Abstract
Cadherins are transmembrane glycoproteins that mediate Ca2+-dependent homophilic cell–cell adhesion and play crucial role during skeletal myogenesis. M-cadherin is required for myoblast fusion into myotubes, but its mechanisms of action remain unknown. The goal of this study was to cast some light on the nature of the M-cadherin–mediated signals involved in myoblast fusion into myotubes. We found that the Rac1 GTPase activity is increased at the time of myoblast fusion and it is required for this process. Moreover, we showed that M-cadherin–dependent adhesion activates Rac1 and demonstrated the formation of a multiproteic complex containing M-cadherin, the Rho-GEF Trio, and Rac1 at the onset of myoblast fusion. Interestingly, Trio knockdown efficiently blocked both the increase in Rac1-GTP levels, observed after M-cadherin–dependent contact formation, and myoblast fusion. We conclude that M-cadherin–dependent adhesion can activate Rac1 via the Rho-GEF Trio at the time of myoblast fusion.
INTRODUCTION
During skeletal muscle development mesodermal precursor cells give rise to committed myoblasts that, after proliferation and migration to the appropriate sites in the embryo, exit the cell cycle, express muscle-specific genes, and fuse into multinucleated myofibers that mature to form multinucleated muscle fibers (Taylor, 2002). Although myoblast fusion is important both during embryonic development and in the maintenance and repair of adult muscles, the mechanisms regulating this process are largely unknown. Myoblast fusion is a multistep process that entails initial recognition and adhesion between myoblasts, their alignment, and finally membrane breakdown and fusion (Doberstein et al., 1997). This process is regulated, at different levels, by a variety of proteins, such as transcription factors or extracellular signaling molecules, including diffusible factors, components of the extracellular matrix, and proteins involved in cell–cell contact (Krauss et al., 2005).
In this later group, M-cadherin plays a prominent role. M-cadherin belongs to the cadherin family of Ca2+-dependent adhesion molecules. Its N-terminal extracellular domain mediates homophilic binding, while the cytoplasmic tail interacts with catenins and is linked to the actin cytoskeleton, thus, coupling the ectodomain interactions to the dynamic intracellular tensile forces (Wheelock and Johnson, 2003b). M-cadherin is found predominantly in developing skeletal muscles and is highly expressed during secondary myogenesis. In mature skeletal muscle, M-cadherin is detectable in satellite cells and on the sarcolemma of myofibers underlying satellite cells (Moore and Walsh, 1993; Rose et al., 1994; Cifuentes-Diaz et al., 1995). M-cadherin is also found at neuromuscular junctions, intramuscular nerves, and in two regions of the CNS, namely the spinal cord and the cerebellum (Cifuentes-Diaz et al., 1996; Bahjaoui-Bouhaddi et al., 1997). M-cadherin–deficient mice do not show defects in skeletal muscle development, probably because of compensation by other cadherins, in particular N-cadherin (Hollnagel et al., 2002). However, data on cultured myoblasts have suggested that M-cadherin could be critical for the fusion of myoblasts to myotubes (Donalies et al., 1991; Pouliot et al., 1994; Zeschnigk et al., 1995; Kuch et al., 1997; Charrasse et al., 2006).
Beside their role in cell recognition, the classical cadherins are adhesion-activated signaling receptors which activate Rho-family GTPases (Wheelock and Johnson, 2003a). The activity of Rho GTPases needs to be tightly controlled to allow myogenesis induction and also myoblast fusion (Luo et al., 1994; Hakeda-Suzuki et al., 2002; Charrasse et al., 2003; Fernandes et al., 2005). RhoA has been reported to positively regulate MyoD expression and skeletal muscle cell differentiation, as it has been demonstrated to be required for serum response factor (SRF)-mediated activation of several muscle-specific gene promoters (Carnac et al., 1998; Wei et al., 1998). On the other hand, Rac1 inhibits myogenesis induction by preventing the withdrawal of myoblasts from the cell cycle (Meriane et al., 2000b, 2002). This coordinated regulation of RhoA and Rac1 during myogenesis induction has been shown to be orchestrated by N-cadherin (Charrasse et al., 2002). Later on during the skeletal muscle differentiation program and in contrast to its inhibitory role in myogenesis induction, Rac1 signaling has been shown to be involved in myoblast fusion, at least in Drosophila (Luo et al., 1994; Hakeda-Suzuki et al., 2002; Fernandes et al., 2005; Erickson et al., 1997; Nolan et al., 1998).
In the present study we show, for the first time, that Rac1 is also involved in mammalian myoblast fusion in the myogenic cell line C2C12. Moreover, we demonstrate that M-cadherin–dependent cell–cell adhesion activates Rac1 by using an M-cadherin ligand, allowing us to mimic M-cadherin–mediated adhesion, or antibodies that specifically recognize the extracellular domain of M-cadherin. Then to elucidate the molecular mechanisms coupling M-cadherin to Rac1 activation, we have analyzed the role of the guanine nucleotide exchange factor (GEF) Trio in the fusion of C2C12 myoblasts. Rho GTPases are activated by the GEF family of proteins promoting the exchange of GDP for GTP (Rossman et al., 2005). We have focused our attention on this Rho-GEF, because the genetical ablation of Trio in mice (trio−/−) showed that Trio is essential for late embryonic development and that it plays a role in skeletal muscle formation and neural tissues organization (O'Brien et al., 2000). Trio contains two Rho-GEF domains: GEFD1, which activates both Rac1 and RhoG, and GEFD2, which acts on RhoA (Bellanger et al., 1998; Blangy et al., 2000). Here, we demonstrate that an inhibition of Trio expression by RNA interference impairs myoblast fusion, as does the inhibition of M-cadherin expression or Rac1 activity. Moreover, Trio knockdown decreases M-cadherin–dependent Rac1 activation. These results shed some light on the mechanisms via which the M-cadherin receptor is functionally coupled to Rac1 activation in C2C12 myoblasts.
MATERIALS AND METHODS
Cell Culture
C2C12 mouse myoblasts were grown as described previously (Charrasse et al., 2006). Stable cell lines derived from C2C12 myoblasts were cultured under the same conditions in medium supplemented with 80 μl hygromycin. The Rac1 inhibitor NSC23766 (Calbiochem, La Jolla, CA) was used at 100 μm and was added 2–16 h after differentiation medium (DM; DMEM/Ham's F-12 supplemented with 2% FCS) addition.
Establishment of Trio Short Interfering RNA Stable Cell Lines
Short interfering RNA (shRNA) constructs were made in pRETROSUPER polymerase III expression vector. To suppress endogenous Trio expression, the oligonucleotide GATCCCCGTGAAGCTATTGATACAGCttcaagagaGCTGTATCAATAGC-TTCACTTTTTGGAAA was inserted into pRETROSUPER. Bold letters correspond to oligonucleotides 3934–3952 of the mouse Trio cDNA sequence (XM980554). As a control, we used the oligonucleotide GATCCCCCTTAATAAGAGAAGCGGAttcaagagaTCCGCTTCTCTTATTAAGTTTTGGAAA, which corresponds to a modified sequence (one base is missing) of the Trio nucleotides 4192–4210. Hygromycin-resistant clones constitutively expressing Trio shRNA were grown in order to harvest retrovirus-containing cell-free supernatants. Infection of C2C12 myoblasts was performed as described (Meriane et al., 2000a). Different clones were isolated by limited dilution and continuously grown in hygromycin.
