Abstract
The role of cyclin B-CDC2 as M phase-promoting factor (MPF) is well established, but the precise functions of cyclin A remain a crucial outstanding issue. Here we show that down-regulation of cyclin A induces a G2 phase arrest through a checkpoint-independent inactivation of cyclin B-CDC2 by inhibitory phosphorylation. The phenotype is rescued by expressing cyclin A resistant to the RNA interference. In contrast, down-regulation of cyclin B disrupts mitosis without inactivating cyclin A-CDK, indicating that cyclin A-CDK acts upstream of cyclin B-CDC2. Even when ectopically expressed, cyclin A cannot replace cyclin B in driving mitosis, indicating the specific role of cyclin B as a component of MPF. Deregulation of WEE1, but not the PLK1-CDC25 axis, can override the arrest caused by cyclin A knockdown, suggesting that cyclin A-CDK may tip the balance of the cyclin B-CDC2 bistable system by initiating the inactivation of WEE1. These observations show that cyclin A cannot form MPF independent of cyclin B and underscore a critical role of cyclin A as a trigger for MPF activation.
INTRODUCTION
Classic cell cycle studies in the 1980s culminated in the purification of M phase-promoting factor (MPF) from Xenopus eggs and starfish oocytes and the identification of its components as cyclin B and CDC2 (also called cyclin-dependent kinase [CDK]-1; reviewed in Doree and Hunt, 2002). Cyclin B accumulates from S phase and forms a complex with CDC2. The complex is kept inactive by phosphorylation of CDC2Thr14/Tyr15 by MYT1 and WEE1 until it is abruptly activated by CDC25 during mitosis. As the cell exits mitosis cyclin B is destroyed via an ubiquitin-mediated mechanism that is catalyzed by the APC/C (reviewed in Fung and Poon, 2005).
Cyclin B-CDC2 catalyzes its own activation by an intricate network of feedback loops. WEE1 is phosphorylated by CDKs, facilitating its degradation by the ubiquitin ligases SCFβ-TrCP and SCFTome-1 (Ayad et al., 2003; Watanabe et al., 2004, 2005; Harvey et al., 2005). The CDC25C phosphatase is activated by multiple CDK phosphorylation (reviewed in Hutchins and Clarke, 2004) and inactivated by phosphorylation on Ser216 (enforced by several kinases, including C-TAK1, CHK1, and CHK2). This phosphorylation creates a 14-3-3 binding site, which masks a proximal nuclear localization sequence and excludes CDC25C from the nucleus. Furthermore, phosphorylation of CDC25CSer214 inhibits the phosphorylation of CDC25CSer216 (Bulavin et al., 2003). Thus MPF is essentially a bistable system (Ferrell, 2002) that becomes autocatalytic once a critical portion is activated.
What triggers the initial activation of MPF has been the subject of immense speculation. PLK1 is a candidate as it can phosphorylate CDC25C at the nuclear-exporting sequence (NES) and promote nuclear accumulation of CDC25C (Toyoshima-Morimoto et al., 2002). PLK1 likewise phosphorylates the NES of cyclin B and increases its nuclear accumulation (Toyoshima-Morimoto et al., 2001; Yuan et al., 2002), coordinating the localization of both cyclin B and CDC25C to the nucleus. Finally, PLK1 can also phosphorylate and inhibit WEE1 (van Vugt and Medema, 2004; Watanabe et al., 2005).
Cyclin A is closely related in sequence to cyclin B, but was not present in the purified MPF fractions from oocytes and eggs. The precise functions of cyclin A still remain poorly defined. Two A-type cyclins, A1 (embryonic form) and A2 (somatic form), are present in higher eukaryotes. Disruption of cyclin A1 in mice leads to only a mild phenotype (a block of the first meiotic division in male mice; Liu et al., 1998), attesting that cyclin A1 is not a component of the essential MPF in embryonic cells. In contrast, disruption of cyclin A2 causes early embryonic lethality (Murphy et al., 1997), suggesting critical cell cycle functions for cyclin A in somatic cells.
Cyclin A accumulates from early S phase and disappears slightly ahead of cyclin B during mitosis (reviewed in Yam et al., 2002). Also unlike cyclin B, cyclin A can bind to both CDC2 and CDK2. The prevailing view is that cyclin A has important functions in S phase, presumably for the loading of CDC45 onto replication origins and for the phosphorylation-dependent degradation of the licensing factor CDC6 (reviewed in Woo and Poon, 2003). Also widely held is that cyclin A has critical functions in mitosis, but its precise role is not fully understood (reviewed in Fung and Poon, 2005).
