Abstract
The proteolytic processing of laminin-5 at the short arm of the γ2 chain (γ2sa) is known to convert this laminin from a cell adhesion type to a motility type. Here, we studied this mechanism by analyzing the functions of γ2sa. In some immortalized or tumorigenic human cell lines, a recombinant γ2sa, in either soluble or insoluble (coated) form, promoted the adhesion of these cells to the processed laminin-5 (Pr-LN5), and it suppressed their migration stimulated by serum or epidermal growth factor (EGF). γ2sa also suppressed EGF-induced tyrosine phosphorylation of integrin β4 and resultant disruption of hemidesmosome-like structures in keratinocytes. γ2sa bound to syndecan-1, and this binding, as well as its cell adhesion activity, was blocked by heparin. By analyzing the activities of three different γ2sa fragments, the active site of γ2sa was localized to the NH2-terminal EGF-like sequence (domain V or LEa). Suppression of syndecan-1 expression by the RNA interference effectively blocked the activities of domain V capable of promoting cell adhesion and inhibiting the integrin β4 phosphorylation. These results demonstrate that domain V of the γ2 chain negatively regulates the integrin β4 phosphorylation, probably through a syndecan-1–mediated signaling, leading to enhanced cell adhesion and suppressed cell motility.
INTRODUCTION
The basement membrane proteins laminins play essential roles not only in the tissue architecture but also in the regulation of cellular functions (Timpl and Brown, 1996). Laminins are large glycoproteins consisting of three different subunits (α, β, and γ chains) linked by disulfide bonds, and each subunit has many functional domains (Aumailley et al., 2005). Therefore, they show a variety of biological activities. For example, laminins regulate cellular adhesion, motility, growth, differentiation, and apoptosis, through interaction with specific receptors on the cell surface (Timpl and Brown, 1996; Colognato and Yurchenco, 2000). The COOH-terminal globular (G) domain of the α chain, which consists of five laminin globular (LG) modules (LG1-5), is a major site to interact with cell surface receptors such as integrins, syndecans, and dystroglycan. The NH2-terminal regions, often called short arms, of the three chains are thought to be mainly involved in the matrix assembly of laminins in basement membranes. Several laminin isoforms, which are produced by different combinations of the α, β, and γ chains, are expressed in a tissue-specific manner during embryonic development as well as in the adult (Miner et al., 1997; Colognato and Yurchenco, 2000).
Laminin-5 (α3β3γ2; laminin-332), abbreviated here as LN5, is a major laminin isoform in the skin basement membrane (Carter et al., 1991; Rousselle et al., 1991) and widely expressed in other epithelial tissues as a relatively minor component (Miner et al., 1997; Mizushima et al., 1998). In vitro, LN5 strongly promotes cellular scattering, migration, and adhesion through the interaction with integrins α3β1, α6β1, and α6β4 (Kikkawa et al., 1994; Rousselle and Aumailley, 1994; Miyazaki, 2006). The cell adhesion activity of LN5 contributes to the tight adhesion of basal keratinocytes to the underlying connective tissue in the skin, which is mediated by the association of LN5 with integrin α6β4 in the hemidesmosome structures (Baker et al., 1996). Therefore, structural defects of LN5 subunits, integrin α6β4, or other hemidesmosome components cause severe skin blistering in humans and experimental animals (Aberdam et al., 1994). In contrast, the cell migration-promoting activity of LN5 is thought to contribute to wound healing (Ryan et al., 1994) and tumor invasion (Pyke et al., 1995). These unique biological activities are likely to depend on its structural feature. All of the three LN5 subunits are truncated in their short arms (NH2-terminal regions), and the β3 and γ2 chains are found only in LN5. However, it is unknown how the apparently opposite functions of LN5, i.e., stable cell adhesion and cell migration, are regulated. In this context, much attention has been focused on the proteolytic processing of LN5 that modulates its biological activities (Miyazaki, 2006).
Human LN5 is synthesized and secreted as a precursor form consisting of a 190-kDa α3 chain, a 135-kDa β3 chain, and a 150-kDa γ2 chain. After secretion, the α3 and γ2 chains undergo specific extracellular proteolytic processing to convert to the mature form containing a 160-kDa α3 chain and a 105-kDa γ2 chain, respectively. For the α3 chain, the 190-kDa α3 chain is almost completely converted to the 160-kDa form immediately after secretion. The proteolytic cleavage of the 190-kDa α3 chain, which occurs between the LG3 and LG4 modules in the G domain (Hirosaki et al., 2000; Tsubota et al., 2000), increases the biological activity of LN5 and laminin-6 (α3β1γ1; laminin-311) (Tsubota et al., 2005; Hirosaki et al., 2002). Structural studies have shown that the major integrin-binding site in LN5 and laminin-6 is located in the LG3 domain of the α3 chain (Hirosaki et al., 2000; Kariya et al., 2003), and the LG4–LG5 domain is important for the matrix assembly of the laminin (Sigle et al., 2004; Tsubota et al., 2005). In contrast, the 150-kDa γ2 chain of LN5 is partially processed to the 105-kDa form in many LN5-producing human cell lines. Bone morphogenic protein-1/mammalian Tolloid metalloproteinase family, including mammalian Tolloid-like-1 and mammalian Tolloid-like-2, are thought to be responsible for the γ2 chain processing in human LN5 (Veitch et al., 2003). In rat LN5, however, the γ2 chain is processed from the 150-kDa form to the 80-kDa mature form by gelatinase A (matrix metalloproteinase [MMP]-2) or membrane type 1-MMP, and this processing enhances the cell motility activity of the LN5 (Giannelli et al., 1997; Koshikawa et al., 2000). In human LN5, the processing of the 150-kDa γ2 chain to the 105-kDa mature form decreases the cell adhesion activity but increases the cell migration activity of LN5 (Ogawa et al., 2004). Furthermore, many immunohistochemical studies have shown that the laminin γ2 chain is overexpressed at the invasion front of human cancers (Pyke et al., 1995; Koshikawa et al., 1999). These results imply that the short arm of the γ2 chain contains an important, functional domain that regulates cellular adhesion and migration. The active site of the γ2 chain may bind some cell surface receptors other than integrins, leading to the modulation of the integrin-mediated signaling. To test these possibilities, we examined the biological functions of the γ2 chain short arm in this study.
MATERIALS AND METHODS
Antibodies and Laminins
Mouse monoclonal antibodies (mAbs) against the human laminin α3 chain (LSα3c4) and γ2 chain (D4B5) were established and characterized previously (Mizushima et al., 1998; Koshikawa et al., 1999). Mouse mAbs against integrin β4 (1A3) and BP180 (233) were generous gifts from Dr. K. Owaribe (Graduate School of Human Informatics, Nagoya University, Nagoya, Japan) (Okumura et al., 2002; Hirako et al., 2003). Other antibodies used and their sources were as follows: a mouse mAb against the human laminin β3 chain from BD Biosciences Transduction Laboratories (Lexington, KY), a mouse mAb against integrin β4 (3E1) from Chemicon International (Temecula, CA), a mouse mAb against integrin β4 (450-11A) from BD Bioscience (San Jose, CA), a polyclonal antibody against epidermal growth factor (EGF) receptor (Ab4) from Oncogene Research Products (Boston, MA), a mouse mAb against EGF receptor (LA1) from Upstate Biotechnology (Lake Placid, NY), and a mouse mAb against phospho-tyrosine (PY-20) from BD Biosciences Transduction Laboratories. A recombinant human LN5 with a 150-kDa nonprocessed γ2 chain (Np-LN5) and a natural LN5 with the 105-kDa processed γ2 chain (Pr-LN5) were prepared as reported previously (Ogawa et al., 2004).
Cells and Culture Conditions
The human bladder carcinoma cell line EJ-1, the Buffalo rat liver cell line BRL and the human epideromoid carcinoma cell line A431 have been used in previous studies (Kariya et al., 2003). The human embryonic kidney cell line HEK293 was obtained from American Type Culture Collection (Manassas, VA). These cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM)/F-12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS). The spontaneously immortalized human keratinocyte line HaCaT (Boukamp et al., 1988), which was a generous gift from Dr. N. E. Fusenig (Deutsches Krebsforschungszentrum, Heidelberg, Germany), was maintained in DMEM supplemented with 10% FCS. The immortalized human mammary epithelial cell line MCF-10A (ATCC CRL-10317) was obtained from American Type Culture Collection and cultured in DMEM/F12 supplemented with 20 ng/ml EGF, 100 ng/ml cholera toxin, 0.01 mg/ml insulin, 500 ng/ml hydrocortisone, and 5% horse serum.
Construction of Expression Vectors and Cell Transfection
cDNAs for the laminin γ2 chain and its NH2-termianl short arm were cloned from a human gastric carcinoma cell line (STKM-1) in our laboratory (Tsubota, Ogawa, and Miyazaki, unpublished data). Briefly, the total RNA was extracted from STKM-1, reverse-transcribed with random primers, and used as a template for further polymerase chain reaction (PCR). The cDNA encoding the laminin γ2 chain (nucleotides 85-3702) was amplified as four overlapping fragments, according to the reported sequence (Kallunki et al., 1992). To construct a cDNA fragment of the γ2 short arm (γ2sa), which consists of the NH2-terminal domains III (or LEb), IV (or L4), and V (or LEa), a SpeI–NdeI fragment (nucleotides 85-1262) and a NdeI–EcoRI fragment (nucleotides 1020-1942) of the γ2 cDNA were ligated into the SpeI–EcoRI site of pBluescript II KS (+). The γ2sa cDNA fragment was finally inserted into the mammalian expression vector pBOS-CITE-Neo, which expresses the inserted cDNA and a neomycin-resistant gene within one mRNA molecule (Ogawa et al., 2004). EJ-1 cells, which expressed none of the laminin α3, β3, and γ2 chains, were transfected with the expression vector by the calcium-phosphate precipitation method and incubated in medium containing 500 μg/ml Geneticin (G-418; Sigma-Aldrich, St. Louis, MO). A Geneticin-resistant EJ-1 cell clone was used as EJ-1/γ2sa cells for the purification of the γ2sa protein.