Differentiation Inhibition Assays
C2C12 cells plated in 35-mm dishes were cultured in DM. Twenty-four hours later, an anti-M-cadherin antibody (Charrasse et al., 2006) or its preimmune serum were added as described previously (Charrasse et al., 2002).
Isolation of Detergent-resistant Membranes
Twenty-four 150-mm dishes of C2C12 cells cultured in DM for 2 d were collected and processed as previously described (Causeret et al., 2005). Fractions were analyzed by immunoblotting for caveolin (Transduction Laboratories, Lexington, KY; 1:5000) and M-cadherin (NanoTools, Munich, Germany; 1/200). For immunoprecipitation experiments, fractions 3–5 were pooled and diluted 5× in 25 mM MOPS, pH 6.5, 150 mM NaCl and 1% Triton X-100, then they were centrifuged at 4°C at 100,000 × g for 18 h. Protein concentration was determined with a BCA protein assay kit (Pierce, Rockford, IL).
Gel Electrophoresis and Immunoblotting
Cell extracts were prepared as described (Mary et al., 2002). Protein, 30 μg, was resolved on polyacrylamide gel (6, 8, and 15%) and transferred onto Immobilon-P or nitrocellulose (only for Trio) membranes. Membranes were then incubated with monoclonal antibodies against β1-integrin (1:2500; from Transduction Laboratories); troponin T (1:1000), myosin (1:2000), or desmin (1:2000) (all from Sigma-Aldrich, St. Louis, MO); or myogenin (1:500, PharMingen, San Diego, CA), α-tubulin (1:100), M-cadherin (1:200, NanoTools), or goat anti-Trio (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA). After washing, membranes were processed as described (Mary et al., 2002).
Immunoprecipitation
Cells were processed as previously described (Mary et al., 2002). 500 μg of protein extracts were immunoprecipitated using a polyclonal anti-Trio antibody (Portales-Casamar et al., 2006), an anti-M-cadherin antibody (Charrasse et al., 2006) or an anti-myc antibody, separated on a polyacrylamide gel, and then transferred onto Immobilon-P. For Rac1 detection, immunoprecipitation products were heated at 80°C for 3 min. in 80 mM Tris, pH 6.8, 2% SDS, 0.2% Bromophenol blue, 10% glycerol, iodoacetamide (19.3 mg/ml). Membranes were probed with M-cadherin and Rac1 (1:250, Transduction Laboratories) monoclonal antibodies and processed as described (Mary et al., 2002).
Preparation of M-cad-Fc–coated Dishes
The M-cad-Fc chimera (a 1794-base pair PCR fragment of M-cadherin that contains the extracellular domain fused to the Fc fragment of human IgG1 subcloned into the pIG1 vector; Williams et al., 1994) was produced in Cos cells after transfection with Jet PEI reagent (Qbiogene, Carlsbad, CA; MP Biomedicals, Solon, OH) and culture in Optimem for 3 d. Supernatant was collected and the concentration of the chimeric protein was estimated by Western blot analysis. Thirty-five- or 100-mm Petri dishes were coated as described previously (Charrasse et al., 2002). Isolated cells were plated onto coated Petri dishes, allowed to set for 4–24 h, and then processed to measure Rac1 activity and to analyze the organization of the F-actin cytoskeleton.
Rho GTPase Activity Assay
C2C12 myoblasts either in proliferating or during the course of differentiation were lysed and processed to measure the total and GTP Rac1 and RhoA levels as described (Charrasse et al., 2002). The PAK-GST protein beads were from Cytoskeleton (Denver, CO).
Immunofluorescence
Cells growing onto 35-mm dishes were fixed in 3.7% formaldehyde in PBS followed by a 5-min permeabilization in 0.1% Triton X-100 in PBS and incubated in PBS containing 0.1% BSA. Anti-troponin T (1:100, Sigma- Aldrich) and anti-myogenin (1:30, Santa Cruz Biotechnology) antibodies were revealed using an Alexa Fluor 546–conjugated goat anti-mouse antibody or an Alexa Fluor 488–conjugated goat anti-rabbit antibody (Molecular Probes, Eugene, OR; Interchim, Lyon, France). Cells were analyzed as described previously (Charrasse et al., 2002).
Cells were stained for F-actin with TRITC-conjugated phalloidin (Sigma-Aldrich, St. Louis, MO) and analyzed with a Metamorph-driven (Molecular Devices, Sunnyvale, CA) spinning-disk confocal microscope (Yokogawa/Perkin Elmer-Cetus, Norwalk, CT) equipped with a krypton/argon ion laser (Melles Griot, Rochester, NY). Images were taken with a PL APO 63× objective (NA 1.32, Leica, Melville, NY) and a Coolsnap HQ camera (Photometrics, Woburn, MA). Stacks of images were captured with a piezo stepper (E662, Physik Instruments, Waldbronn, Germany) with a 0.2-μm Z step. Stacks were then restored with the Huygens deconvolution software (Scientific Volume Imaging) and the restored images were viewed in 3D with MetaMorph.
Time-Lapse Imaging
C2C12 cells were transfected with actin-RFP and isolated cells were plated onto Mcad-Fc coated Petri dishes for 24 h and analyzed for the dynamic of the F-actin cytoskeleton. Time-lapse epifluorescence microscopy was performed as previously described (Mary et al., 2002). The exposure time is 1500 ms. Fluorescent images were restored using a maximum likelihood estimation (MLE) deconvolution algorithm (Huygens, Scientific Volume, Imaging, Hilversrum, The Netherlands). The restored images were saved as Tif files and further compiled into QuickTime movies using Montpellier RIO Imaging Cell Image Analyzer program (Baecker and Travo, 2006).
RESULTS
Rac1 GTPase Activity Increases at the Onset of Myoblast Fusion
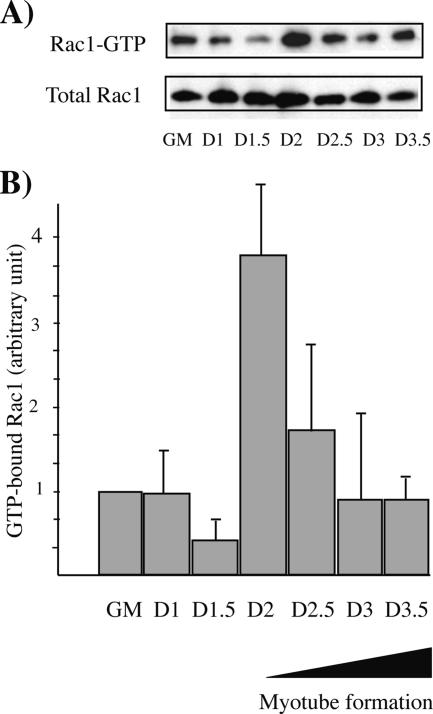
In Drosophila, studies using loss-of-function Rac mutations or the expression of active and dominant negative mutants of Rac1 have suggested an involvement of this protein in myoblast fusion (Luo et al., 1994; Hakeda-Suzuki et al., 2002; Fernandes et al., 2005). Thus, we measured Rac1 activity by using pulldown assays at different times after the shift of mammalian C2C12 myoblasts to DM (Figure 1, A and B). Rac1-GTP levels are increased after 2 d in DM, which corresponds to the onset of the fusion process as described previously (Charrasse et al., 2006).