Understanding the functions of cyclin A has implication on the fundamental question of how different cyclins can promote distinct points in the cell cycle. Several lines of evidence suggest that the specificity of different cyclins is due to intrinsic substrate specificity or subcellular localization (an alternative model proposes that differences in the timing and levels of expression of cyclins dictate their specificity; reviewed in Miller and Cross, 2001). For example, the yeast M phase cyclin (Clb2p) cannot replace the functions of the S phase cyclins (Clb5p and Clb6p), and Clb5p cannot complement the M phase cyclins (Clb1p–Clb4p). Indeed, owning to an interaction between a hydrophobic patch in Clb5 and the substrates' RXL motif, Clb5p can phosphorylate some substrates more efficiently than Clb2p (Loog and Morgan, 2005). In Xenopus egg extracts, relocating cyclin B-CDC2 from the cytoplasm to the nucleus allows the complex to promote S phase functions, suggesting that localization can also be an important determinant of cyclin specificity (Moore et al., 2003).
To decipher the role of cyclin A in somatic cells, we are now able to approach the problem using reverse genetics based on RNA interference (RNAi). With the aim of distinguishing whether cyclin A itself is a component of MPF or is part of the machinery that activates MPF, cyclin A and cyclin B were down-regulated, and their requirement for mitosis was studied. We found that the G2 delay induced by the absence of cyclin A was caused by the inactivation of cyclin B-CDC2 through inhibitory phosphorylation. This phenotype could be reversed by expressing RNAi-resistant cyclin A. Increased expression of cyclin B or a nonphosphorylatable CDC2 could overcome the cyclin A arrest. In contrast, we found that ectopically expressed cyclin A could not replace cyclin B in driving mitosis. Finally, we present evidence that among the regulators of CDC2 phosphorylation, deregulation of WEE1 allowed activation of cyclin B-CDC2 even in the absence of cyclin A. These observations underscore a critical role of cyclin A as a trigger for MPF activation.
MATERIALS AND METHODS
Materials
All reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless stated otherwise.
DNA Constructs
Short hairpin RNA (shRNA) were expressed from mU6pro (a gift from Dr. David Turner, University of Michigan, Ann Arbor, MI; Yu et al., 2002). Specific shRNA constructs were created by annealing the following pairs of primers into BbsI-XbaI-cut mU6pro: 5′TTTGGTAGCAGAGTTTGTGTACATTCAAGAGATGTACACAAACTCTGCTACTTTTT3′ and 5′CTAGAAAAAGTAGCAGAGTTTGTGTACATCTCTTGAATGTACACAAACTCTGCTAC3′ (corresponded to positions 823–841 of human cyclin A2 open reading frame [ORF]); 5′TTTGGATGGAGCTGATCCAAACCTTCAAGAGAGGTTTGGATCAGCTCCATCTTTTT3′ and 5′CTAGAAAAAGATGGAGCTGATCCAAACCTCTCTTGAAGGTTTGGATCAGCTCCATC3′ (corresponded to positions 478–496 of human cyclin B1 ORF); 5′TTTGGTCCCGGTATACAACAGAATTCAAGAGATTCTGTTGTATACCGGGACTTTTT3′ and 5′CTAGAAAAAGTCCCGGTATACAACAGAATTCAAGAGATTCTGTTGTATACCGGGAC3′ (corresponded to positions 876–894 of human WEE1 ORF).
Plasmids expressing FLAG-tagged CDC2 (Chow et al., 2003b), CDK2 (Yam et al., 2000), CDC2AF (Chow et al., 2003b), CDK2AF (Chow et al., 2003b), cyclin A (Yam et al., 2000), cyclin B (Fung et al., 2005), cyclin F (Yam et al., 2000), p21CIP1/WAF1 (Poon and Hunter, 1998), 3HA-tagged CDC25A (Chow et al., 2003a), CDC25B (Chow et al., 2003b), GST-tagged CDC25A (Chow et al., 2003a), CDK2K33R (Poon et al., 1993), WEE1NΔ215 (Poon and Hunter, 1995), 3C protease (Fung et al., 2005), and histone H2B-GFP (Chow et al., 2003b) were constructed or obtained from sources as previously described. 3HA-CDC25CS216A in pCAGGS and myc-PLK1 in pRcCMV were gifts from Katsumi Yamashita (Kanazawa University, Japan).