The following three additional γ2 cDNA fragments were prepared by PCR by using the γ2sa cDNA as a template: γ2pf cDNA (nucleotides 199-1419) encoding the full length of domains V and IV and a small sequence of domain III, γ2dV cDNA (nucleotides 199-705) encoding only domain V, and γ2dIII cDNA (nucleotides 1420-1935) encoding most of domain III. The primer sequences used in the PCR were as follows: γ2pf sense, 5′-TAGGTACCTGTGATTGCAATGGGAAG-3′; γ2pf antisense, 5′-ATCTCGAGCCCCTGAATAACAATCTCCTGT-3′; γ2dV sense, 5′-TAGGTACCTGTGATTGCAATGGGAAG-3′; γ2dV antisense, 5′-ATCTCGAGCGCAGCTGGCTGAATG-3′; γ2dIII sense, 5′-TAGGTACCGATGAGAATCCTGAC-3′; and γ2dIII antisense 5′-ATCTCGAGCTGCTCCATGCTCAC-3′. The sense primers contained an additional KpnI site, whereas the antisense primers contained an additional XhoI site to allow insertion to an expression vector. The PCR products were then inserted into the T-easy vector (Promega, Madison, WI). These constructs was subcloned into the KpnI–XhoI sites of the mammalian expression vector pSectag2B (Invitrogen, Carlsbad, CA), which expresses a secreted protein linked with a histidine hexamer at the COOH terminus. The cDNA constructs were transfected into the human embryonic kidney cell line HEK293. Zeocine-resistant cell clones were then selected for high-level secretion of the deletion proteins.
Preparation of LN5 and Recombinant Proteins of Laminin γ2 Short Arm
A natural human LN5 consisting of a 160-kDa mature α3 chain, a 135-kDa β3 chain, and a 105-kDa processed γ2 chain, tentatively named Pr-LN5, was prepared from the conditioned medium of the human squamous cell carcinoma line HSC-4 as described previously (Ogawa et al., 2004). A recombinant human LN5 consisting of a 160-kDa α3 chain, a 135-kDa β3 chain, and a 150-kDa, nonprocessed γ2 chain, tentatively named Np-LN5, was prepared from the conditioned medium of the human gastric carcinoma line HSC-4 expressing an exogenous, mutated γ2 chain (Ogawa et al., 2004). Briefly, these LN5 proteins were purified by successively applying the serum-free conditioned media to molecular-sieve chromatography on a Sepharose 4B column and to immunoaffinity chromatography with an anti-γ2-chain mAb (D4B5), according to the method published previously (Hirosaki et al., 2000). The γ2 short arm protein (γ2sa) was purified from the serum-free conditioned medium of EJ-1/γ2sa cells by essentially the same method as described above. To purify three γ2 short arm fragments, HEK293 cell lines transfected with γ2pf, γ2dV, or γ2dIII cDNA were grown to confluence in serum-containing medium and then incubated in serum-free medium for 2 d (Kariya et al., 2003). The serum-free conditioned media containing secreted His-tagged proteins (γ2pf, γ2dV, or γ2dIII) were collected, concentrated 500-fold by ammonium sulfate precipitation at 80% saturation, and dialyzed against 50 mM phosphate, pH 7.8, buffer containing 300 mM NaCl. Proteins from 1 liter of the conditioned medium were applied to a NiSO4-conjugated chelating Sepharose column (2 ml) (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). The column was washed with 20 ml of the same phosphate buffer, and the remaining proteins on the column were successively eluted with 3 ml of 50, 100, 300 and 500 mM imidazole in 100 mM phosphate buffer, pH 6.0, containing 300 mM NaCl. The majority of γ2pf, γ2dV, and γ2dIII proteins were eluted at 300 mM imidazole and dialyzed against Ca2+/Mg2+-free phosphate-buffered saline (PBS). The γ2pf and γ2dV fractions were finally applied to a heparin-Sepharose column (1 ml) (HiTrap Heparin HP column; GE Healthcare) and eluted with a NaCl gradient. γ2pf and γ2dV were eluted at 0.36–0.40 M NaCl and 0.30–0.4 M NaCl, respectively. γ2dIII was finally purified using the immunoaffinity column of the anti-γ2-chain mAb (D4B5) as described above. Approximately 500 μg of γ2pf, 200 μg of γ2dV, and 550 μg of γ2dIII were purified from 1 liter of the conditioned media, respectively.
Assays of Cell Adhesion Activity
The cell adhesion assay was performed as described previously (Ogawa et al., 2004). Microtiter plates (96-well; Corning Life Sciences, Acton, MA) were coated with appropriate concentrations of substrate proteins in PBS at 4°C overnight and then blocked with 1.2% (wt/vol) bovine serum albumin (BSA) at 37°C for 1.5 h. Cells were suspended in serum-free medium at a density of 2∼4 × 105 cells/ml, and a 100-μl aliquot was inoculated per well of the plates. After incubation at 37°C for 1 h, nonadherent cells were removed after gentle agitation, and adherent cells were fixed with 2.5% (wt/vol) glutaraldehyde and stained with 0.0005% (wt/vol) Hoechst 33342 in 0.001% (wt/vol) Triton X-100 for 1.5 h. The fluorescent intensity of each well of the plates was measured using a CytoFluor 2350 fluorometer (Millipore, Bedford, MA).
Assays of Cell Migration
Cell migration speed was assayed as reported previously (Hirosaki et al., 2000). Each well of 24-well plastic plates was coated with indicated concentrations of test substrates and then blocked with BSA as described above. EJ-1 cells (1.0 × 104 cells in DMEM/F-12 plus 1% FCS) were inoculated per well of the plates. After preincubation for 1 h at 37°C, cell movement was monitored for 10 h by using a time-lapse video, and the cell migration distance was determined by measuring the total length of the random path that each cell covers, using a video micrometer (VM-30; Olympus, Tokyo, Japan).
Identification of Membrane Receptor of γ2sa
Membrane proteins of EJ-1 cells were collected according to the method of Hoffman et al. (1998) and then incubated with a γ2sa-conjugated beads, which had been prepared by binding the purified γ2sa to Affigel-10 beads (Bio-Rad, Hercules, CA), in PBS buffer at 4°C overnight. The incubated beads were washed with the buffer several times, and γ2sa-bound proteins were eluted from the beads with 1 M NaCl. The eluted proteins were precipitated by 10% (wt/vol) trichloroacetic acid and dissolved in 20 mM HEPES-NaOH, pH 7.5, buffer supplemented with a protease inhibitor mixture (Wako Pure Chemicals, Osaka, Japan). To identify heparan sulfate proteoglycans (HSPGs), protein samples (2 μg) were treated with 0.5 U/ml heparitinase (Seikagaku Kogyo, Tokyo, Japan) at 37°C for 4 h and analyzed by immunoblotting with the mouse anti-Δheparan-sulfate mAb 3G10 (Seikagaku Kogyo), which reacts with a heparan sulfate neo-epitope generated by the heparitinase digestion, with the anti-syndecan-1 mAb B-B4 (AbD; Serotec, Oxford, United Kingdom), the goat anti-syndecan-2 polyclonal antibody L-18 (Santa Cruz Biotechnology, Santa Cruz, CA), or the goat anti-syndecan-4 polyclonal antibody N-19 (Santa Cruz Biotechnology).
Analysis of Phosphorylation of Integrin β4 in γ2sa-treated Cells
Cells were serum starved for 48 h, harvested, and suspended in serum-free medium at a density of 1 × 106 cells/ml, and a 2-ml aliquot was inoculated per 60-mm culture dish (Sumibe Medical, Tokyo, Japan), which had been precoated with 1 μg/ml LN5 and blocked with BSA as described above. After incubation at 37°C for 2 h, nonadherant cells were removed by washing the cultures with PBS, and the remaining adherent cells were further incubated in serum-free medium supplemented with 50 ng/ml EGF in the presence or absence of γ2sa (0.4 or 0.8 μg/ml) for 10 min. The cells were then lysed in a lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride and 1% Triton X-100), harvested, and centrifuged at 15,000 × g for 10 min. The resultant supernatants were incubated with an anti-mouse immunoglobulin G antibody affinity gel (ICN, Aurora, OH) conjugated with the anti-integrin-β4 mAb 3E1 at 4°C for 12 h. The immunoprecipitates thus obtained were extensively washed with the lysis buffer, dissolved in the SDS-buffer containing 2-mercaptoethanol, and subjected to immunoblotting with the anti-phospho-tyrosine antibody PY-20. In some experiments, cell lysates were directly subjected to the immunoblotting.
Immunofluorescence Microscopy of Hemidesmosome-like Structures
HaCaT cells were incubated in serum-free DMEM for 48 h. The serum-starved cells were trypsinized, washed with the medium containing 1 mg/ml soybean trypsin inhibitor, and suspended in the serum-free DMEM at a density of 2.5 × 105 cells/ml. A 250-μl portion of the cell suspension was inoculated per well of eight-well Lab-Tek chamber slides (Nalge Nunc, Naperville, IL), which had previously been coated with LN5, and incubated at 37°C for 3 h. Adherent cells were further treated with EGF and/or γ2sa as described above. The cultures were then rinsed with cooled PBS, fixed in 10% (wt/vol) Formalin in PBS for 15 min, and washed three times with PBS. The cells were permeabilized with 0.2% (vol/vol) Triton X-100 in PBS for 15 min, blocked with 10% horse serum in PBS for 15 min, and then incubated with a primary antibody diluted in 3% horse serum in PBS at 4°C for 12 h. A fluorescein isothiocyanate-coupled secondary antibody (Vector Laboratories, Burlingame, CA) was used for detection. Fluorecsence images were obtained using a fluorescence microscope (model BZ-8000; Keyence, Osaka, Japan).