Figure 1.
Variation of Rac1 activity during myogenesis. (A) The level of GTP-bound Rac1 was measured using GST fused to the Rac-binding domain of the Rac1 effector PAK (GST-CRIB) in lysates obtained from C2C12 myoblasts in growth medium (GM) or differentiation medium (DM) collected at the indicated times (1–3.5 d after addition of the DM). Rac1 was detected by immunoblotting. GM, growth medium; D, day. (B) Three independent experiments were analyzed by densitometry, as described in Materials and Methods. The histogram represents the GTP-bound Rac1 normalized to the amount of total protein.
The Activation of Rac1 Is Required for Myoblast Fusion
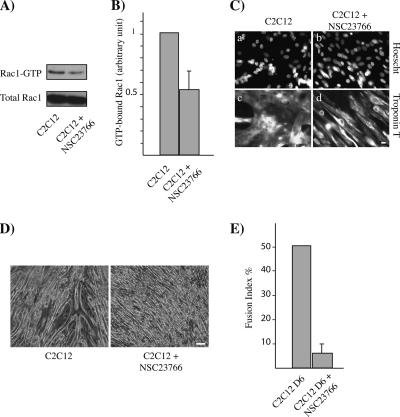
We next investigated the role of Rac1 activity in myoblast fusion by using NSC23766, a chemical inhibitor of Rac1 (Gao et al., 2004). We first tested whether this Rac1 inhibitor is efficient in C2C12 myoblasts. As shown in Figure 2, A and B, NSC23766 strongly decreased Rac1 activity, when added to proliferative C2C12 myoblasts. A decrease in Rac1 activity was also measured 2 d after DM addition (data not shown). We then analyzed whether this Rac1 inhibitor could affect the fusion process. Because we know that Rac1 inhibition impairs myogenesis induction (Meriane et al., 2000b), the addition of the Rac1 inhibitor was performed at least 2 h after DM addition. C2C12 myoblasts treated with NSC23766 expressed muscle-specific proteins, such as myogenin (data not shown) and troponin T (Figure 2C). However, we were unable to detect any significant myotube formation in NSC23766-treated myoblasts 6 d after DM addition, and cells remained aligned and elongated (Figure 2D). Consistently, the fusion index was dramatically reduced after NSC23766 treatment (Figure 2E). Similarly reduced myoblast fusion was observed also when the Rac1 inhibitor was added between 2 and 48 h after DM addition. Its effect was reversible because its removal allowed myoblast fusion (data not shown). Moreover, we could show a similar effect in primary myoblasts as well, where the addition of NSC23766 inhibited again the fusion into myotubes (data not shown). Taken together, these data suggest that the increase of Rac1 activity at the onset of myoblast fusion is crucial for myotube formation.
Figure 2.
Effect of the inhibition of Rac1 activity on myoblast fusion. (A) The level of GTP-bound Rac1 was measured in lysates obtained form C2C12 myoblasts either untreated (left) or treated for 5 h with the Rac1 inhibitor NSC23766 (right). Rac1 was detected by immunoblotting. (B) The histogram is representative of three independent experiments that were analyzed by densitometry. (C) C2C12 myoblasts were grown up to 80% confluence and shifted to DM without (a and c) or with the Rac1 inhibitor (b and d). Cells were fixed and stained for troponin T expression. Bar, 10 μm. (D) Phase-contrast images of C2C12 myoblasts and myotubes after 6 d in DM in absence (a) or in presence of the Rac1 inhibitor (b). Bar, 30 μm. (E) The fusion index, i.e., the number of nuclei in multinucleated myotubes divided by the total number of nuclei, was calculated for the conditions described in D. Cells with a minimum of three nuclei were considered as myotubes. The histogram represents the fusion index calculated from five independent experiments, where at least 7000 nuclei in each experiment were counted using the MRI Cell Image Analyzer program (Baecker and Travo, 2006).
Inhibition of M-Cadherin Homophilic Association Specifically Impairs the Fusion Process
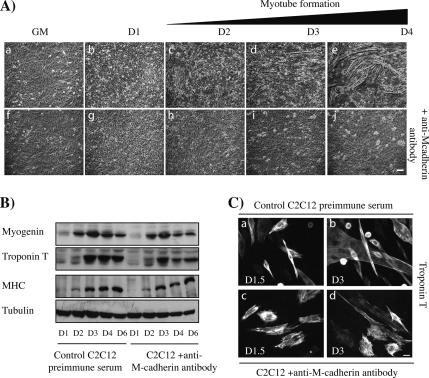
To be able to specifically interfere with M-cadherin–dependent cell–cell contacts, we used an antibody raised against the extracellular domain of the M-cadherin molecule (Charrasse et al., 2006). We first analyzed whether this antibody could efficiently block M-cadherin–dependent signaling. We thus treated C2C12 myoblasts with this anti-M-cadherin antibody and followed myoblast differentiation by time-lapse imaging and analysis of the expression of the muscle-specific proteins myogenin, troponin T, and myosin heavy chain (MHC). As shown in Figure 3, addition of the anti-M-cadherin antibody specifically inhibited myotube formation (Figure 3A) without affecting the expression of the muscle-specific proteins (Figure 3B). Comparable results were obtained with three different antisera (data not shown), whereas the use of preimmune serum did not have any effect. These data suggest that, as previously reported, M-cadherin is required for myoblast fusion (Zeschnigk et al., 1995; Charrasse et al., 2006), and they validate the use of this anti-M-cadherin antibody to specifically interfere with the activation of this adhesive receptor.
Figure 3.
Inhibition of M-cadherin dependent cell–cell contacts prevents myotube formation without affecting the expression of myogenic markers. (A) C2C12 myoblasts were grown up to 80% confluency (a and f) and shifted in DM for 4 d (b–e, g–j). Cells were treated with an anti-M-cadherin antibody (f–j). Phase contrast images show the presence of myotubes in control C2C12 myoblasts (c–e), whereas C2C12 myoblasts treated with the anti-M-cadherin antibody did not fuse (h–j). Bar, 30 μm. (B) Protein extracts from myoblasts treated as in A were immunoblotted to detect myogenin, troponin T, and MHC expression. (C) C2C12 myoblasts treated as in A were fixed at the indicated times and stained for troponin T expression. Bar, 10 μm.