The NcoI-BamHI fragment from FLAG-cyclin B in pUHD-P1 (Fung et al., 2005) was ligated into pUHD-P1/3C (Fung et al., 2005) to create a construct that expressed cyclin B tagged with FLAG followed by a 3C protease cleavage site. The cyclin E cDNA was amplified by PCR with the oligonucleotides 5′GGAATTCATGAAGGAGGACGGCGG3′ and 5′GGAATTCTCACGCCATTTCCGG3′; the PCR product was cut with EcoRI and put into pUHD-P1 (Yam et al., 1999a) to create FLAG-cyclin E in pUHD-P1. The CDC25C cDNA was amplified by PCR with the primers 5′GACCATGGCTACGGAACTCTTCT3′ and 5′TGCTCGAGTGGGCTCATGTCCTTCAC3′ or 5′TCCTCGAGCATCTGAGTGATAG3′; the PCR products were cut with NcoI-XhoI and ligated into pGEX-KG to create GST-CDC25C and GST-CDC25CCΔ272, respectively. PCR was then performed on GST-CDC25C in pGEX-KG using a pGEX-KG forward primer and 5′TTTCCATGGTCATCACCGAAACGCGCGAG3′; the product was cut with NcoI and ligated into pUHD-P2 (Yam et al., 2000) to create HA-CDC25C in pUHD-P2. FLAG-tagged CDC25C and CDC25CS216A will be described elsewhere (unpublished results).
GST-WEE1NΔ215 in pGEX-KG was amplified by PCR with a vector forward primer and 5′TCACTCGAGGTATATAGTAAGGCTGA3′; the PCR product was cut with NdeI-XhoI and ligated into pET21b (Novagene, Madison, WI) to produce WEE1NΔ371-H6 in pET21b. The XbaI site of this construct was inserted the XbaI fragment of WEE1K328R in pSLX-CMV (Chow et al., 2003b) to produce WEE1K328R-H6 in pET21b. Site-directed mutagenesis was carried out with Quik-Change site-directed mutagenesis kit (Stratagene, La Jolla, CA) with the following oligonucleotides and their antisense: 5′CCAGAAGTAGCGGAATTCGTCTACATTACAGA3′ (for introduction of silence mutations into cyclin A to create a shRNA-resistant construct), 5′AAGATGGAGCCGACCCGAACCT3′ (for shRNA- resistant cyclin B), and 5′GGAGAGGAAGAAGGACCTGTGTGGGA3′ (for PLK1T210D mutation). Cyclin A/shRNA in pKAR1 was constructed as described (Ma et al., 2007).
Cell Culture
HtTA1 cells are HeLa cells (human cervical carcinoma) expressing the tTA tetracycline repressor chimera (Yam et al., 2000). Cells were grown in DMEM supplemented with 10% vol/vol calf serum (Invitrogen, Carlsbad, CA) in a humidified incubator at 37°C in 5% CO2. Cells were transfected with the calcium phosphate precipitation method (Ausubel et al., 1991). The efficiency of transient transfection in HtTA1 cells was typically above 50%. For transfection of shRNA-expressing plasmids, plasmids containing resistant genes to blasticidin or puromycin were cotransfected and cells were grown in medium containing the respective antibiotics for 36 h to enrich the transfected cells. Selection medium was washed out, and the cells were grown in normal medium for another 12 h. Cell-free extracts were prepared as previously described (Poon et al., 1995). Cell number was measured over time with a method previously described (Chow et al., 2003b). Colonies were fixed with methanol:acetic acid (2:1 vol/vol) and visualized by staining with 2% wt/vol crystal violet in 20% methanol. To measure the mitotic index, cells were transfected with a green fluorescent protein (GFP)-tagged histone H2B marker, and the chromosomes were examined under fluorescence microscopy. Unless stated otherwise, cells were treated with the following reagents at the indicated final concentration: Adriamycin (200 ng/ml; Calbiochem, San Diego, CA), blasticidin (5 μg/ml; Invitrogen), caffeine (5 mM), doxycycline (2 μg/ml), hydroxyurea (1.5 mM), nocodazole (0.1 μg/ml), puromycin (1 μg/ml), and SB203580 (10 μM). To release cells from the nocodazole block, cells were resuspended in PBS and collected by centrifugation for three times before replating in normal growth medium. Double thymidine synchronization was performed as previously described (Arooz et al., 2000) except that cells were transfected after the release from the first thymidine block. The cells were washed after 10 h, incubated in thymidine and blasticidin for 21 h before the release of the second thymidine block. Ionizing radiation was delivered with a cesium137 source from a MDS Nordion Gammacell 1000 Elite Irradiator (Ottawa, Ontario, Canada). HtTA1 cells stably expressing cyclin A/shRNA in pKAR1 were generated as described (Ma et al., 2007). Stable cyclin A/shRNA-expressing clones based on U2OS/Tet-ON cells (Clontech, Palo Alto, CA) were generated similarly except that the cells were grown in the presence of 10% vol/vol fetal bovine serum and selected in the presence of doxycycline.