SDS-Polyacrylamide Gel Electrophoresis (PAGE) and Immunoblotting Analyses
SDS-PAGE was performed on 5, 6, or 10% polyacrylamide gels under reducing or nonreducing conditions. In analyses of purified proteins, separated proteins were stained with a Wako silver staining kit II (Wako Pure Chemicals). In immunoblotting analysis, proteins resolved by SDS-PAGE were transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore) and visualized using an enhanced chemiluminescence (ECL) Western blotting kit (GE Healthcare) with specific antibodies.
Suppresion of Syndecan-1 Expression by RNA Interference
A predesigned small interference RNA (siRNA) corresponding to the target sequence for human syndecan-1 and a control short RNA (#4611) were obtained from Applied Biosystems (Foster City, CA). The target sequence was 5′-GGAGGAAUUCUAUGCCUGA-tt-3′ (#16704, sense) (Beauvais et al., 2004). To transfect these RNAs, EJ-1 cells were inoculated the day before transfection at a cell density of 20–30% saturation in 60-mm culture dishes and treated with the control RNA or the siRNA by using the HiPerFect reagent (QIAGEN, Tokyo, Japan) according to the manufacturer's protocol. Three days later, the cells were used for the analysis of syndecan-1 expression by immunoblotting and other experiments.
Determination of Protein Concentrations
Protein concentrations were determined using a Bio-Rad protein assay kit with BSA as a standard, unless otherwise noted.
RESULTS
Biological Activity of Laminin γ2 Chain Short Arm
The γ2 chain of human LN5 is cleaved at a specific site of the short arm by extracellular proteinases (Figure 1A). Previously, we found that a recombinant human LN5 with a 150-kDa γ2 chain (Np-LN5) has an ∼5-times higher cell adhesion activity and an ∼2-times lower cell migration activity than the mature LN5 with the 105-kDa γ2 chain (Pr-LN5). This suggests that the short arm of the γ2 chain contains a functional domain that regulates cellular adhesion and migration. To examine this possibility, we constructed an expression vector for the short arm of laminin γ2 chain, and we introduced it into the human bladder carcinoma cell line EJ-1 (Figure 1B). The γ2 short arm, named γ2sa, was purified from the conditioned medium of the EJ-1 cells by affinity chromatography on an anti-laminin-γ2 mAb (D4B5) column. The purified material exhibited a single band of 70 kDa on SDS-PAGE under reducing conditions (Figure 1C).
Figure 1.
Domain structures of LN5 and recombinant γ2 proteins. (A) Schematic structure of LN5 molecule (α3β3γ2) and its proteolytic processing sites. Top closed arrow, cleavage site in the human laminin γ2 chain to produce the 105-kDa chain and the 45-kDa γ2 fragment (γ2pf); open arrow, second cleavage site in the rat laminin γ2 chain to produce the 80-kDa chain and the 25- and 45-kDa fragments; bottom arrow, cleavage site in the human laminin α3 chain to produce the 160-kDa α3 chain and a LG4–5 fragment; LG, laminin carboxyl-terminal globular domain of the α3 chain. Roman numerals indicate the domains of the γ2 chain: domains III (or LEb), IV (or L4), and V (or LEa). (B) Schematic diagram of the structures of the full-length human laminin γ2 chain and recombinant γ2 proteins. Numerals indicate the positions of amino acid residues from the NH2-terminus. Shadow boxes indicate the signal sequence. (C) SDS-PAGE of the purified γ2 chain short arm (γ2sa) on a 10% gel under reducing condition. Proteins were stained with silver.
The purified γ2sa protein was assayed for some biological activities exhibited by LN5 (Figure 2). When only the purified γ2sa was coated on plastic plates, it did not support adhesion of EJ-1 cells at all (Figure 2A). However, when plastic plates were sequentially coated with constant concentrations of Pr-LN5 and then with various concentrations of γ2sa, the cell adhesion was promoted in a dose-dependent manner to γ2sa. The coating of plastic plates with 0.8 μg/ml γ2sa decreased the effective concentration of Pr-LN5 for the half-maximal cell adhesion (ED50) more than threefold (Figure 2B). Furthermore, the synergistic cell adhesion activity of γ2sa with Pr-LN5 was observed even when γ2sa was directly added to the culture medium in the plates pre-coated with Pr-LN5 (Figure 2C). Similar results were obtained with BRL rat liver cells (data not shown). These results indicate that γ2sa supports efficient cell adhesion in synergy with Pr-LN5, and this effect is not due to the increase in the coating efficiency of Pr-LN5.
Figure 2.
Cell adhesion activity of γ2sa in presence or absence of Pr-LN5. (A) Effect of varied concentrations of γ2sa with or without fixed concentrations of Pr-LN5 on adhesion of EJ-1 cells to plastic plates. Plates (96-well) were precoated without (○) or with 0.1 μg/ml (▴), 0.2 μg/ml (■), or 0.4 μg/ml (•) of Pr-LN5 and then coated with the indicated concentrations of γ2sa, followed by blocking with BSA. EJ-1 cells were plated into each well in serum-free medium and incubated at 37°C for 1 h. After the incubation, relative numbers of adherent cells were determined as described in Materials and Methods. Each value represents the mean ± SD (bar) of triplicate assays. (B) Effect of varied concentrations of Pr-LN5 with or without a fixed concentration of γ2sa on adhesion of EJ-1 cells to plastic plates. Pr-LN5 was coated at the indicated concentrations without (○) or with (•) 0.8 μg/ml γ2sa on 96-well plates. The adhesion of EJ-1 cells onto the plates was measured as described above. (C) Effect of addition of soluble γ2sa on adhesion of EJ-1 cells to Pr-LN5. Plastic plates were coated with 0.3 mg/ml Pr-LN5 and blocked with BSA. After EJ-1 cells were plated on the plates, γ2sa was directly added to the serum-free culture medium and incubated for 1 h. Adherent cells were quantified as described above.
The activity of γ2sa was also examined with respect to the migration of EJ-1 cells on the Pr-LN5 substrate, by using a culture medium containing 1% FCS. When increasing concentrations of γ2sa was coated with a constant concentration of Pr-LN5 on culture plates, it dose-dependently suppressed the migration of EJ-1 cells (Figure 3A). When γ2sa was directly added to the culture medium, it also suppressed the migration of EJ-1 cells on the Pr-LN5 substrate (Figure 3B). Similar inhibitory effects of soluble γ2sa were observed with respect to the migration of the mammary epithelial cell line MCF10A, the epidermoid carcinoma cell line A431, and the rat liver cell line BRL on Pr-LN5 (Figure 3B). The cell migration assays shown above were performed in the presence of 1% FCS in culture medium. When serum-starved EJ-1 cells were seeded in a serum-free medium, they migrated poorly even on the Pr-LN5 substrate. Under these conditions, soluble γ2sa did not affect the cell migration (Figure 3C). However, when the cell migration was stimulated by EGF, soluble γ2sa again significantly inhibited the cell migration on Pr-LN5. All of these results were consistent with our previous result that the nonprocessed LN5 (Np-LN5) had a higher cell adhesion activity but a lower cell migration activity than the processed LN5 (Pr-LN5) (Ogawa et al., 2004).
Figure 3.
Inhibitory effect of γ2sa on cell migration on Pr-LN5 substrate. (A) Effect of insoluble (or coated) γ2sa on migration of EJ-1 cells on Pr-LN5 substrate. Plates (24-well) were cocoated with the indicated concentrations of γ2sa and 0.3 μg/ml Pr-LN5 and then blocked with BSA. EJ-1 cells (10,000 cells) were inoculated per well in DMEM/F-12 medium supplemented with 1% FCS. After preincubation for 1 h to allow cell spreading, the migration of EJ-1 cells was monitored by video for 10 h. Each point represents the mean ± SD of the cell migration speed of 20 cells. The cell migration speed at 0.8 μg/ml γ2sa is significantly lower than the control value in the absence of γ2sa (p < 0.001). Other experimental conditions are described in Materials and Methods. (B) Effect of soluble γ2sa on migration of four kinds of cell lines. EJ-1 (○), BRL (•), A431 (▴), or MCF-10A (Δ) cells were inoculated into each well precoated with 0.3 μg/ml Pr-LN5, and the indicated concentration of γ2sa was added to the culture medium containing 1% FCS. After preincubation for 1 h, the cell migration speed was measured as described above. In the four cell lines, the cell migration speeds at 0.8 μg/ml γ2sa are significantly lower than the respective control value (p < 0.001). (C) Effect of soluble γ2sa on EGF-induced migration of EJ-1 cells on Pr-LN5 substrate in serum-free medium. EJ-1 cells were inoculated into each well, which had been coated with 0.3 μg/ml Pr-LN5, in serum-free DMEM/F-12 medium without (○) or with (▴) 0.05 μg/ml EGF, and the indicated concentration of γ2sa was added to the culture medium. The cell migration speed was measured as described above. The differences in the cell migration speeds between 0.0 and 0.4 or 0.8 μg/ml γ2sa are statistically significant in the presence of EGF (p < 0.001) but insignificant in its absence (p > 0.05).
