides are extremely potent inhibitors of M-current, mediated by P2Y1 receptors, with an IC50 for ADPβS of 84 nm. Although ADP itself inhibited M-current somewhat less completely, it is probably still strongly active at submicromolar concentrations, because the IC50 for M-current inhibition by ADP in sympathetic neurons was even lower, at 6.9 nm (Brown et al., 2000). It is reasonable to suppose that such concentrations could readily be achieved through breakdown of any released ATP by ectonucleotidases (Zimmermann and Braun, 1996). Thus, it has been reported recently that hypercapnia releases ATP from the ventral medulla to give a concentration of 3.8 μm as registered by a biosensor in contact with the outer surface of the ventral medulla (Gourine et al., 2005). Likewise, hypercapnia increases the extracellular concentration of adenosine in hippocampal slices by ∼0.7 μm (Dulla et al., 2005).
There are two available sources for nucleotide release here. First, ATP is synaptically released onto hippocampal pyramidal neurons, in which it activates P2X receptors to induce an inward synaptic current that contributes (along with glutamate) to the fast EPSC (Pankratov et al., 1998). Thus, after trains of afferent stimuli, there may be sufficient build-up of ATP (with conversion to ADP) to activate P2Y1 receptors and inhibit the M-current. Inhibition of the M-current will increase neuronal firing as observed here and also can generate a slow EPSP (Gahwiler and Brown, 1985). This would be comparable with the slow EPSP induced by glutamate, acting via metabotropic glutamate receptors, after trains of afferent stimuli (Charpak and Gahwiler, 1991). Thus, physiological consequences of M-current inhibition via P2Y1 receptors might be similar to those produced by activation of metabotropic group 1 glutamate receptors. If so, there may be some segregation of the two responses, because we noted that the two receptors were not always expressed on the same neurons, as judged from responses of M-currents to ADPβS and DHPG. A second source of ATP is through release from neighboring glial cells (Newman, 2003), whence it can produce both tonic and activity-dependent effects on hippocampal neurons (Koizumi et al., 2003; J. M. Zhang et al., 2003; Bowser and Khakh, 2004).
Footnotes
This work was supported by the Wellcome Trust. R.C.Y.C. held a Royal Society International Postdoctoral Fellowship. We thank Joanna Reilly for tissue culture and Dr. Steve Marsh for help with Ca2+ imaging experiments.
References
- Aiken SP, Lampe BJ, Murphy PA, Brown BS. Reduction of spike frequency adaptation and blockade of M-current in rat C1 pyramidal neurons by linopirdine (Dup-996), a neurotransmitter release enhancer. Br J Pharmacol. 1995;115:1163–1168. doi: 10.1111/j.1476-5381.1995.tb15019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger BE, Sim JA, Brown DA. Single-channel activity correlated with medium-duration, Ca-dependent K current in cultured rat hippocampal neurones. Neurosci Lett. 1994;168:23–28. doi: 10.1016/0304-3940(94)90407-3. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Simon J, Webb TE. Nucleotide receptors in the nervous system. An abundant component using diverse transduction mechanisms. Mol Neurobiol. 1997;15:103–129. doi: 10.1007/BF02740631. [DOI] [PubMed] [Google Scholar]
- Bofill-Cardona E, Vartian N, Nanoff C, Freissmuth M, Boehm S. Two different signaling mechanisms involved in the excitation of rat sympathetic neurons by uridine nucleotides. Mol Pharmacol. 2000;57:1165–1172. [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci. 2004;24:8606–8620. doi: 10.1523/JNEUROSCI.2660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown DA, Filippov AK, Barnard EA. Inhibition of potassium and calcium currents in neurones by molecularly-defined P2Y receptors. J Auton Nerv Syst. 2000;81:31–36. doi: 10.1016/s0165-1838(00)00150-8. [DOI] [PubMed] [Google Scholar]
- Calvert JA, Atterbury-Thomas AE, Leon C, Forsythe ID, Gachet C, Evans RJ. Evidence for P2Y1, P2Y2, P2Y6 and atypical UTP-sensitive receptors coupled to rises in intracellular calcium in mouse cultured superior cervical ganglion neurons and glia. Br J Pharmacol. 2004;143:525–532. doi: 10.1038/sj.bjp.0705959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak S, Gahwiler BH. Glutamate mediates a slow synaptic response in hippocampal slice cultures. Proc Biol Sci; 1991. pp. 221–226. [DOI] [PubMed] [Google Scholar]