Flow Cytometry
Bromodeoxyuridine (BrdU) incorporation followed by flow cytometry analysis was performed as previously described (Chow et al., 2003b). Bivariate analysis of DNA content and histone H3Ser10 phosphorylation was performed as previously described (Siu et al., 2004).
Expression and Purification of Recombinant Proteins
Coupled transcription-translation reactions in the presence of [35S]methionine in rabbit reticulocyte lysate were performed according to the manufacturer's instructions (Promega, Madison, WI). Expression and purification of GST-tagged proteins were performed as previously described (Poon et al., 1995).
Kinase and Phosphatase Assays
Histone H1 kinase assays were performed as previously described (Poon and Hunter, 1995). Phosphorylation was detected and quantified with a PhosphorImager (Amersham Biosciences, Piscataway, NJ). The conditions for GST-CDC25A treatment were as previously described (Poon et al., 1997). The kinase activity of PLK1 was assayed as previously described (Poon et al., 1997) except that GST-CDC25CCΔ272 was used as a substrate. The kinase activity of WEE1 was assayed using a kinase-dead GST-CDK2K33R substrate as previously described (Poon et al., 1997). To assay the mobility shifts of WEE1, in vitro–translated WEE1K328R was incubated at 25°C for 15 min with cell extracts prepared with hypotonic buffer as previously described (Yam et al., 2000).
To assay CDC25C phosphatase activity, FLAG-3C-cyclin B (a 3C protease cleavage site was present between FLAG and cyclin B) was expressed in HtTA1 cells. Cell extracts were prepared and the recombinant cyclin B was immunoprecipitated with FLAG antiserum. The immunoprecipitates were washed with kinase buffer (Chow et al., 2003b) and incubated with 1 μg of GST-WEE1NΔ215 (in 10 μl kinase buffer supplemented with 1 mM ATP) at 25°C for 30 min. The immunoprecipitates were washed three times with bead buffer (Poon et al., 1993), once with the buffer 50 mM HEPES, pH 7.2, 20 mM EGTA, 1 mM DTT, and cleaved with recombinant 3C protease (Fung et al., 2005). The eluent was added to CDC25C immunoprecipitates and incubated at 25°C for 1 h. The supernatant was made to 80 mM β-glycerophosphate and 15 mM MgCl2, and the histone H1 kinase activity was assayed.
Antibodies and Immunological Methods
Immunoblotting and immunoprecipitation were performed as described (Poon et al., 1995). Indirect immunofluorescent microscopy was performed as described previously (Yam et al., 1999b) except that cells were fixed by ice-cold methanol and blocked with 3% bovine serum albumin in PBS. Monoclonal antibodies A17 against CDC2 (Siu et al., 2004), AN4.3 against CDK2 (Chow et al., 2003b), E23 against cyclin A2 (Yam et al., 2001), 12CA5 against hemagglutinin (HA) tag (Fung et al., 2002), M2 against FLAG tag (Fung et al., 2002), polyclonal antibodies against CDC2 (Li et al., 2002), CDK2 (Siu et al., 2004), cyclin A (Yam et al., 1999a), cyclin B (Arooz et al., 2000), and FLAG (Yam et al., 2000) were obtained from sources as previously described. Antibodies against CDC25A, CDC25B, phosphorylated ATMSer1981, CDC2Tyr15, CDC2Thr161, p38, phospho-p38Thr180/Thr182, and phospho-CHK1Ser345 were obtained from Cell Signaling Technology (Beverly, MA). Antibodies against Aurora A and TPX2 were obtained from BioLegend (San Diego, CA). Monoclonal antibodies against CHK1 (sc-8408), CDC25C (sc-13138), cyclin B1 (sc-245), cyclin E (sc-247), lamin A/C (sc-7292), polyclonal antibodies against CDC25C (sc-327), phospho-CHK2Thr68 (sc-16297R), cyclin F (sc-952), p21 (sc-397), phospho-histone H3Ser10 (sc-8656R), PLK1 (sc-6355), securin (sc-227722), and WEE1 (sc-325) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
RESULTS
Down-Regulation of Cyclin A by shRNA Induces a Cell Cycle Arrest at G2 Phase
Down-regulation of cyclin A was achieved with shRNA. Transfection of a plasmid that expressed the shRNA specifically abolished the expression of recombinant cyclin A, but not CDK2, from cotransfected plasmids (Supplementary Figure S1A). To see if endogenous cyclin A could also be down-regulated, cells were transfected with control plasmids or cyclin A shRNA plasmids. The endogenous cyclin A was efficiently suppressed by the transfection of cyclin A shRNA plasmids but not the control plasmids (Figure 1A). The down-regulation was specific as the abundance of cyclin B and CDC2 was not affected.