Effect of γ2sa on EGF-induced Phosphorylation of Integrin β4
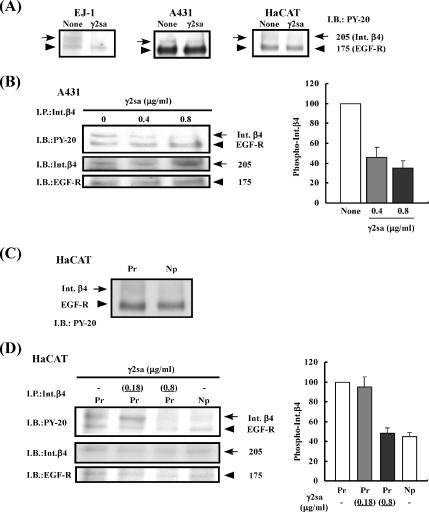
The results shown above demonstrated that the short arm of laminin γ2 chain had a stimulatory activity on cell adhesion and an inhibitory activity on cell migration in the presence of Pr-LN5. In addition, the activity of soluble γ2sa suggested that it might bind to a receptor other than integrins, leading to the inhibition of the EGF-induced signal transduction. To examine this possibility, we analyzed the phosphorylation levels of EGF receptor and related proteins by using three kinds of cell lines, EJ-1, A431, and the keratinocyte line HaCaT. Serum-starved cells were incubated on the Pr-LN5 substrate for 90 min and then treated with EGF in the presence or absence of soluble γ2sa. Treatment of these cell lines with EGF induced tyrosine phosphorylation of EGF receptor (data not shown). When the tyrosine phosphorylation levels were compared between the cell lysates from the γ2sa-treated and nontreated cells, there was no significant difference in the tyrosine phosphorylation level of the 175-kDa EGF receptor (Figure 4A). However, the phosphorylation of a 205-kDa band seemed to slightly decrease by the γ2sa treatment in all of the three cell lines.
Figure 4.
Inhibitory effect of γ2sa on EGF-induced phosphorylation of integrin β4. (A) Serum-starved EJ-1, A431, or HaCaT cells were suspended in serum-free DMEM/F-12 medium and inoculated into culture dishes that had previously been coated with 0.5 μg/ml Pr-LN5 and then blocked with BSA. After incubation for 1.5 h, the cultures were added with 0.05 μg/ml EGF and without (None) or with 0.4 μg/ml soluble γ2sa (γ2sa). After further incubation for 10 min, cell lysate was prepared from each culture. The samples were subjected to SDS-PAGE and subsequent immunoblotting (I.B.) with a mAb against PY-20. The 175-kDa band was identified as EGF receptor (EGF-R), and the 205-kDa band was identified as integrin β4 (Int.β4), as shown below. Other experimental conditions are described in Materials and Methods. (B) A431 cells were treated with 0.4 or 0.8 μg/ml γ2sa and 0.05 μg/ml EGF on the Pr-LN5 substrate. Integrin β4 present in the cell lysates was subjected to immunoprecipitation (I.P.) with the anti-integrin β4 mAb 3E1. The immunoprecipitates were analyzed by immunoblotting with the antibodies against PY-20, integrin β4 (Int.β4) and EGF-R (left). Relative intensity of the 205-kDa immunosignal for the integrin β4 phosphorylation was determined using the NIH Image software (right). Each value represents the mean intensity ± SD of three separate experiments. The relative intensity of the control culture (None) was regarded as 100. (C and D) Serum-starved HaCaT cells were treated with 0.18 or 0.8 μg/ml soluble γ2sa in addition to 0.05 μg/ml EGF in culture dishes that had been pre-coated with 1 μg/ml Pr-LN5 (Pr) or Np-LN5 (Np) as described above. The tyrosine phosphorylation of integrin β4 (arrows) and EGF receptor (arrowheads) in the cell lysates were analyzed before (C) and after (D) immunoprecipitation with the anti-integrin β4 mAb 3E1.
EGF receptor is known to be associated with integrin β4 (Mainiero et al., 1995; Mainiero et al., 1996). To identify the 205-kDa phosphorylated protein, immunoprecipitation was performed using A431 cells treated with γ2sa plus Pr-LN5. An antibody against integrin β4 immunoprecipitated both the 205-kDa phosphorylated protein and EGF receptor (Figure 4B). The 205-kDa phosphorylated protein was identified as integrin β4 by immunoblotting (Figure 4B, left). The quantitative analysis of the integrin β4 phosphorylation clearly showed that the tyrosine phosphorylation of integrin β4 was dose-dependently decreased by γ2sa (Figure 4B, right). Similar results were obtained when the cell lysate was immunoprecipitated with an anti-EGF-receptor polyclonal antibody (data not shown). These results demonstrate that γ2sa inhibits the EGF-induced phosphorylation of integrin β4 in the presence of Pr-LN5.
To further investigate the role of γ2sa in the regulation of integrin functions, we compared the phosphorylation of integrin β4 in HaCaT cells incubated in three different conditions, i.e., Pr-LN5 alone, Pr-LN5 plus γ2sa, and Np-LN5 alone (Figure 4, C and D). When Pr-LN5 and Np-LN5 were compared using their total cell lysates, the cells incubated on Np-LN5 showed a slightly lower level of integrin β4 phosphorylation compared with those on Pr-LN5 (Figure 4C). There was no significant difference in the phosphorylation level of EGF receptor between the cells incubated on Pr-LN5 and those on Np-LN5. The differential phosphorylation of integrin β4 between Pr-LN5 and Np-LN5 was more clearly shown by the analysis after immunoprecipitation with the anti-integrin-β4 antibody (Figure 4D). When cells were incubated on Pr-LN5 with or without the same molar concentration (0.18 μg/ml) of soluble γ2sa, the treatment with γ2sa did not significantly affect the EGF-induced phosphorylation of integrin β4 (Figure 4D). However, when the concentration of γ2sa was increased to 0.8 μg/ml, it significantly decreased the phosphorylation of integrin β4 compared with the cells incubated on Pr-LN5 alone. These results suggest that the changes of the biological activities of LN5 induced by the proteolytic cleavage of the γ2 short arm may be mediated by the increased phosphorylation of integrin β4. The results also imply that the effect of γ2sa is greater in its presence within the LN5 molecule than in the released form.
Effect of Laminin γ2 Chain Short Arm on Disassembly of Hemidesmosome-like Structures Induced by EGF
Because tyrosine phosphorylation of integrin β4 is known to disrupt hemidesmosomes (Mainiero et al., 1997; Mariotti et al., 2001), we next examined the effect of γ2sa on hemidesmosome structures of HaCaT keratinocytes. Under an immunofluorescent microscope, hemidesmosomes look like punctuated structures, which tend to coalesce around circular areas (Mariotti et al., 2001). Immunofluorescent staining with an anti-BP180 antibody revealed many hemidesmosome-like, punctuated structures in the cells treated with Pr-LN5 alone, Pr-LN5 plus soluble γ2sa, and NP-LN5 alone (Figure 5A). When these cells were additionally treated with EGF, the punctuated structures of the cells on Pr-LN5 became faint and diffused. However, the immunostaining patterns of the cells on Np-LN5 and those on Pr-LN5 with soluble γ2sa were scarcely affected by the EGF treatment. In contrast, the immunofluorescent staining with the anti-integrin-β4 antibody of HaCaT cells showed no significant difference among the three different conditions regardless of the EGF treatment (Figure 5B). This suggested that the association of LN5 with integrin α6β4 was maintained even after the EGF treatment, although the interaction between integrin α6β4 and BP230/180 in the cells on Pr-LN5 was lost after the EGF treatment. These results demonstrate that the short arm of γ2 chain prevents the disassembly of hemidesmosome-like structure induced by EGF.
Figure 5.
Effect of γ2sa on disassembly of hemidesmosome-like structures induced by EGF. Serum-starved HaCaT cells were inoculated in serum-free medium and incubated for 8 h on culture dishes that had been coated with 1.0 μg/ml Pr-LN5 or Np-LN5. The cultures were then treated for 10 min with 0.4 μg/ml soluble γ2sa (γ2sa) in the presence (+EGF) or absence (None) of 0.05 μg/ml EGF. The treated cells were subjected to immnofluorescence staining with an anti-BP180 mAb (A) and the anti-integrin-β4 mAb (B). Other experimental conditions are described in Materials and Methods.
Identification of Membrane Receptor for γ2sa
The results shown above suggest that γ2sa binds to a cell surface receptor, leading to the enhancement of cell adhesion. We speculated that γ2sa might interact with an HSPG on cell surface, because the short arm of the γ2 chain has been reported to contain heparin-binding sites (Sasaki et al., 2001).
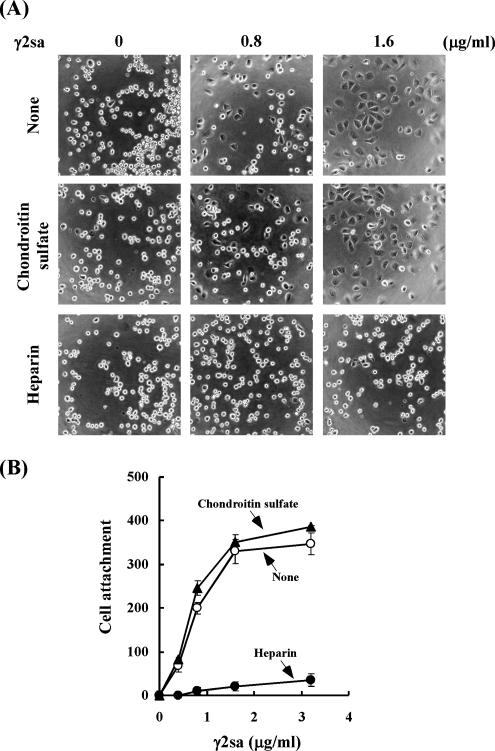
To examine this possibility, effect of heparin on the cell adhesion activity of γ2sa was examined using EJ-1 cells. As shown above, γ2sa promoted the cell adhesion in synergy with Pr-LN5. Heparin almost completely blocked the cell adhesion activity of γ2sa (Figure 6, A and B). However, chondroitin sulfate, a different type of sulfated glycosaminoglycans, failed to block the cell adhesion. These results suggest that γ2sa specifically interacts with heparan sulfates.