- Charpak S, Gahwiler BH, Do KQ, Knopfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990;347:765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Cooper EC, Aldape KD, Abosch A, Barbaro NM, Berger MS, Peacock WS, Jan YN, Jan LY. Colocalization and coassembly of two human brain M-type potassium channel subunits that are mutated in epilepsy. Proc Natl Acad Sci USA. 2000;97:4914–4919. doi: 10.1073/pnas.090092797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzblanca H, Koh DS, Hille B. Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc Natl Acad Sci USA. 1998;95:7151–7156. doi: 10.1073/pnas.95.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenquelli B, Staley KJ, Masimo SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;46:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Webb TE, Barnard EA, Brown DA. P2Y2 nucleotide receptors expressed heterologously in sympathetic neurons inhibit both N-type Ca2+ and M-type K+ currents. J Neurosci. 1998;18:5170–5179. doi: 10.1523/JNEUROSCI.18-14-05170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Stemkowski PL, Light PE, Smith PA. Experiments to test the role of phosphatidylinositol 4,5-bisphosphate in neurotransmitter-induced M-channel closure in bullfrog sympathetic neurons. J Neurosci. 2003;23:4931–4941. doi: 10.1523/JNEUROSCI.23-12-04931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine A1-receptor-mediated inhibition of evoked acetylcholine release in the rat hippocampus does not depend on protein kinase C. Acta Physiol Scand. 1990;140:245–255. doi: 10.1111/j.1748-1716.1990.tb08996.x. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Brown DA. Functional innervation of cultured hippocampal neurones by cholinergic afferents from co-cultured septal explants. Nature. 1985;313:577–579. doi: 10.1038/313577a0. [DOI] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels. J Gen Physiol. 2003;122:17–31. doi: 10.1085/jgp.200208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Illes P, Ribeiro AJ. Molecular physiology of P2 receptors in the central nervous system. Eur J Pharmacol. 2004;483:5–17. doi: 10.1016/j.ejphar.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Gachet C, Inoue K, Kato F. Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J Neurosci. 2004;24:10835–10845. doi: 10.1523/JNEUROSCI.3028-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H, Kugelgen I, Starke K. P2-receptor-mediated inhibition of noradrenaline release in the rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:707–715. doi: 10.1007/pl00005003. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci USA. 2003;100:11023–11028. doi: 10.1073/pnas.1834448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Stausberg P, Adelmann G, Chessell IP, Dietrich D. Ecto-nucleotidases and nucleoside transporters mediate activation of adenosine receptors on hippocampal mossy fibers by P2X7 receptor agonist 2′-3′-O-(4-benzoylbenzoyl)-ATP. J Neurosci. 2004;24:7128–7139. doi: 10.1523/JNEUROSCI.2093-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence IJ, Statland JM, Grinspan ZM, McBain CJ. Cell type-specific muscarinic signalling in mouse hippocampal stratum oriens interneurons. J Physiol (Lond) 2006;570:595–610. doi: 10.1113/jphysiol.2005.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol (Lond) 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol (Lond) 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Lancaster B, Nicoll RA. Voltage-clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987;7:733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Diao L, Illes P, Zahniser NR, Larson GA, Johansson B, Fredholm BB, Dunwiddie TV. Modulation of hippocampal glutamatergic transmission by ATP is dependent on adenosine A1 receptors. J Pharmacol Exp Ther. 2002;303:356–363. doi: 10.1124/jpet.102.036731. [DOI] [PubMed] [Google Scholar]
- Moore D, Chambers J, Waldvogel H, Faull R, Emson P. Regional and cellular distribution of the P2Y1 purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol. 2000;421:374–384. doi: 10.1002/(sici)1096-9861(20000605)421:3<374::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Moran-Jimenez MJ, Matute C. Immunohistochemical localization of the P2Y1 purinergic receptor in neurons and glial cells of the central nervous system. Brain Res Mol Brain Res. 2000;78:50–58. doi: 10.1016/s0169-328x(00)00067-x. [DOI] [PubMed] [Google Scholar]