M-Cadherin–dependent Cell–Cell Contact Formation Activates Rac1 in C2C12 Myoblasts
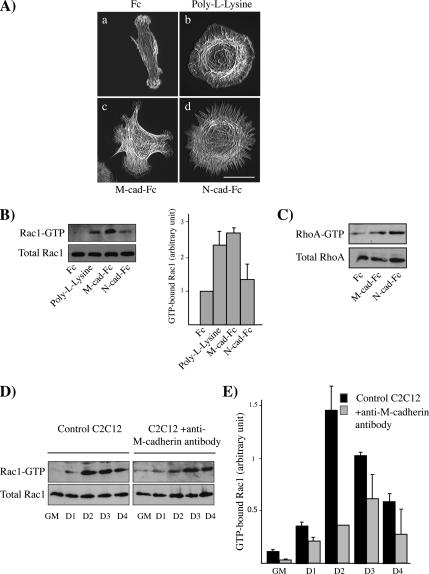
Both M-cadherin and Rac1 activities are required for myoblast fusion. Because cadherins induce a downstream signaling cascade that involves activation of Rho GTPases, we wondered whether M-cadherin–dependent cell–cell contacts control Rac1 activity. For this we used the organization of the F-actin cytoskeleton as a functional read-out. Rac1 mediates local actin polymerization, leading to the formation of ruffles and lamellipodia, whereas RhoA induces the formation of stress fibers (Ridley and Hall, 1992). The dynamic organization of the F-actin cytoskeleton was analyzed in C2C12 myoblasts plated onto dishes coated with either anti-Fc antibody (Fc), poly-l-lysine (PL), or M-cad-Fc or N-cad-Fc ligand, which allowed us to mimic N- or M-cadherin–mediated adhesion. Lamellipodia were detected in myoblasts plated on PL and Mcad-Fc ligand–coated dishes, whereas some stress fibers were detected in myoblasts plated on N-cad-Fc ligand–coated surfaces, as previously reported (Charrasse et al., 2002; Figure 4A and Supplementary Online Videos). These results suggest that Rac1 could be activated by the M-cadherin engagement and, thus, led us to assess Rac1 activity by pulldown assays (Figure 4B). Twenty-four hours after plating, we observed a marked Rac1 activation in C2C12 myoblasts plated on surfaces coated with PL or M-cad-Fc. No such Rac1 activity was observed when C2C12 myoblasts were plated on N-cad-Fc–coated surfaces. RhoA activity was not significantly increased in C2C12 cells plated on M-cad-Fc–coated surfaces (Figure 4C), in contrast to what happens in N-cad-Fc–coated ones (Charrasse et al., 2002).
Figure 4.
M-cadherin–dependent cell–cell contacts increases Rac1 activity. (A) Four hours after plating onto surfaces coated with anti-Fc antibody (a), poly-l-lysine (b), M-cad-Fc (c), or N-cad-Fc (d), C2C12 myoblasts were stained with rhodamine-labeled phalloidin to analyze the F-actin distribution. Bar, 10 μm. (B) The level of GTP-bound Rac1 was measured in lysates obtained from C2C12 cells 24 h after being plated on surfaces coated with either anti-Fc antibody, poly-l-lysine, M-cad-Fc, or N-cad-Fc. Rac1 was detected by immunoblotting. The histogram represents the GTP-bound Rac1 normalized for the amount of total Rac1 protein. The results are presented as mean of three independent experiments. (C) The level of GTP-bound RhoA was measured in lysates that were obtained from C2C12 myoblasts 24 h after being plated on surfaces coated with either anti-Fc antibody, M-cad-Fc, or N-cad-Fc. RhoA was detected by immunoblotting. (D) The level of GTP-bound Rac1 was measured in lysates obtained form C2C12 myoblasts in GM or DM as indicated either left untreated (left) or incubated with an anti-M-cadherin antibody (right). (E) The histogram represents the GTP-bound Rac1 level normalized for the amount of total Rac1 protein. Two independent experiments were analyzed.
We then wanted to known whether the blockade of cell–cell adhesion mediated by M-cadherin could affect the Rac1 activation observed in C2C12 myoblasts after 2 d in DM. We thus measured Rac1 activation at different times after the shift to DM in control versus anti-M-cadherin–treated C2C12 myoblasts by using pulldown assays (Figure 4, D and E). Rac1 activation was again observed at the onset of myoblast fusion in C2C12 myoblasts; however, its activation was decreased and delayed in the cells treated with the anti-M-cadherin antibody. Nevertheless, because cells treated with the anti-M-cadherin antibody do not fuse, the residual Rac1 activity is either not sufficient to allow myoblast fusion and/or appears too late in the sequence of event to allow myoblast fusion. Altogether, these data demonstrate that M-cadherin activates Rac1 in myoblasts and that inhibition of M-cadherin–dependent cell–cell adhesion decreases Rac1 activation at the onset of myoblast fusion.
The Rho-GEF Trio Is required for Myotube Formation
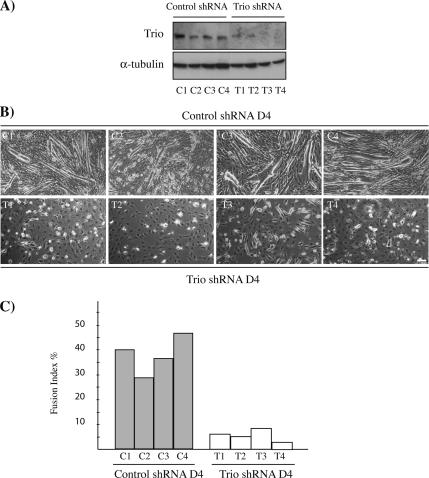
The increase in Rac1 activity induced by the M-cadherin activation led us to look for Rho-GEFs that might be involved in this M-cadherin signaling via Rac1 during myotube formation. Among the Rho-GEFs described, Trio has retained our attention because 1) it has been functionally linked to Rac1 activation (Debant et al., 1996; Blangy et al., 2000) and 2) its loss-of-function mutation in mice causes skeletal muscle defects due to myoblast fusion deficiency (O'Brien et al., 2000). We first assessed whether Trio was required for myoblast fusion in C2C12 myoblasts. For this purpose, we made use of the RNA interference technology to knockdown Trio expression. We generated by retroviral infection stable C2C12 cell lines in which the expression of Trio was inactivated by RNA interference (Trio shRNA). As a control, we made C2C12 cell lines stably expressing a shRNA in which Trio sequence was mutated (control shRNA). Trio silencing was analyzed by Western blot in different clones (Figure 5A). Trio protein levels were strongly decreased in the four Trio shRNA clones used, in comparison with the control shRNA ones. Trio and control shRNA clones were grown to 80% confluency and shifted to DM for 4 d. Although numerous myotubes were observed in the control shRNA C2C12 cells (Figure 5B, top panels, C1–C4), only few tiny myotubes were visible in the Trio shRNA clones (Figure 5B, bottom panels, T1–T4). Although the level of Trio was variable in the different control shRNA clones, the expression level was sufficient to allow the fusion process, suggesting that a threshold of Trio signaling is required for myoblast fusion. Similar results were obtained both with the selected Trio shRNA clones and with a pool of cells collected before cloning by serial dilution, demonstrating that these effects were not due to clonal variations (data not shown). The quantification of the fusion index, which was performed at D4 in both control and Trio shRNA clones, demonstrated that Trio is required for myotube formation (Figure 5C). No myotubes were observed in Trio shRNA even after 7 d in DM (data not shown), indicating that myoblast-to-myotube transition is efficiently blocked and not simply delayed.
Figure 5.
Inhibition of Trio expression by RNA interference prevents myotube formation. (A) Four clones of control shRNA (C1–C4) or Trio shRNA (T1–T4) were analyzed for Trio and α-tubulin expression by Western blot. (B) Phase-contrast images of the clones described in A after 4 d in DM (D4). DM was added to cells at 80% confluency. Bar, 30 μm. (C) The histogram represents the fusion index, i.e., the number of nuclei in multinucleated myotubes divided by the total number of nuclei, calculated for control and Trio shRNA clones at D4. The results are representative of three independent experiments, where at least 3000 nuclei/experiment were counted.