Figure 1.
Down-regulation of cyclin A induces a cell cycle arrest at G2 phase. (A) Down-regulation of endogenous cyclin A with shRNA in HeLa cells. Cells were transfected with either control vectors or cyclin A shRNA-expressing plasmids. After enriching the transfected cells (Materials and Methods), more than 90% of cells expressed a cotransfected GFP-tagged histone H2B (data not shown). Cell-free extracts were prepared and subjected to immunoblotting for cyclin A and cyclin B. Uniform loading of lysates was confirmed by immunoblotting for CDC2. The slight increase of cyclin B in the cyclin A–depleted cells did not occur in all the experiments. (B) Disruption of cyclin A inhibits cell growth. Cells were transfected with empty vector or cyclin A shRNA plasmids. The cell number was measured at different time points after washing out the selection medium (Materials and Methods). (C) Cyclin A shRNA induces a cell cycle arrest at G2/M. Cells were transfected with either control or cyclin A shRNA plasmids and treated with buffer or nocodazole for 16 h as indicated. Half of the nocodazole-treated cells were released into nocodazole-free medium and incubated for another 5 h. The cells were labeled with BrdU for 1 h before harvesting, and the cell cycle distribution was analyzed with flow cytometry. (D) Cells progress through S phase but not mitosis in the absence of cyclin A. HtTA1 cells were cotransfected with GFP-histone H2B and either control or cyclin A shRNA plasmids. Thymidine was immediately added for 19 h to block the cells in S phase. The cells were then released from the thymidine block and harvested at the indicated time points. The cell cycle distribution of transfected (GFP-positive) cells was analyzed by flow cytometry. (E) Knockdown of cyclin A abolishes entry into mitosis. Cells expressing control vector or cyclin A shRNA were either mock-treated or treated with nocodazole for 12 h. Bivariate analysis of DNA content (x-axis) and histone H3Ser10 phosphorylation (y-axis) was carried out by flow cytometry. The percentage of phospho-histone H3Ser10- positive cells (boxed region) is indicated. (F) Down-regulation of cyclin A does not affect cyclin B expression but increases CDC2 phosphorylation. Control or cyclin A shRNA-expressing cells were exposed to nocodazole and harvested at the indicated time points. Cell extracts were prepared and the expression of the indicated proteins was detected by immunoblotting. The asterisk indicates the position of the slower migrating, phosphorylated form of CDC2.
Expression of the cyclin A shRNA inhibited cell growth, as indicated by the number of attached cells over several days (Figure 1B) as well as by their ability to form colonies over a longer period of time (Supplementary Figure S1B). To see at which cell cycle phase the cells were arrested by the shRNA, the cells were pulsed with BrdU before processed for flow cytometry analysis. Figure 1C shows that the cyclin A knockdown cells exhibited a significant reduction of the G1 population in comparison to the control cells. Longer incubation further eroded the S phase (data not shown), suggesting the shRNA caused a significant delay at G2/M. Releasing the cells from a thymidine block indicated that mainly G2/M, but not S phase progression, was affected by the absence of cyclin A (Figure 1D). Trapping the cells in G2/M with nocodazole also confirmed that the cells were not arrested in S phase (Figure 1C). As expected, control cells could be effectively released into G1 from the nocodazole-mediated block. In contrast, the majority of shRNA-expressing cells remained in G2/M after replating in nocodazole-free medium. These data indicate that cyclin A ablation caused predominantly a G2/M arrest.