- Moskvina E, Unterberger U, Boehm S. Activity-dependent autocrine-paracrine activation of neuronal P2Y receptors. J Neurosci. 2003;23:7479–7488. doi: 10.1523/JNEUROSCI.23-20-07479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandaran E, Jang SY, Moro S, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, Boyer JL, Jacobson KA. Synthesis, biological activity, and molecular modeling of ribose modified deoxyadenosine bisphosphate analogues as P2Y1 receptor ligands. J Med Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiishi T, Saitoh Y, Yukawa A, Saji M, Ren Y, Shirao T, Miyamoto H, Nakata H, Sekino Y. High level of adenosine A1 receptor-like immunoreactivity in the CA2/CA3a region of the adult rat hippocampus. Neuroscience. 1999;93:955–967. doi: 10.1016/s0306-4522(99)00179-7. [DOI] [PubMed] [Google Scholar]
- O’Kane EM, Stone TW. Characterisation of ATP-induced facilitation of transmission in rat hippocampus. Eur J Pharmacol. 2000;409:159–166. doi: 10.1016/s0014-2999(00)00785-8. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur J Neurosci. 1998;10:3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8:51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- Roche JP, Westenbroek R, Sorom AJ, Hille B, Mackie K, Shapiro MS. Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K+ channels. Br J Pharmacol. 2002;137:1173–1186. doi: 10.1038/sj.bjp.0704989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in rat hippocampus. J Neurosci. 2005;25:6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD, Goldsworthy J, Johnson BG, Salhoff CR, Baker SR. 3,5-dihydroxyphenylglycine is a highly selective agonist for phosphoinositide-linked metabotropic glutamate receptors in the rat hippocampus. J Neurochem. 1994;63:769–772. doi: 10.1046/j.1471-4159.1994.63020769.x. [DOI] [PubMed] [Google Scholar]
- Shah M, Haylett DG. Ca2+ channels involved in the generation of the slow afterhyperpolarization in cultured rat hippocampal pyramidal neurons. J Neurophysiol. 2000;83:2554–2561. doi: 10.1152/jn.2000.83.5.2554. [DOI] [PubMed] [Google Scholar]
- Shah MM, Mistry M, Marsh SJ, Brown DA, Delmas P. Molecular correlates of the M-current in cultured rat hippocampal neurons. J Physiol (Lond) 2002;544:29–37. doi: 10.1113/jphysiol.2002.028571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25:7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Vigne P, Eklund KM, Michel AD, Carruthers AM, Humphrey PPA, Frelin C, Barnard EA. Activity of adenosine diphosphates and triphosphates on a P2YT-type receptor in brain capillary endothelial cells. Br J Pharmacol. 2001;132:173–182. doi: 10.1038/sj.bjp.0703816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singec I, Knoth R, Ditter M, Volk B, Frotscher M. Neurogranin is expressed by principal cells but not interneurons in the rodent and monkey neocortex and hippocampus. J Comp Neurol. 2004;479:30–42. doi: 10.1002/cne.20302. [DOI] [PubMed] [Google Scholar]
- Stemkowski PL, Tse FW, Peuckmann V, Ford CP, Colmers WF, Smith PA. ATP-inhibition of M current in frog sympathetic neurons involves phospholipase C but not Ins P3, Ca2+, PKC, or Ras. J Neurophysiol. 2002;88:277–288. doi: 10.1152/jn.2002.88.1.277. [DOI] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- Tung EKK, Choi RCY, Siow NL, Jiang JXS, Karen KY, Ling KKY, Simon J, Barnard EA, Tsim KWK. P2Y2 receptor activation regulates the expression of acetylcholinesterase and acetylcholine receptor genes at vertebrate neuromuscular junctions. Mol Pharmacol. 2004;66:794–806. doi: 10.1124/mol.104.003269. [DOI] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Webb TE, Simon J, Barnard EA. Regional distribution of [35S]2′-deoxy 5′-O-(1-thio) ATP binding sites and the P2Y1 messenger RNA within the chick brain. Neuroscience. 1998;84:825–837. doi: 10.1016/s0306-4522(97)00478-8. [DOI] [PubMed] [Google Scholar]
- Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, Brown DA, Marsh SJ. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25:3400–3413. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, Logothetis DE. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Braun N. Extracellular metabolism of nucleotides in the nervous system. J Auton Pharmacol. 1996;16:397–400. doi: 10.1111/j.1474-8673.1996.tb00062.x. [DOI] [PubMed] [Google Scholar]