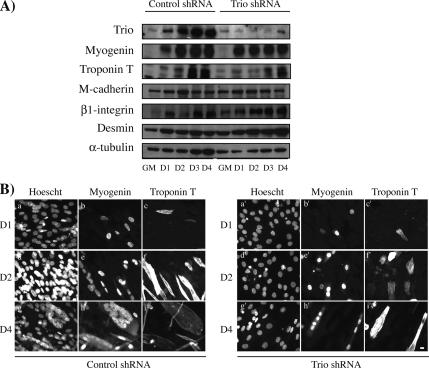
We next examined whether Trio silencing could affect the expression of muscle-specific proteins by using Western blot analysis and immunocytochemistry (Figure 6, A and B, respectively). Interestingly, the expression of myogenin and troponin T was unaffected by Trio gene silencing in all Trio shRNA clones (Figure 6 and data not shown), suggesting that the induction of these genes does not require Trio, as also observed for M-cadherin, β1-integrin, desmin, and α-tubulin. These data indicate that although Trio is dispensable for myogenesis induction, it is necessary for myotube formation.
Figure 6.
Inhibition of Trio expression by RNA interference does not affect myogenesis induction. (A) Trio shRNA interferes with Trio protein expression without affecting myogenin, troponin T, M-cadherin, β1-integrin, desmin, and α-tubulin expression. Expression of these proteins was analyzed by Western blot during differentiation of control shRNA (clone C3) and Trio shRNA (clone T1) clones. (B) myogenin (b, b′, e, e′, h, h′) and troponin T (c, c′, f, f′, i, and i′) expression was analyzed by immunocytochemistry during differentiation in control shRNA (clone C3; a–i) and Trio shRNA (clone T1; a′–i′) clones. DNA was stained with Hoechst dye (a, a′, d, d′, g, and g′). Bar, 10 μm.
The Rho-GEF Trio and Rac1 Are Complexed with M-Cadherin at the Onset of Myoblast Fusion
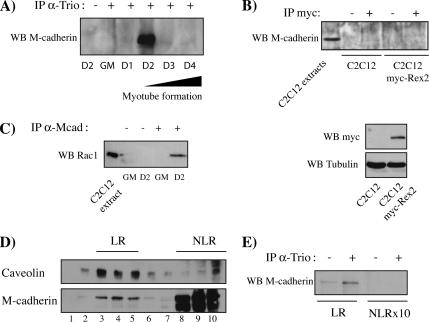
We next examined whether Trio was associated with M-cadherin complexes. Immunoprecipitation using an anti-Trio antibody was performed on samples of C2C12 myoblasts throughout myogenesis. M-cadherin was coimmunoprecipitated with Trio at the onset of myoblast fusion (Figure 7A). Similar results were obtained using enriched plasma membrane preparations (data not shown). Rex2, another Rho-GEFs, was not detected in association with the M-cadherin complex (Figure 7B). Similar absence of an association with M-cadherin was obtained for the Rho-GEF LARG (data not shown). In addition, we detected a strong association of Rac1 with the M-cadherin complex at day 2 after the shift to DM (Figure 7C) as observed for Trio (Figure 7A). RhoA was not detected with the M-cadherin complex (data not shown).
Figure 7.
The Rho-GEF Trio and Rac1 are complexed with M-cadherin at the onset of myoblast fusion. (A) Cell lysates of C2C12 myoblasts cultured in GM or in DM (D1–D4) were immunoprecipitated using an anti-Trio antibody and immunoblotted for the presence of M-cadherin. (B) Cell lysates of C2C12 myoblasts expressing or not Rex2 were immunoprecipitated using an anti-myc antibody and immunoblotted for the presence of M-cadherin (top). Cell lysates of C2C12 myoblasts expressing or not Rex2 were immunoblotted using an anti-myc antibody (bottom). (C) Cell lysates of C2C12 myoblasts cultured in GM or 2 d after DM addition (D2) were immunoprecipitated using an anti-M-cadherin antibody and immunoblotted for the presence of Rac1. (D) C2C12 myoblasts 2 d after DM addition were lysed in 1% Triton X-100 and fractionated on a sucrose gradient. Thirty microliters of each fraction were analyzed for caveolin and M-cadherin distribution by immunoblotting. Fractions 3–5 correspond to lipid rafts (LR) and fractions 8–10 correspond to nonlipid rafts (NLR). (E) Fifty micrograms of LR (pooled fractions 3–5) and 500 μg of NLR (pooled fractions 8–10) fractions were immunoprecipitated using an anti-Trio antibody and immunoblotted for the presence of M-cadherin.
We next analyzed whether this association might occur in the lipid raft microdomains that concentrate molecules in a microenvironment of the membrane to facilitate signal transduction (Simons and Toomre, 2000). We first analyzed whether M-cadherin is found in lipid rafts of C2C12 myoblasts, as does N-cadherin (Causeret et al., 2005), by isolating detergent-resistant membranes (DRMs; Simons and Ikonen, 1997). As shown in Figure 7D, a fraction of M-cadherin was found in DRMs as well as caveolin, a well-known DRM marker. Fractions 3–5, corresponding to lipid rafts (LR), and 8–10, corresponding to nonlipid rafts (NLR), were pooled and immunoprecipitated using an anti-Trio antibody. M-cadherin was coimmunoprecipitated with Trio exclusively in the LR fractions, albeit 10 times more NLR proteins were used (Figure 7E).
Trio Knockdown Impairs M-Cadherin–dependent Rac1 Activation and Rac1 Association to the M-Cadherin Complex
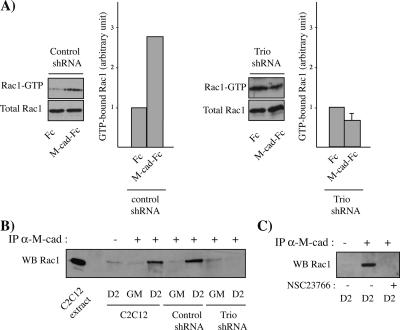
We next analyzed whether Trio is involved in M-cadherin–dependent Rac1 activation. Rac1 activity was measured by pulldown assays in Trio shRNA C2C12 myoblasts plated on dishes coated with either the anti-Fc antibody alone or the M-cad-Fc ligand. We did not observe any increase in Rac1 activation in Trio shRNA C2C12 myoblasts plated on M-cad-Fc–coated dishes compared with those plated on anti-Fc antibody (Figure 8A). This result contrasts with the GTPase activation observed after M-cadherin engagement, which was detected in control shRNA (Figure 8) and wild-type C2C12 myoblasts (Figure 4B), and indicates that Trio is involved in Rac1 activation in this process. Unexpectedly we observed a higher basal Rac1 activity in Trio knockdown myoblasts than in the wild-type C2C12 cells (Figure 8A), probably because of a compensatory mechanism. In any case this Rac1 activity is not sufficient or correctly localized to allow the fusion process to occur.
Figure 8.