Figure 6.
Effects of heparin and chondroitin sulfate on adhesion of EJ-1 cells to culture plates coated with both Pr-LN5 and γ2sa. (A) Plastic plates were coated with 0.3 μg/ml Pr-LN5 and then with 0.8 or 1.6 μg/ml γ2sa, followed by blocking with BSA. EJ-1 cells were suspended in serum-free medium supplemented without (None) or with 20 μg/ml heparin or 20 μg/ml chondroitin sulfate ABC (Chondroitin sulfate), inoculated on the precoated plates, and incubated at 37°C for 1 h. The resultant cultures were examined under a phase-contrast microscope. Original magnification, 300×. (B) EJ-1 cells were incubated in serum-free medium without (○) or with heparin (•) or chondroitin sulfate (▴) on plates that had been coated with 0.3 μg/ml Pr-LN5 and the indicated concentrations of γ2sa. Adherent cells were quantified as shown in Figure 2. Other experimental conditions are described in Materials and Methods.
To identify the membrane receptor of γ2sa, a surface-biotinylated membrane fraction of EJ-1 cells was applied to the control column or a γ2sa-conjugated column. When biotinylated molecules bound to the column were eluted with 1.0 M NaCl and analyzed by SDS-PAGE, the eluate from the γ2sa column specifically showed a broad band at a molecular mass range >200 kDa (Figure 7A). When the bound materials were subjected to immunoblotting with an anti-ΔHSPG antibody after treating with heparitinase, a major band of 66 kDa and a minor band of 60 kDa were detected. Immunoblotting with an anti-syndecan-1 antibody also showed immunoreactive bands at the same positions, indicating that both 66- and 60-kDa bands were the major cell surface HSPG syndecan-1. The amount of syndecan-1 bound to the column increased by increasing the amount of γ2sa conjugated to the beads, but the syndecan-1 binding to the γ2sa column was reduced by the addition of heparin (Figure 7B). These results, together with the data in Figure 6, strongly suggest that syndecan-1 is a receptor of γ2sa in EJ-1 cells.
Figure 7.
Identification of receptor for γ2 short arm. (A) Surface membrane proteins of EJ-1 cells were biotinylated with Biotin-OSu (Dojindo, Kumamoto, Japan), and the labeled proteins were passed through a γ2sa-conjugated column (γ2sa) or a control column without γ2sa (Cont.). Bound materials were eluted with 1 M NaCl, separated by nonreducing SDS-PAGE on a 5–12% gradient gel, and electrotransferred to a nitrocellulose membrane. The transferred membrane proteins were detected with the horseradish peroxidese-avidin D/ECL method. The arrow indicates molecules specifically bound to the γ2sa column. (B) The labeled membrane proteins were passed through columns conjugated with 0, 0.25, or 1.0 μg/ml γ2sa in the absence (−) or presence (+) of 1.0 μg/ml heparin. Bound materials were eluted from the column, treated with heparitinase, and immunoblotted with an anti-Δheparan sulfate antibody (top) and an anti-syndecan-1 antibody (bottom). Arrowheads, syndecan-1 core proteins and their apparent molecular sizes in kilodaltons.
Identification of Active Site of γ2 Chain
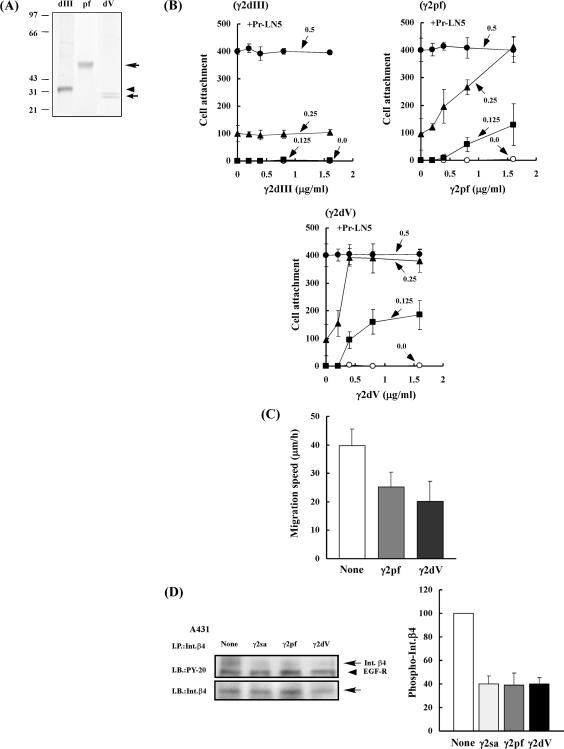
To localize the active site in the short arm of the γ2 chain, we expressed three histidine-tagged proteins containing a part of the NH2-terminal region, γ2pf, γ2dV, and γ2dIII, in HEK293 cells (Figure 1B). γ2pf corresponds to a natural NH2-terminal proteolytic fragment released by the processing of the γ2 chain and contains domains V, IV, and a short sequence of domain III, whereas γ2dV and γ2dIII contains only domain V and domain III, respectively. These recombinant proteins were purified by affinity chromatographies on a nickel-chelating column and then on a heparin-Sepharose column (for γ2pf and γ2dV) or an anti-γ2 mAb column (for γ2dIII). The purified γ2pf and γ2dIII showed a single, broad band of 60 and 30 kDa, respectively, whereas γ2dV showed double bands of 28 and 25 kDa on SDS-PAGE under reducing conditions (Figure 8A). These purified proteins contained little contaminating proteins.
Figure 8.
Biological activities of three NH2-terminal γ2 fragments: γ2pf, γ2dV, and γ2dIII. (A) SDS-PAGE of purified γ2dIII (dIII), γ2pf (pf), and γ2dV (dV) on a 12.5% gel under reducing conditions. Proteins were stained with Coomassie brilliant blue R-250. (B) Effects of varied concentrations of γ2dIII, γ2pf, and γ2dV on adhesion of EJ-1 cells to Pr-LN5. Plates (96-well) were coated without (○) or with 0.125 μg/ml (■), 0.25 μg/ml μg/ml (▴), or 0.5 μg/ml (•) of Pr-LN5 and then with the indicated concentrations of γ2dIII (top left), γ2pf (top right), or γ2dV (bottom). After blocking with BSA, EJ-1 cells were plated on each substrate and incubated at 37°C for 1 h. Relative numbers of adherent cells were determined as described in Figure 2 and Materials and Methods. Each value represents the mean ± SD of triplicate assays. One micorgram per milliliter is equivalent to ∼32.3 nM for γ2dIII, 16.7 nM for γ2pf, and 35.7 nM for γ2dV. (C) Inhibitory effects of γ2pf and γ2dV on migration of EJ-1 cells on Pr-LN5 substrate. Plates (24-well) were coated with 0.3 μg/ml Pr-LN5 and then with 0.8 μg/ml (13.3 nM) γ2pf or 0.4 μg/ml (14.3 nM) γ2dV, followed by blocking with BSA. The cell migration speed on the plates was determined as described in Figure 3. (D) Inhibitory effects of γ2pf and γ2dV on EGF-induced phosphorylation of integrin β4. Serum-starved A431 cells were inoculated into culture dishes precoated with 1 μg/ml Pr-LN5 and incubated without or with 0.8 μg/ml (13.3 nM) γ2pf, 0.4 μg/ml (14.3 nM) γ2dV, or 0.8 μg/ml (11.4 nM) γ2sa in serum-free medium supplemented with 0.05 μg/ml EGF for 10 min. The tyrosine phosphorylation of integrin β4 was analyzed after immunoprecipitation. Other experimental conditions are described in Figure 4 and Materials and Methods.
The cell adhesion activities of these proteins were assayed in the presence or absence of Pr-LN5 (Figure 8B). When only the purified γ2pf, γ2dV, or γ2dIII was coated on plastic plates, neither one supported adhesion of EJ-1 cells. When plastic plates were sequentially coated with constant concentrations of Pr-LN5 and then with various concentrations of γ2pf, γ2dV, or γ2dIII, both γ2pf and γ2dV dose-dependently promoted cell adhesion, but γ2dIII did not support cell adhesion. On the plates coated with 0.25 μg/ml Pr-LN5, the maximum cell adhesion was attained at 1.6 μg/ml (26.7 nM) γ2pf or 0.4 μg/ml (14.3 nM) γ2dV. Thus, γ2dV seemed to have a higher activity than γ2pf. The cell adhesion activity of γ2dV was efficiently blocked by heparin, suggesting that the activity was mediated by syndecan-1 (data not shown).
Next, inhibitory effects of γ2pf and γ2dV on cell migration activity of Pr-LN5 were assayed. When γ2pf and γ2dV were cocoated at nearly the same molar concentration with Pr-LN5, both of them significantly suppressed the migration of EJ-1 cells (Figure 8C). The inhibitory activity of γ2dV seemed to be slightly higher than that of γ2pf.
Furthermore, we examined effects of γ2pf and γ2dV on the tyrosine phosphorylation of integrin β4 by using A431 cells (Figure 8D). As expected, both γ2pf and γ2dV suppressed EGF-induced tyrosine phosphorylation of integrin β4 on the plates precoated with Pr-LN5.