To distinguish whether cyclin A shRNA induced an arrest in G2 or M phase, we next analyzed the phosphorylation of histone H3Ser10 (which usually begins before prophase and is required for chromosome condensation; Hendzel et al., 1997). As expected, histone H3Ser10 phosphorylation was low in control cells (∼3%), and increased to ∼25% after 12 h of nocodazole treatment (Figure 1E). In contrast, histone H3Ser10 phosphorylation remained at background in cyclin A shRNA-expressing cells even in the presence of nocodazole, indicating that cyclin A–depleted cells were predominantly in G2 phase.
It is noteworthy that Figure 1E also shows that elimination of cyclin A promoted cell death, as indicated by an increase of cells possessing sub-G1 DNA content. The sub-G1 population could reach more than 20% after prolonged culturing (our unpublished data). Experiments in this article were performed before substantial cell death started to occur.
To further confirm that knockdown of cyclin A inhibited histone H3Ser10 phosphorylation, control or shRNA-expressing cells were exposed to nocodazole and harvested at different time points (Figure 1F). As before, depletion of cyclin A did not affect the expression of cyclin B and CDC2. Consistent with the G2 arrest, there was no accumulation of cyclin E (a G1 cyclin) or histone H3Ser10 phosphorylation. Interestingly, there was a decrease in cyclin F, which normally accumulates at the similar time as cyclin A in the cell cycle (Fung et al., 2002). Similar results were obtained when more synchronized cells were used (released from a double thymidine block, Supplementary Figure S2). Finally, direct counting of mitotic figures also confirmed that knockdown of cyclin A inhibited mitosis (Figure 2A).
Figure 2.
Down-regulation of cyclin A or cyclin B disrupts mitosis through different mechanisms. (A) Expression of cyclin A or cyclin B shRNA depletes the mitotic population. Cells were transfected with control vector or shRNA-expressing plasmids. A construct expressing GFP-tagged histone H2B was cotransfected as a DNA marker. The mitotic index of the transfected (GFP-positive) cells were measured using fluorescence microscopy (300 cells were counted per sample). The mean and SD of three independent experiments are shown. (B) Differential effects on CDC2Tyr15 and CDC2Thr161 phosphorylation by the knockdown of cyclin A or cyclin B. Control vector, cyclin A shRNA, cyclin B shRNA, or both shRNA were expressed in HtTA1 cells. The cells were either mock-treated or treated with nocodazole for 12 h. Cell-free extracts were prepared, and the expression of the indicated proteins was detected by immunoblotting. (C) Cells were transfected with control vector or cyclin B shRNA and mock-treated or treated with nocodazole as in B. Cells expressing phospho-histone H3Ser10 were detected by flow cytometry as described in Figure 1E. Phospho-histone H3Ser10 was also detected by immunoblotting (bottom panel).
To confirm that the G2 arrest was not due to nonspecific effects of the shRNA, silence mutations were introduced into the cyclin A cDNA to render it resistant to the shRNA. We found that the shRNA-induced G2 arrest could be rescued with the shRNA-reflective cyclin A, indicating that the effect was specific to cyclin A disruption (Supplementary Figure S3). We have also verified the role of cyclin A by generating cell lines that stably express cyclin A shRNA and the corresponding rescue cDNA. Both shRNA and recombinant cyclin A were engineered into the same plasmid, with the cDNA under the control of doxycycline (Supplementary Figure S4A). Cells were transfected and selected in the presence of the compensatory cyclin A. Individual clones were isolated that expressed FLAG-cyclin A but were deficient in the expression of endogenous cyclin A (Supplementary Figure S4B). Cyclin A expression was then eliminated by addition of doxycycline (lanes 3 and 4). In agreement with the transient transfection data, switching off the rescue cyclin A in the shRNA background significantly increased the G2/M population (Supplementary Figure S4C). We think that probably because of the leakiness of the doxycycline-controlled promoter, the knockdown phenotype of this system was less stringent.
Collectively, these results indicate that cyclin A knockdown does not affect cyclin B expression but prevents mitotic entry.