Trio knockdown impairs M-cadherin–dependent Rac1 activation and its association to the M-cadherin complex. (A) The level of GTP-bound Rac1 was measured in lysates obtained 24 h after plating control shRNA (clone C3) or Trio shRNA C2C12 on surfaces coated with either anti-Fc antibody or M-cad-Fc. Rac1 was detected by immunoblotting. The histograms represent the amount of GTP-bound Rac1 normalized for the amount of total Rac1 protein. The results are presented as mean of three independent experiments performed by using three different Trio shRNA clones or is the densitometric analysis of the autoradiogram shown for control shRNA. (B) Cell lysates of parental, control shRNA (clone C3), or Trio shRNA (clone T1) C2C12 myoblasts cultured in GM or 2 d after DM addition (D2) were immunoprecipitated using an anti-M-cadherin antibody and immunoblotted for the presence of Rac1. (C) Cell lysates of C2C12 myoblasts cultured in DM supplemented or not with the Rac1 inhibitor NSC33766 for 2 d (D2) were immunoprecipitated using an anti-M-cadherin antibody and immunoblotted for the presence of Rac1.
We next investigated whether Trio was required for the association of Rac1 to the M-cadherin complex. Immunoprecipitation using an anti-M-cadherin antibody was performed on samples of parental, control shRNA, and Trio shRNA C2C12 myoblasts cultured in growth medium (GM) or after 2 d in DM. Rac1 was coimmunoprecipitated with M-cadherin at the onset of myoblast fusion in parental C2C12 and control shRNA clones, but not in Trio knockdown cells (Figure 8C). Finally, we analyzed the Rac1 and Trio association with the M-cadherin complex in C2C12 myoblasts treated with the specific Rac1 inhibitor NSC23766. As shown in Figure 8D, Rac1 association with M-cadherin was lost in NCS23766-treated C2C12 myoblasts.
DISCUSSION
Cellular interactions mediated by members of the cadherin family of Ca2+-dependent cell–cell adhesion molecules have been shown to play important roles throughout myogenesis. N-cadherin–dependent intercellular adhesion has a major role in cell cycle exit and in the induction of the skeletal muscle differentiation program (Seghatoleslami et al., 2000; Charrasse et al., 2002). M-cadherin is expressed later than N-cadherin. Its expression increases at the onset of secondary myogenesis, and this protein has been implicated in terminal myoblast differentiation, particularly in myoblast fusion (Zeschnigk et al., 1995). Although the signal transduction pathways, which are elicited by N-cadherin and which control myogenesis induction, are in the process of being elucidated (Charrasse et al., 2002), no details are available for the M-cadherin–dependent signaling. As cadherin adhesive receptors are well-known regulators of the Rho GTPase activity, we have analyzed their contribution to the M-cadherin–dependent signaling. Our results establish that Rac1 is required for myoblast fusion to occur in C2C12 myoblasts. Indeed, we have observed that inhibition of Rac1 activity impairs myoblast fusion. This observation is supported also by the increase in Rac1 activity at the onset of myoblast fusion. This contrasts with RhoA GTPase, which must be down-regulated to allow fusion to occur (Charrasse et al., 2006) and highlights that the maintenance of a dynamic balance between RhoA and Rac1 activities is crucial for both myogenesis induction (Charrasse et al., 2002) and myoblast fusion. Rac1 has been previously involved in myoblast fusion in Drosophila (Luo et al., 1994; Hakeda-Suzuki et al., 2002; Fernandes et al., 2005) and in this study, we provide the first demonstration of its requirement in mammalian myoblast fusion in mouse C2C12 myoblasts. In addition, we demonstrate that Rac1 is activated by the M-cadherin adhesive receptor during myoblast fusion, thus deciphering a new cross-talk between a cell adhesion receptor and a Rho GTPase. This also suggests that the timing of Rac1 activation during the differentiation process might lead to antagonistic biological effects. Indeed, Rac1 activation at the beginning of the differentiation process inhibits myogenesis induction by preventing the withdrawal of myoblasts from the cell cycle (Meriane et al., 2000b, 2002). Along the same line, we have observed that the expression of R-cadherin in myoblasts also activates Rac1 and causes inhibition of myogenesis induction and impairment of cell cycle exit (unpublished results). In contrast, we show here that M-cadherin–dependent Rac1 activation, once the differentiation process is engaged, positively regulates myoblast fusion.
The mechanisms underlying the cross-talk between cadherin ligation and the modulation of Rho GTPases activity is still unclear. Typical regulators of Rho GTPases are GEFs and GTPase-activating proteins (GAPs). The function of M-cadherin and Rac1 in myoblast fusion may involve the GEF Trio, because Trio loss-of-function mouse showed that Trio is essential for late embryonic development and particularly for secondary myoblast fusion (O'Brien et al., 2000). Moreover, M-cadherin has been shown to accumulate at the areas of contact between fusing secondary myoblasts and myotubes (Cifuentes-Diaz et al., 1995). The Trio protein contains two putative GEF domains: one specific for RhoG and Rac1 (GEFD1) and the other for RhoA (GEFD2; Debant et al., 1996; Blangy et al., 2000). The activity of GEFD2 in the whole Trio protein has not been demonstrated, and it is assumed that the Trio biological effects are due to Rac1 activation. Here we show that 1) Trio is required for myoblast fusion and 2) that Trio is associated with M-cadherin at the time of fusion. Whether the association of Trio with the M-cadherin complex is direct or the result of an interaction in cis with partners in the membrane remains to be determined. Indeed, one can envisage that Trio may interact with the cadherin–catenin complexes, as it has been reported for the Rho-GEF Vav2 (Noren et al., 2000). Moreover, Trio was originally isolated as a binding partner of the LAR transmembrane tyrosine phosphatase (Debant et al., 1996), and we were able to show that LAR sediments with M-cadherin at the time of myoblast fusion (data not shown). Furthermore, members of the immunoglobulin superfamily, such as CDO and BOC, have been shown to interact with cadherin in cis and to positively regulate myogenesis (Kang et al., 2003). In addition, Neogenin, a receptor for the Netrin family of secreted ligands which interacts with CDO, promotes myotube formation (Kang et al., 2004). Netrins and their receptors are well-known regulators of axon guidance, and they recruit the Rho-GEF Trio (Forsthoefel et al., 2005). Another interesting point to assess will be to verify whether the Trio GEFD1 may activate Rac1 either directly or through RhoG, which we have previously described as a target of TrioGEFD1 and an upstream activator of Rac1 (Gauthier-Rouviere et al., 1998; Blangy et al., 2000).
Other pathways that end with the activation of Rac1 might also be controlled by M-cadherin. In particular, the Drosophila myoblast city (mbc), which encodes a cytoskeleton-associated protein with homology to the human DOCK180 protein, was shown to be an upstream regulator of Rac1 activity and to be essential for myoblast fusion (Erickson et al., 1997; Nolan et al., 1998). The DOCK180/Elmo complex might play a role in Rac1 activation via either RhoG or Arf6 (Katoh and Negishi, 2003; Santy et al., 2005). The small GTPase Arf6, as well as the Arf6-GEF Loner, is also involved in myoblast fusion in Drosophila (Chen et al., 2003). Further studies will be necessary to precisely identify the proteins that associated with or are activated by the M-cadherin multiproteic complex and are coordinately involved in the promyogenic signaling.