Cationic Nature of the γ2 Short Arm Proteins
As described above, the HSPG syndecan-1 was identified as a possible receptor of γ2sa, and both γ2pf and γ2dV had similar biological activities to γ2sa. Because HSPGs and heparin bind cationic proteins, the biological activities of the γ2 short arm proteins are likely to depend on their cationic nature. Therefore, we compared heparin-binding activity between γ2pf and γ2dV. When each protein was applied to a heparin-agarose column and the bound protein was eluted by a NaCl gradient, γ2pf and γ2dV were eluted at 0.36∼0.4 M NaCl and 0.3∼0.33 M NaCl, respectively. This indicates that γ2dV has slightly lower heparin-binding affinity than γ2pf.
To further test whether the activities of the γ2 short arm proteins simply depend on their cationic nature, we compared effects of γ2pf and a highly cationic peptide, poly-l-lysine. When only poly-l-lysine or γ2pf was coated on plastic plates, poly-l-lysine, but not γ2pf, supported adhesion of EJ-1 to the plates (data not shown). When cocoated with Pr-LN5, both poly-l-lysine and γ2pf promoted the adhesion of EJ-1 cells dose-dependently (Figure 9A). The cell adhesion activity was stronger for poly-l-lysine than γ2pf. However, their adhesion activities were comparable when A431 cells were used instead of EJ-1 cells (Figure 9B).
Figure 9.
Effects of γ2pf and poly-l-lysine on cell adhesion, cell migration, and phosphorylation of integrin β4. (A and B) Effect on adhesion of EJ-1 (A) and A431 (B) cells to Pr-LN5. Plates (96-well) were precoated with 0.25 μg/ml (A) or 0.125 μg/ml (B) Pr-LN5 and then coated with the indicated concentrations of γ2pf (•) and poly-l-lysine (▴), followed by blocking with BSA. The adhesion of EJ-1 (A) and A431 (B) cells onto the plates was measured as described in Figure 2A. (C) Effect on migration of A431 cells. A431 cells were inoculated into each well precoated with 0.3 μg/ml Pr-LN5, and 0.4 μg of γ2sa or poly-l-lysine (PL) was added to the culture medium containing 1% FCS. After preincubation for 1 h, the cell migration speed was measured as described in Figure 3A. (D) Effect on integrin β4 phosphorylation of A431 cells. A431 cells were treated with 0.8 μg/ml γ2pf or PL and with 0.01 μg/ml EGF on the Pr-LN5 substrate. Integrin β4 present in the cell lysates was immunoprecipitated with the anti-integrin β4 mAb 3E1. The immunoprecipitates were analyzed by immunoblotting with the mAb against PY-20. Left, immunoblotting patterns. Right, quantitative analysis of the integrin β4 phodphorylation. Other experimental conditions are described in Figure 4 and Materials and Methods.
Next, effects of γ2pf and poly-l-lysine were compared on the migration activity of A431 cells (Figure 9C). As shown in Figure 3, γ2pf significantly inhibited the cell migration on the Pr-LN5 substrate, but poly-l-lysine showed no significant effect. Furthermore, we compared effects of γ2pf and poly-l-lysine on the phosphorylation of integrin β4 in A431 cells on the Pr-LN5 substrate (Figure 9D). In good accordance with the results of cell migration, γ2pf suppressed EGF-stimulated phosphorylation of integrin β4, whereas poly-l-lysine did not significantly affect the phosphorylation of integrin β4 or EGF receptor.
All these results indicate that although the cationic nature, or heparin-binding activity, is prerequisite but not sufficient for the biological activities of the γ2 short arm proteins, especially for the suppression of the integrin β4 phosphorylation and cell migration, it is clear that the γ2 short arm has a specific activity.
Suppression of Syndecan-1 Expression by RNA Interference
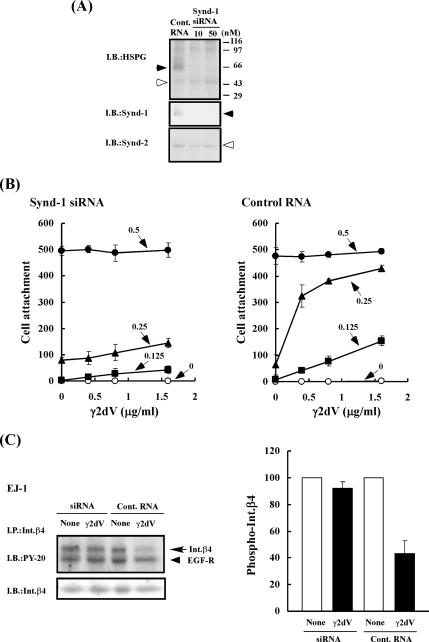
To further confirm the specific interaction between domain V of the γ2 chain and syndecan-1, we carried out an RNA interference experiment to block the expression of syndecan-1 gene. An siRNA and a control RNA were transfected into EJ-1 cells. Some HSPGs, including syndecans-1 and -2, were detected in control cells by immunoblotting (Figure 10A). Syndecan-4 was undetectable by immunoblotting (data not shown). Transfection with siRNA efficiently blocked the expression of syndecan-1 in EJ-1 cells, which was a major HSPG band in the control cells. The siRNA treatment did not significantly affect the amounts of syndecan-2 and other HSPG species. The cell adhesion activity of γ2dV in the presence of Pr-LN5 was assayed with the control and siRNA-treated cells 3 d after the RNA transfection. γ2dV negligibly promoted the adhesion of the EJ-1 cells treated with the siRNA (Figure 10B, left), whereas it clearly promoted the adhesion of the control cells (Figure 10B, right).
Figure 10.
Effect of suppression of syndecan-1 expression by RNA interference on cell adhesion and integrin β4 phosphorylation. (A) Changes of HSPGs in EJ-1 cells after transfection with a control RNA (Cont. RNA) and a syndecan-1 siRNA (siRNA). EJ-1 cells were grown in 60-mm dishes, transfected with either 50 nM Cont. RNA or with 10 or 50 nM siRNA and further incubated for 72 h. The cultured cells (1 × 106) were used for the determination of syndecan-1 expression. Total cell lysates (200 μg of protein) were digested by heparitinase, resolved by SDS-PAGE, and transferred to a PVDF membrane. Total HSPG species, syndecan-1 (Synd-1) and syndecan-2 (Synd-2) present in the cell lysates were detected by immunoblotting with the anti-Δheparansulfate mAb, the anti-syndecan-1 mAb, or the anti-syndecan-2 antibody, as described in Figure 7B. The protein concentrations of the cell lysates were determined by the Lowry–Folin method. (B) Effect of syndecan-1 knockdown on γ2dV-dependent adhesion of EJ-1 cells to Pr-LN5. The cell adhesion activity of γ2dV in the presence of Pr-LN5 was assayed as described in Figure 2, by using EJ-1 cells that had been transfected with 50 nM control RNA (Control RNA; right) or 50 nM syndecan-1 siRNA (siRNA; left) as shown above. (C) Effect of syndecan-1 knockdown of EJ-1 cells on γ2dV-mediated suppression of integrin β4 phosphorylation. Serum-starved EJ-1 cells, which had been transfected with 50 nM control RNA (Cont. RNA) or 50 nM syndecan-1 siRNA (siRNA), were inoculated into culture dishes precoated with 1 μg/ml Pr-LN5 and incubated without or with 0.8 μg/ml γ2dV in serum-free medium supplemented with 0.1 μg/ml EGF for 15 min. The tyrosine phosphorylation of integrin β4 was analyzed after immunoprecipitation as described in Figure 4. Left, immunoblots. Right, quantitative analysis of phosphorylated integrin β4. Other experimental conditions are described in Materials and Methods.
Furthermore, we examined whether the syndecan-1 knockdown affects the integrin β4 phosphorylation of the EJ-1 cells (Figure 10C). Although γ2dV suppressed the EGF-induced tyrosine phosphorylation of integrin β4 in the control cells, it showed little effect on the siRNA-transfected cells. These results clearly show that the biological effects of γ2dV are mediated by syndecan-1 but not other HSPGs, at least in EJ-1 cells.
DISCUSSION
The present study demonstrates that the short arm of laminin γ2 chain (γ2sa) in both soluble and insoluble (coated) forms promotes cell adhesion but suppresses cell migration on the processed LN5 substrate. These activities of γ2sa are consistent with our previous finding that the nonprocessed LN5 (Np-LN5) is more adhesive but less active with respect to the cell motility activity than Pr-LN5 (Ogawa et al., 2004). Interestingly, γ2sa suppressed the EGF-dependent tyrosine phosphorylation of integrin β4, and it seemed to stabilize hemidesmosome structures. All these activities of γ2sa seem to be related with one another and mediated by its specific binding to syndecan-1 as a receptor. Furthermore, our results strongly suggest that the NH2-terminal sequence domain V is responsible for these biological activities of γ2sa.
Proteolytic processing of extracellular matrix proteins modulates their biological activities (Schenk and Quaranta, 2003). Giannelli et al. (1997) reported that when a rat LN5 with the unprocessed 150-kDa γ2 chain was treated with gelatinase A (MMP-2), the digest containing the LN5 with an 80-kDa processed γ2 chain stimulated cell migration more strongly than the untreated LN5 in the Boyden chamber assay. Recently, the same group showed that a 21-kDa recombinant rat γ2 fragment containing only the laminin-type EGF-like domain III, which could be produced by cleavage at two sites (Figure 1A), binds and activates the EGF receptor to stimulate cell migration (Schenk and Quaranta, 2003). They suggested that the domain III fragment of γ2 chain, rather than the LN5 with the 80-kDa truncated γ2 chain, is responsible for the enhanced cell migration activity. Consistent with our results, a recombinant protein containing domains III–V, which corresponds to our γ2sa, was unable to activate the EGF receptor. In human LN5, however, the production of the 80-kDa γ2 chain and the 21-kDa domain III fragment is thought to be very rare, if any, under ordinary conditions, because the proteolytic processing site of rat γ2 chain to produce the 80-kDa γ2 chain is not conserved in the human γ2 chain (Veitch et al., 2003). The 150-kDa γ2 chain of human LN5 is processed mainly to the 105-kDa γ2 form, releasing a 45-kDa fragment containing domains IV–V, which corresponds to γ2pf in this study (Figure 1A). Our results indicate that the elevated cell migration activity of Pr-LN5 results from the loss of the 45-kDa fragment (domains IV–V) or domain V, which suppresses cell migration but promotes cell adhesion.