The G2 Arrest Induced by Cyclin A Knockdown Is Not Due to Mitotic Slippage
It is conceivable that the cyclin A shRNA-induced apparent G2 arrest and reduction of histone H3Ser10 phosphorylation could be due to mitotic slippage (also called adaptation). During slippage, cells exit mitosis without cytokinesis, producing G1 cells with 4N DNA contents. It is unlikely that mitotic slippage occurred in the absence of cyclin A, as cyclin B remained stabilized (Figure 1, A and F). To further ensure that cyclin A knockdown cells were not in a pseudo G1, we also examined the expression of several potential APC/C targets. As expected, Aurora A, cyclin B, securin, and TPX2 accumulated during nocodazole-mediated mitotic block, but were destroyed when released into G1 (Supplementary Figure S5). Importantly, these proteins were stabilized in cyclin A knockdown cells. Consistent with the G2 arrest, the mitotic mobility shifts of securin were reduced without cyclin A. These data suggest that in the absence of cyclin A, cells were arrested in G2 phase rather than in a tetraploid G1 state.
Disruption of Cyclin B Prevents Mitotic Entry But Does Not Affect the Activity of Cyclin A-CDK2
To compare the functions of cyclin A versus cyclin B, we also disrupted the expression of cyclin B with shRNA. Figure 2B shows that cyclin B expression could be effectively and specifically suppressed. Not surprisingly, knockdown of cyclin B (either in the presence or absence of cyclin A) reduced histone H3Ser10 phosphorylation. Flow cytometry analysis also indicated that depletion of cyclin B reduced the number of cells expressing phospho-histone H3Ser10 (Figure 2C; albeit the sensitivity and linear range of the two assays appear to differ). The mitotic index was likewise diminished in the absence of cyclin B (Figure 2A). Staining for lamin A/C indicated that the nuclear envelope was intact after the knockdown of either cyclin A or cyclin B (Supplementary Figure S6). As controls, the nuclear envelope was broken down in the mitotic population of the control cells. These results indicate mitosis was disrupted in the absence of either cyclin A or cyclin B.
Given that cyclin B knockdown did not affect the abundance of cyclin A (Figure 2B; see also Figure 5), we next investigated if the kinase activity of cyclin A-CDK was modulated. Figure 3A shows that the kinase activities associated with cyclin A or CDK2 were not reduced after cyclin B down-regulation. As expected, ablation of cyclin A (or cyclin A and cyclin B together) abolished the kinase activities. Taken together, these data show that blocking the accumulation of cyclin B disrupts mitosis without affecting the activity of cyclin A.
Figure 5.
Cyclin A cannot replace the M phase–promoting functions of cyclin B. (A) Histone H3 phosphorylation in cyclin B–ablated cells cannot be restored by cyclin A overexpression. Control or cyclin B shRNA was coexpressed with empty vector or FLAG-tagged cyclins as indicated. The cells were trapped in mitosis with nocodazole for 12 h before harvesting (except lanes 1 and 2). Cell-free extracts were prepared, and the expression of the indicated proteins was detected by immunoblotting. The recombinant cyclins were detected with an antibody against FLAG. (B) Reintroduction of cyclin B into cyclin B shRNA-treated cells restores mitosis. Control or cyclin B shRNA was coexpressed with empty vector or cyclin B. The cyclin B construct contained silence mutations that rendered resistance to the shRNA. Lysates were prepared, and the expression of cyclin A, cyclin B, and phospho-histone H3Ser10 was detected by immunoblotting. (C) Overexpression of nonphosphorylatable CDKs cannot rescue mitosis in cyclin B–depleted cells. Control or cyclin B shRNA was coexpressed with empty vector or FLAG-tagged CDK2, CDK2AF, CDC2, or CDC2AF as indicated. Nocodazole was then added for 12 h to enrich mitotic cells (except lanes 1 and 2). Cell lysates were prepared, and the expression of cyclin A, cyclin B, and phospho-histone H3Ser10 was detected by immunoblotting. The recombinant CDKs were detected with an antibody against FLAG. (D) Mitosis in cyclin B–ablated cells cannot be restored by cyclin A overexpression. Control or cyclin B shRNA was coexpressed with empty vector or FLAG-tagged cyclins or CDKs as indicated. The cyclin B construct contained silence mutations that rendered resistance to the shRNA. The mitotic index was measured as described in Figure 2A (top panel). After nocodazole was added for 12 h to trap cells in mitosis, cell extracts were prepared and the kinase activity of CDC2 was assayed (bottom panel). (E) Cyclin A is active but cannot drive mitosis in the absence of cyclin B. Control or cyclin B shRNA was coexpressed with empty vector or FLAG-cyclin A and FLAG-CDC2AF. Nocodazole was added for 16 h to trap cells in mitosis before cell-free extracts were prepared. CDC2 was immunoprecipitated, and the kinase activity was assayed. The samples were also immunoblotted with antibodies against phospho-histone H3Ser10 and FLAG.