In conclusion, we propose that M-cadherin might be involved both in the myoblast recognition and in the induction of localized intracellular signaling pathways conducting to Rac1 activation, a prerequisite for the cytoskeletal rearrangements necessary for myoblast fusion (Clark et al., 2002; Musa et al., 2003). Indeed, Rac1 is a well-known regulator of the dynamic organization of the cortical actin cytoskeleton (Hall, 1998, 2005) and was found localized to discrete sites along plasma membrane of fusion competent myoblast in Drosophila (Chen et al., 2003). Interestingly, the actin cytoskeleton is extensively reorganized during myoblast fusion as the bundles of actin stress fibers disappear and nonmuscle actin is found under the plasma membrane (Swailes et al., 2004). Rac1 has also been involved in the organization of the microtubule cytoskeleton, a structure that is reorganized and participates to myoblast fusion and also associates with M-cadherin (Saitoh et al., 1988; Kaufmann et al., 1999; Musa et al., 2003; Wittmann et al., 2004). Further studies are required to address how Rac1 might be involved in these cytoskeletal changes during myoblast fusion.
Cell therapy by means of transplantation of fusion competent myoblasts may help to treat devastating muscle diseases and muscular atrophy. Unfortunately, the inability of the injected myoblasts to fuse efficiently with host myofibers represents at the moment a major limitation of cell therapy (Skuk and Tremblay, 2000). Further understanding of the signaling pathways which control the myoblast fusion could improve future therapeutic strategies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sylvain De Rossi, Julien Cau, and Pierre Travo for constant support (http://www.crbm.cnrs-mop.fr/platform/page_web_rio/index.htm). We thank Guillaume Fargier and Fanny Dubuquoy for technical support and Philippe Fort and Camille Auziol for helpful discussions. All authors are members of the CNRS research network GDR2823. This work was supported by the Association Française contre les Myopathies, the Agence Nationale de la Recherche (ANR), and the Association Française pour la Recherche contre le Cancer.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-08-0766) on March 1, 2007.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Baecker V., Travo P. Cell Image Analyzer—a visual scripting interface for ImageJ and its usage at the microscopy facility Montpellier RIO Imaging. Proceedings of the ImageJ User and Developer Conference; 2006. pp. 105–110. [Google Scholar]
- Bahjaoui-Bouhaddi M., Padilla F., Nicolet M., Cifuentes-Diaz C., Fellmann D., Mege R. M. Localized deposition of M-cadherin in the glomeruli of the granular layer during the postnatal development of mouse cerebellum. J. Comp. Neurol. 1997;378:180–195. [PubMed] [Google Scholar]
- Bellanger J. M., Lazaro J. B., Diriong S., Fernandez A., Lamb N., Debant A. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene. 1998;16:147–152. doi: 10.1038/sj.onc.1201532. [DOI] [PubMed] [Google Scholar]
- Blangy A., Vignal E., Schmidt S., Debant A., Gauthier-Rouviere C., Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J. Cell Sci. 2000;113(Pt 4):729–739. doi: 10.1242/jcs.113.4.729. [DOI] [PubMed] [Google Scholar]
- Carnac G., Primig M., Kitzmann M., Chafey P., Tuil D., Lamb N., Fernandez A. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting myf5 in mouse myoblasts [In Process Citation] Mol. Biol. Cell. 1998;9:1891–1902. doi: 10.1091/mbc.9.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causeret M., Taulet N., Comunale F., Favard C., Gauthier-Rouviere C. N-cadherin association with lipid rafts regulates its dynamic assembly at cell-cell junctions in C2C12 myoblasts. Mol. Biol. Cell. 2005;16:2168–2180. doi: 10.1091/mbc.E04-09-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S., Causeret M., Comunale F., Bonet-Kerrache A., Gauthier-Rouviere C. Rho GTPases and cadherin-based cell adhesion in skeletal muscle development. J. Muscle Res. Cell Motil. 2003;24:309–313. [PubMed] [Google Scholar]
- Charrasse S., Comunale F., Grumbach Y., Poulat F., Blangy A., Gauthier-Rouviere C. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol. Biol. Cell. 2006;17:749–759. doi: 10.1091/mbc.E05-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S., Meriane M., Comunale F., Blangy A., Gauthier-Rouviere C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J. Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. H., Pryce B. A., Tzeng J. A., Gonzalez G. A., Olson E. N. Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell. 2003;114:751–762. doi: 10.1016/s0092-8674(03)00720-7. [DOI] [PubMed] [Google Scholar]
- Cifuentes-Diaz C., Goudou D., Padilla F., Facchinetti P., Nicolet M., Mege R. M., Rieger F. M-cadherin distribution in the mouse adult neuromuscular system suggests a role in muscle innervation. Eur. J. Neurosci. 1996;8:1666–1676. doi: 10.1111/j.1460-9568.1996.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Cifuentes-Diaz C., Nicolet M., Alameddine H., Goudou D., Dehaupas M., Rieger F., Mege R. M. M-cadherin localization in developing adult and regenerating mouse skeletal muscle: possible involvement in secondary myogenesis. Mech. Dev. 1995;50:85–97. doi: 10.1016/0925-4773(94)00327-j. [DOI] [PubMed] [Google Scholar]
- Clark P., Dunn G. A., Knibbs A., Peckham M. Alignment of myoblasts on ultrafine gratings inhibits fusion in vitro. Int. J. Biochem. Cell Biol. 2002;34:816–825. doi: 10.1016/s1357-2725(01)00180-7. [DOI] [PubMed] [Google Scholar]
- Debant A., Serra-Pages C., Seipel K., O'Brien S., Tang M., Park S. H., Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc. Natl. Acad. Sci. USA. 1996;93:5466–5471. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doberstein S. K., Fetter R. D., Mehta A. Y., Goodman C. S. Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J. Cell Biol. 1997;136:1249–1261. doi: 10.1083/jcb.136.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donalies M., Cramer M., Ringwald M., Starzinski-Powitz A. Expression of M-cadherin, a member of the cadherin multigene family, correlates with differentiation of skeletal muscle cells. Proc. Natl. Acad. Sci. USA. 1991;88:8024–8028. doi: 10.1073/pnas.88.18.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson M. R., Galletta B. J., Abmayr S. M. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J. Cell Biol. 1997;138:589–603. doi: 10.1083/jcb.138.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J. J., Atreya K. B., Desai K. M., Hall R. E., Patel M. D., Desai A. A., Benham A. E., Mable J. L., Straessle J. L. A dominant negative form of Rac1 affects myogenesis of adult thoracic muscles in Drosophila. Dev. Biol. 2005;285:11–27. doi: 10.1016/j.ydbio.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Forsthoefel D. J., Liebl E. C., Kolodziej P. A., Seeger M. A. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development. 2005;132:1983–1994. doi: 10.1242/dev.01736. [DOI] [PubMed] [Google Scholar]