Integrin α6β4 is an essential component of hemidesmosome structures, which support the stable adhesion of basal epithelial cells to the basement membrane. Integrin α6β4 is associated with EGF receptor, and the activation of EGF receptor induces the phosphorylation of integrin β4 through the Src family kinase Fyn or Yes (Mariotti et al., 2001). The phosphorylation of the integrin β4 tail promotes the hemidesmosome disassembly, which is essential for the epithelial cell migration and invasion (Mainiero et al., 1997; Mariotti et al., 2001). The activity of γ2sa to suppress the EGF-stimulated phosphorylation of integrin β4 and subsequent disassembly of the hemidesmosome structures well explains the molecular mechanism by which γ2sa promotes cell adhesion but suppresses cell migration in the presence of Pr-LN5.
Our results indicated that the biological activities of γ2sa were mediated by its direct binding to a HSPG molecule(s) on cell surface. The cell surface HSPG family syndecan is well known to cooperate with integrins to regulate actin cytoskeletal organization and cell adhesion (Couchman, 2003; Beauvais and Rapraeger, 2004). EJ-1 cells expressed syndecan-1, syndecan-2, and some other unidentified HSPGs. However, when the membrane extract of EJ-1 cells was applied to a γ2sa-conjugated column, only syndecan-1 was adsorbed to the column. In addition, the down-regulation of syndecan-1 expression by a specific siRNA effectively blocked the activities of γ2sa to promote cell adhesion and inhibit EGF-induced phosphorylation of integrin β4. These results clearly indicate that syndecan-1 is a major membrane receptor of γ2sa, and the suppression of the EGF-stimulated phosphorylation of integrin β4 by γ2sa is mediated by its specific binding to syndecan-1. Syndecan-1 is thought to play an important role in maintaining normal epithelial phenotypes and morphology. This epithelial type syndecan is down-regulated in several epithelial cancers (Beauvais and Rapraeger, 2004). Loss of cell surface syndecan-1 causes epithelia to transform into mesenchyme-like cells (Kato et al., 1995). Syndecan-1 cooperates with integrin αvβ3 to regulate cytoskeletal organization and cell spreading (Beauvais and Rapraeger, 2003). Despite many past studies on syndecans, no reports have described interaction of any syndecan with β4 integrins as far as we know. It is expected that the suppression of the integrin β4 phosphorylation by γ2sa contributes to the stable cell adhesion through integrin α6β4. Because not only an insoluble, or coated, form but also a soluble form of γ2sa blocked the integrin β4 phosphorylation, a γ2sa-stimulated syndecan-1 signaling, rather than the direct interaction between syndecan-1 and integrin β4, is expected to negatively regulate the integrin β4 phosphorylation. Further studies are required to clarify this novel mechanism. It has been reported that the γ2 short arm contains binding sites for heparin/sulfatide, fibulins-1 and -2, and nidogen-1 (Sasaki et al., 2001; Gagnoux-Palacios et al., 2001). A recent study has demonstrated that the binding of laminins to sulfated glycolipids (sulfatides) via the LG domain on cell surface plays an important role in the basement membrane assembly (Li et al., 2005). Although our results demonstrate the important roles of syndecan-1 as a receptor of the γ2 short arm, it cannot be excluded that the γ2 short arm may also bind to other membrane molecules including other HSPGs and sulfatides to regulate cellular adhesion and migration.
Many heparin-binding proteins, including growth factors, extracellular components, and anticoagulants, are known to interact with syndecans, mainly through their heparan sulfate residues. Presumably, the syndecan-mediating signaling varies depending on the kind of ligand. Laminins contain several heparin-binding sites. The COOH-terminal LG domain of laminin α1 chain in laminins-1 and -2 contains a heparin-binding site that interacts with syndecan-1 (Hoffman et al., 1998). In LN5, a recombinant LG4 protein of the laminin α3 chain binds syndecans-1 and -4 (Hirosaki et al., 2002). The LG4–5 fragment, which is released from the precurosr LN5 by proteolytic processing (Tsubota et al., 2000), supports weak cell adhesion by itself and promotes cell spreading in synergy with Pr-LN5 (Hirosaki et al., 2002). However, it remains to be investigated whether the α3 LG4–5 fragment suppresses the integrin β4 phosphorylation. In this study, the γ2 short arm proteins showed heparin-binding activity, but they did not support cell adhesion by itself. This may be due to their weaker affinity to HSPGs compared with the α3 LG4–5 fragment. Theoretical isoelectric point (pI) value calculated from the amino acid sequence is higher for domain IV (pI = 9.9) than for domain V (pI = 7.7). In good accordance with these pI values, the affinity to heparin was lower in γ2dV than γ2pf. However, our results suggested that domain V, rather than domain IV, might be responsible for the unique biological activity of γ2sa. In contrast to these fragments, the domain III fragment γ2dIII, which has been reported to bind and activate the EGF receptor (Schenk and Quaranta, 2003), scarcely promoted the cell adhesion to Pr-LN5. Furthermore, poly-l-lysine promoted the cell adhesion but suppressed neither the EGF-stimulated integrin β4 phosphorylation nor cell migration. All these results also suggest that the unique activities of γ2 short arm are mediated by a specific interaction between domain V and syndecan-1. It is noted that an NH2-terminal EGF repeat (repeat 1) (pI = 9.3) of domain V contains a cluster of basic amino acid residues (amino acid no. 67–80; pI = 10.7). Such a specific sequence of γ2sa may contribute to its binding to syndecan-1 on cell surface.
In the present study, we showed that the γ2 short arm had activities to inhibit both the integrin β4 phoshorylation and cell motility. Np-LN5 showed a lower level of integrin β4 phoshorylation than Pr-LN5. It is expected that Np-LN5 binds both to integrins via the α3 LG1–3 domain and to syndecan-1 via the γ2 short arm, especially domain V. This implies that LN5 regulates cellular activities by binding two or more receptors on cell surface simultaneously. The two or multiple interactions between the functional domains of LN5 molecule and receptors seem to synergistically regulate cytoskeleton and complex cellular functions. The proteolytic cleavage producing the 45-kDa NH2-terminal fragment (γ2pf) and the 105-kDa γ2 chain is known to occur both in vitro and in vivo (Sasaki et al., 2001). The increase of the cell migration activity of LN5 by the γ2 chain processing may be important in some pathological conditions such as wound healing and tumor invasion through basement membranes, where epithelial cells or tumor cells must actively migrate. Alternatively, it is unclear whether the γ2pf fragment regulates cellular adhesion and migration in vivo. γ2sa and γ2pf were required ∼10 times as much as Pr-LN5 in molar ratio to induce its biological activities. We have reported previously that a monomeric laminin γ2 chain is overexpressed at the invasion fronts of gastric carcinomas and lung adenocarcinomas and secreted by carcinoma cells in vitro (Koshikawa et al., 1999; Kagesato et al., 2001). Therefore, it seems likely that the γ2 fragment produced by invading tumor cells, in cooperation with Pr-LN5 or other integrin-binding matrix proteins, may support the invasive growth of tumor cells. This possibility should be investigated in further studies.
ACKNOWLEDGMENTS
We thank Professor K. Owaribe for the generous gift of antibodies. We also thank M. Maeda, Y. Kaneko, T. Mori, and M. Chino for technical assistance and Drs. H. Yasumitsu, S. Higashi, T. Hirosaki, and K. Yamamoto for helpful discussion. This study was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Ministry of Welfare and Labor of Japan.
Abbreviations used:
- γ2dV
recombinant γ2 protein containing only domain V
- γ2pf
recombinant γ2 protein containing mainly domains V and IV
- γ2sa
recombinant protein of laminin γ2 short arm
- BSA
bovine serum albumin
- FCS
fetal calf serum
- HSPG
heparan sulfate proteoglycan
- LN5
laminin-5
- mAb
monoclonal antibody
- Np-LN5
recombinant laminin-5 with a nonprocessed, mutated γ2 chain
- Pr-LN5
natural laminin-5 with a processed γ2 chain.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0806) on February 21, 2007.