- Gao Y., Dickerson J. B., Guo F., Zheng J., Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier-Rouviere C., Vignal E., Meriane M., Roux P., Montcourier P., Fort P. RhoG GTPase controls a pathway that independently activates Rac1 and Cdc42Hs. Mol. Biol. Cell. 1998;9:1379–1394. doi: 10.1091/mbc.9.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda-Suzuki S., Ng J., Tzu J., Dietzl G., Sun Y., Harms M., Nardine T., Luo L., Dickson B. J. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Hollnagel A., Grund C., Franke W. W., Arnold H. H. The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Mol. Cell. Biol. 2002;22:4760–4770. doi: 10.1128/MCB.22.13.4760-4770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. S., Feinleib J. L., Knox S., Ketteringham M. A., Krauss R. S. Promyogenic members of the Ig and cadherin families associate to positively regulate differentiation. Proc. Natl. Acad. Sci. USA. 2003;100:3989–3994. doi: 10.1073/pnas.0736565100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. S., Yi M. J., Zhang W., Feinleib J. L., Cole F., Krauss R. S. Netrins and neogenin promote myotube formation. J. Cell Biol. 2004;167:493–504. doi: 10.1083/jcb.200405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H., Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- Kaufmann U., Kirsch J., Irintchev A., Wernig A., Starzinski-Powitz A. The M-cadherin catenin complex interacts with microtubules in skeletal muscle cells: implications for the fusion of myoblasts. J. Cell Sci. 1999;112(Pt 1):55–68. doi: 10.1242/jcs.112.1.55. [DOI] [PubMed] [Google Scholar]
- Krauss R. S., Cole F., Gaio U., Takaesu G., Zhang W., Kang J. S. Close encounters: regulation of vertebrate skeletal myogenesis by cell-cell contact. J. Cell Sci. 2005;118:2355–2362. doi: 10.1242/jcs.02397. [DOI] [PubMed] [Google Scholar]
- Kuch C., Winnekendonk D., Butz S., Unvericht U., Kemler R., Starzinski-Powitz A. M-cadherin-mediated cell adhesion and complex formation with the catenins in myogenic mouse cells. Exp. Cell Res. 1997;232:331–338. doi: 10.1006/excr.1997.3519. [DOI] [PubMed] [Google Scholar]
- Luo L., Liao Y. J., Jan L. Y., Jan Y. N. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- Mary S., Charrasse S., Meriane M., Comunale F., Travo P., Blangy A., Gauthier-Rouviere C. Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Mol. Biol. Cell. 2002;13:285–301. doi: 10.1091/mbc.01-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriane M., Charrasse S., Comunale F., Mery A., Fort P., Roux P., Gauthier-Rouviere C. Participation of small GTPases Rac1 and Cdc42Hs in myoblast transformation. Oncogene. 2002;21:2901–2907. doi: 10.1038/sj.onc.1205396. [DOI] [PubMed] [Google Scholar]
- Meriane M., Roux P., Primig M., Fort P., Gauthier-Rouviere C. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell. 2000a;11:2513–2528. doi: 10.1091/mbc.11.8.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriane M., Roux P., Primig M., Fort P., Gauthier-Rouviere C. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell. 2000b;11:2513–2528. doi: 10.1091/mbc.11.8.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Walsh F. S. The cell adhesion molecule M-cadherin is specifically expressed in developing and regenerating, but not denervated skeletal muscle. Development. 1993;117:1409–1420. doi: 10.1242/dev.117.4.1409. [DOI] [PubMed] [Google Scholar]
- Musa H., Orton C., Morrison E. E., Peckham M. Microtubule assembly in cultured myoblasts and myotubes following nocodazole induced microtubule depolymerisation. J. Muscle Res. Cell Motil. 2003;24:301–308. [PMC free article] [PubMed] [Google Scholar]
- Nolan K. M., Barrett K., Lu Y., Hu K. Q., Vincent S., Settleman J. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 1998;12:3337–3342. doi: 10.1101/gad.12.21.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren N. K., Liu B. P., Burridge K., Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien S. P., Seipel K., Medley Q. G., Bronson R., Segal R., Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc. Natl. Acad. Sci. USA. 2000;97:12074–12078. doi: 10.1073/pnas.97.22.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portales-Casamar E., Briancon-Marjollet A., Fromont S., Triboulet R., Debant A. Identification of novel neuronal isoforms of the Rho-GEF Trio. Biol. Cell. 2006;98:183–193. doi: 10.1042/BC20050009. [DOI] [PubMed] [Google Scholar]
- Pouliot Y., Gravel M., Holland P. C. Developmental regulation of M-cadherin in the terminal differentiation of skeletal myoblasts. Dev. Dyn. 1994;200:305–312. doi: 10.1002/aja.1002000405. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. Distinct patterns of actin organization regulated by the small GTP-binding proteins Rac and Rho. Cold Spring Harb. Symp. Quant. Biol. 1992;57:661–671. doi: 10.1101/sqb.1992.057.01.072. [DOI] [PubMed] [Google Scholar]
- Rose O., Rohwedel J., Reinhardt S., Bachmann M., Cramer M., Rotter M., Wobus A., Starzinski-Powitz A. Expression of M-cadherin protein in myogenic cells during prenatal mouse development and differentiation of embryonic stem cells in culture. Dev. Dyn. 1994;201:245–259. doi: 10.1002/aja.1002010308. [DOI] [PubMed] [Google Scholar]
- Rossman K. L., Der C. J., Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Saitoh O., Arai T., Obinata T. Distribution of microtubules and other cytoskeletal filaments during myotube elongation as revealed by fluorescence microscopy. Cell Tissue Res. 1988;252:263–273. doi: 10.1007/BF00214368. [DOI] [PubMed] [Google Scholar]
- Santy L. C., Ravichandran K. S., Casanova J. E. The DOCK180/Elmo complex couples ARNO-mediated Arf6 activation to the downstream activation of Rac1. Curr. Biol. 2005;15:1749–1754. doi: 10.1016/j.cub.2005.08.052. [DOI] [PubMed] [Google Scholar]
- Seghatoleslami M. R., Myers L., Knudsen K. A. Upregulation of myogenin by N-cadherin adhesion in three-dimensional cultures of skeletal myogenic BHK cells. J. Cell Biochem. 2000;77:252–264. doi: 10.1002/(sici)1097-4644(20000501)77:2<252::aid-jcb8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Skuk D., Tremblay J. P. Progress in myoblast transplantation: a potential treatment of dystrophies. Microsc. Res. Tech. 2000;48:213–222. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<213::AID-JEMT9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Swailes N. T., Knight P. J., Peckham M. Actin filament organization in aligned prefusion myoblasts. J. Anat. 2004;205:381–391. doi: 10.1111/j.0021-8782.2004.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. V. Muscle differentiation: how two cells become one. Curr. Biol. 2002;12:R224–R228. doi: 10.1016/s0960-9822(02)00757-1. [DOI] [PubMed] [Google Scholar]
- Wei L., Zhou W., Croissant J. D., Johansen F. E., Prywes R., Balasubramanyam A., Schwartz R. J. RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J. Biol. Chem. 1998;273:30287–30294. doi: 10.1074/jbc.273.46.30287. [DOI] [PubMed] [Google Scholar]
- Wheelock M. J., Johnson K. R. Cadherin-mediated cellular signaling. Curr. Opin. Cell Biol. 2003a;15:509–514. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Wheelock M. J., Johnson K. R. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 2003b;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- Williams E. J., Furness J., Walsh F. S., Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Wittmann T., Bokoch G. M., Waterman-Storer C. M. Regulation of microtubule destabilizing activity of Op18/stathmin downstream of Rac1. J. Biol. Chem. 2004;279:6196–6203. doi: 10.1074/jbc.M307261200. [DOI] [PubMed] [Google Scholar]
- Zeschnigk M., Kozian D., Kuch C., Schmoll M., Starzinski-Powitz A. Involvement of M-cadherin in terminal differentiation of skeletal muscle cells. J. Cell Sci. 1995;108(Pt 9):2973–2981. doi: 10.1242/jcs.108.9.2973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.