REFERENCES
- Aberdam D., et al. Herlitz's junctional epidermolysis bullosa is linked to mutations in the gene (LAMC2) for the gamma 2 subunit of nicein/kalinin (LAMININ-5) Nat. Genet. 1994;6:299–304. doi: 10.1038/ng0394-299. [DOI] [PubMed] [Google Scholar]
- Aumailley M., et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Baker S. E., Hopkinson S. B., Fitchmun M., Andreason G. L., Frasier F., Plopper G., Quaranta V., Jones J. C. Laminin-5 and hemidesmosomes: role of the alpha3 chain subunit in hemidesmosome stability and assembly. J. Cell Sci. 1996;109:2509–2520. doi: 10.1242/jcs.109.10.2509. [DOI] [PubMed] [Google Scholar]
- Beauvais D. M., Burbach B. J., Rapraeger A. C. The syndecan-1 ectodomain regulates αvb3 integrin activity in human mammary carcinoma cells. J. Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais D. M., Rapraeger A. C. Syndecan-1-mediated cell spreading requires signaling by alphaVbeta3 integrins in human breast carcinoma cells. Exp. Cell Res. 2003;286:219–232. doi: 10.1016/s0014-4827(03)00126-5. [DOI] [PubMed] [Google Scholar]
- Beauvais D. M., Rapraeger A. C. Syndecans in tumor cell adhesion and signaling. Reprod. Biol. Endocrinol. 2004;2:1–12. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Ryan M. C., Gahr P. J. Epiligrin, a new cell adhesion ligand for integrin alpha3 beta1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Colognato H., Yurchenco P. D. Form and function: the laminin family of heterotrimers. Dev. Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Couchman J. R. Syndecans: proteoglycan regulators of cell-surface microdomains. Nat. Rev. Mol. Cell Biol. 2003;4:926–937. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- Gagnoux-Palacios L., Allegra M., Spirito F., Pommeret O., Romero C., Ortonne J. P., Meneguzzi G. The short arm of the laminin gamma2 chain plays a pivotal role in the incorporation of laminin 5 into the extracellular matrix and in cell adhesion. J. Cell Biol. 2001;153:835–850. doi: 10.1083/jcb.153.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli G., Falk-Marzillier J., Schiraldi O., Stetler-Stevenson W. G., Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Hirako Y., Yoshino K., Zillikens D., Owaribe K. Extracellular cleavage of bullous pemphigoid antigen 180/Type XVII collagen and its involvement in hemidesmosomal disassembly. J. Biochem. 2003;133:197–206. doi: 10.1093/jb/mvg024. [DOI] [PubMed] [Google Scholar]
- Hirosaki T., Mizushima H., Tsubota Y., Moriyama K., Miyazaki K. Structural requirement of carboxyl-terminal globular domains of laminin alpha3 chain for promotion of rapid cell adhesion and migration by laminin-5. J. Biol. Chem. 2000;275:22495–22502. doi: 10.1074/jbc.M001326200. [DOI] [PubMed] [Google Scholar]
- Hirosaki T., Tsubota Y., Kariya Y., Moriyama K., Mizushima H., Miyazaki K. Laminin-6 is activated by proteolytic processing and regulates cellular adhesion and migration differently from laminin-5. J. Biol. Chem. 2002;277:49287–49295. doi: 10.1074/jbc.M111096200. [DOI] [PubMed] [Google Scholar]
- Hoffman M. P., Nomizu M., Roque E., Lee S., Jung D. W., Yamada Y., Kleinman H. K. Laminin-1 and laminin-2 G-domain synthetic peptides bind syndecan-1 and are involved in acinar formation of a human submandibular gland cell line. J. Biol. Chem. 1998;273:28633–28641. doi: 10.1074/jbc.273.44.28633. [DOI] [PubMed] [Google Scholar]
- Kagesato Y., Mizushima H., Koshikawa N., Kitamura H., Hayashi H., Ogawa N., Tsukuda M., Miyazaki K. Sole expression of laminin gamma 2 chain in invading tumor cells and its association with stromal fibrosis in lung adenocarcinomas. Jpn. J. Cancer Res. 2001;92:184–192. doi: 10.1111/j.1349-7006.2001.tb01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallunki P., Sainio K., Eddy R., Byers M., Kallunki T., Sariola H., Beck K., Hirvonen H., Shows T. B., Tryggvason K. A truncated laminin chain homologous to the B2 chain: structure, spatial expression, and chromosomal assignment. J. Cell Biol. 1992;119:679–693. doi: 10.1083/jcb.119.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya Y., Tsubota Y., Hirosaki T., Mizushima H., Puzon-McLaughlin W., Takada Y., Miyazaki K. Differential regulation of cellular adhesion and migration by recombinant laminin-5 forms with partial deletion or mutation within the G3 domain of alpha3 chain. J. Cell. Biochem. 2003;88:506–520. doi: 10.1002/jcb.10350. [DOI] [PubMed] [Google Scholar]
- Kato M., Saunders S., Nguyen H., Bernfield M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol. Biol. Cell. 1995;6:559–576. doi: 10.1091/mbc.6.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y., Umeda M., Miyazaki K. Marked stimulation of cell adhesion and motility by ladsin, a laminin-like scatter factor. J. Biochem. 1994;116:862–869. doi: 10.1093/oxfordjournals.jbchem.a124608. [DOI] [PubMed] [Google Scholar]
- Koshikawa N., Giannelli G., Cirulli V., Miyazaki K., Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa N., Moriyama K., Takamura H., Mizushima H., Nagashima Y., Yanoma S., Miyazaki K. Overexpression of laminin gamma2 chain monomer in invading gastric carcinoma cells. Cancer Res. 1999;59:5596–5601. [PubMed] [Google Scholar]
- Li S., Liquari P., McKee K. K., Harrison D., Patel R., Lee S., Yurchenco P. D. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J. Cell Biol. 2005;169:179–189. doi: 10.1083/jcb.200501098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F., Pepe A., Wary K. K., Spinardi L., Mohammadi M., Schlessinger J., Giancotti F. G. Signal transduction by the alpha 6 beta 4 integrin: distinct beta 4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F., Murgia C., Wary K. K., Curatola A. M., Pepe A., Blumemberg M., Westwick J. K., Der C. J., Giancotti F. G. The coupling of alpha6beta4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F., Pepe A., Yeon M., Ren Y., Giancotti F. G. The intracellular functions of alpha6beta4 integrin are regulated by EGF. J. Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti A., Kedeshian P. A., Dans A., Curatola A. M., Gagnoux-Palacios L., Giancotti F. G. EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J. Cell Biol. 2001;155:447–458. doi: 10.1083/jcb.200105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. H., Patton B. L., Lentz S. I., Gilbert D. J., Snider W. D., Jenkins N. A., Copeland N. G., Sanes J. R. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alphα3 isoform. J. Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K. Laminin-5 (laminin-332): unique biological activity and role in tumor growth and invasion. Cancer Sci. 2006;97:91–98. doi: 10.1111/j.1349-7006.2006.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima H., Koshikawa N., Moriyama K., Takamura H., Nagashima Y., Hirahara F., Miyazaki K. Wide distribution of laminin-5 gamma 2 chain in basement membranes of various human tissues. Horm. Res. 1998;2:7–14. doi: 10.1159/000053118. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Tsubota Y., Maeda M., Kariya Y., Miyazaki K. Regulation of biological activity of laminin-5 by proteolytic processing of gamma2 chain. J. Cell. Biochem. 2004;92:701–714. doi: 10.1002/jcb.20112. [DOI] [PubMed] [Google Scholar]
- Okumura M., Yamakawa H., Ohara O., Owaribe K. Novel alternative splicings of BPAG1 (bullous pemphigoid antigen 1) including the domain structure closely related to MACF (microtubule actin cross-linking factor) J. Biol. Chem. 2002;277:6682–6687. doi: 10.1074/jbc.M109209200. [DOI] [PubMed] [Google Scholar]
- Pyke C., Salo S., Ralfkiaer E., Romer J., Dano K., Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139. [PubMed] [Google Scholar]
- Rousselle P., Aumailley M. Kalinin is more efficient than laminin in promoting adhesion of primary keratinocytes and some other epithelial cells and has a different requirement for integrin receptors. J. Cell Biol. 1994;125:205–214. doi: 10.1083/jcb.125.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P., Lunstrum G. P., Keene D. R., Burgeson R. E. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J. Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. C., Tizard R., VanDevanter D. R., Carter W. G. Cloning of the LamA3 gene encoding the α3 chain of the adhesive ligand epiligrin. Expression in wound repair. J. Biol. Chem. 1994;269:22779–22787. [PubMed] [Google Scholar]
- Sasaki T., Gohring W., Mann K., Brakebusch C., Yamada Y., Fassler R., Timpl R. Short arm region of laminin-5 gamma2 chain: structure, mechanism of processing and binding to heparin and proteins. J. Mol. Biol. 2001;314:751–763. doi: 10.1006/jmbi.2001.5176. [DOI] [PubMed] [Google Scholar]
- Schenk S., Quaranta V. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol. 2003;13:366–375. doi: 10.1016/s0962-8924(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Sigle R. O., Gil S. G., Bhattacharya M., Ryan M. C., Yang T. M., Brown T. A., Boutaud A., Miyashita Y., Olerud J., Carter W. G. Globular domains 4/5 of the laminin α3 chain mediate deposition of precursor laminin 5. J. Cell Sci. 2004;117:4481–4494. doi: 10.1242/jcs.01310. [DOI] [PubMed] [Google Scholar]
- Timpl R., Brown J. C. Supramolecular assembly of basement membranes. Bioessays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- Tsubota Y., Mizushima H., Hirosaki T., Higashi S., Yasumitsu H., Miyazaki K. Isolation and activity of proteolytic fragment of laminin-5 alpha3 chain. Biochem. Biophys. Res. Commun. 2000;278:614–620. doi: 10.1006/bbrc.2000.3851. [DOI] [PubMed] [Google Scholar]
- Tsubota Y., Yasuda C., Kariya Y., Ogawa T., Hirosaki T., Mizushima H., Miyazaki K. Regulation of biological activity and matrix assembly of laminin-5 by COOH-terminal, LG4–5 domain of alpha3 chain. J. Biol. Chem. 2005;280:14370–14377. doi: 10.1074/jbc.M413051200. [DOI] [PubMed] [Google Scholar]
- Veitch D. P., et al. Mammalian tolloid metalloproteinase, and not matrix metalloprotease 2 or membrane type 1 metalloprotease, processes laminin-5 in keratinocytes and skin. J. Biol. Chem. 2003;278:15661–15668. doi: 10.1074/jbc.M210588200. [DOI] [PubMed] [Google Scholar]