Figure 3.
Cyclin A is required for the dephosphorylation and activation of CDC2 during mitosis. (A) Cyclin A-CDK2 remains active in cyclin B knockdown cells. Cyclin shRNA constructs were expressed as indicated. Cell-free extracts were prepared and subjected to immunoprecipitation with either cyclin A or CDK2 antiserum. The histone H1 kinase activity was assayed and detected with phosphorimagery. (B) Down-regulation of cyclin A or cyclin B inhibits CDC2 activity. Cells were transfected with constructs expressing specific shRNA and exposed to nocodazole (12 h) as indicated. Cell-free extracts were prepared and subjected to immunoprecipitation with a CDC2 antiserum, and the histone H1 kinase activity was assayed. (C) CDC2 from cyclin A–depleted cells can be reactivated by CDC25. Cells were transfected with vector or cyclin A shRNA plasmids and treated with nocodazole (12 h) to trap the cells in mitosis. Cell lysates were prepared, and cyclin B was immunoprecipitated. The immunoprecipitates were mock-treated or incubated with GST-CDC25A for 30 min, and immunoblotted with antibodies against cyclin B, phospho-CDC2Tyr15, and CDC2. Total lysates were also loaded as controls. The kinase activity associated with the immunoprecipitates were at the same time assayed using histone H1 as a substrate (bottom panels). (D) Nonphosphorylatable CDC2 can override the cyclin A shRNA-induced arrest. Control or cyclin A shRNA was coexpressed with empty vector or FLAG-tagged CDK2, CDK2AF, CDC2, or CDC2AF as indicated. Nocodazole was then added for 12 h to enrich mitotic cells (except lanes 1 and 2). Cell lysates were prepared, and the expression of cyclin A, cyclin B, and phospho-histone H3Ser10 was detected by immunoblotting. The recombinant CDKs were detected with an antibody against FLAG. (E) Nonphosphorylatable CDC2 restored cyclin B–associated kinase activity after cyclin A knockdown. Control or cyclin A shRNA was coexpressed with empty vector or FLAG-tagged CDKs or CDKAF mutants as indicated. The cells were trapped in mitosis with nocodazole for 12 h before harvested. Cell-free extracts were prepared, cyclin B was immunoprecipitated, and the histone H1 kinase activity was assayed. (F) Overexpression of CDC2AF stimulates mitosis in the absence of cyclin A. Control or cyclin A shRNA was coexpressed with empty vector or FLAG-tagged CDKs or CDKAF mutants as indicated. The mitotic index was measured as described in Figure 2A.
Cyclin A Drives the Dephosphorylation and Activation of Cyclin B-CDC2 Complexes during Mitosis
We next sought to elucidate the molecular basis of G2 arrest after cyclin A or cyclin B were eliminated. Figure 3B shows that CDC2 was inactive after the knockdown of cyclin A and/or cyclin B. Similarly, cyclin B immunoprecipitates lacked kinase activity after cyclin A knockdown (Figure 3, C and E). This inactivity of cyclin B-CDC2 was likely to be caused by posttranslational mechanisms because both proteins were present (Figure 2B) and could form a complex (Figure 3C). Moreover, CDC2Thr161 was still phosphorylated after cyclin A knockdown (Figure 2B), indicating that a loss of activating phosphorylation was not the underlying mechanism (as expected, CDC2Thr161 was not phosphorylated in the absence of cyclin B).
As the CDK inhibitor p21CIP1/WAF1 can bind to cyclin B-CDC2 under some conditions, a possible mechanism of cyclin B-CDC2 inactivation after cyclin A knockdown may be due to the redistribution of p21CIP1/WAF1 from cyclin A-CDK to cyclin B-CDC2. We think this unlikely because although a recombinant FLAG-p21CIP1/WAF1 could bind both cyclin A and cyclin B, there was no increase in binding to cyclin B in the presence of cyclin A shRNA (Supplementary Figure S7A). This conclusion is further confirmed by experiments with endogenous p21CIP1/WAF1 in U2OS cells (Supplementary Figure S7B). As expected, cyclin A was found in the p21CIP1/WAF1-immunoprecipitates before but not after cyclin A knockdown. As a control, CDK2 was associated with p21CIP1/WAF1 both before and after cyclin A down-regulation. In contrast, cyclin B was not detected in the p21CIP1/WAF1-immunoprecipitates in either cases, indicating that no substantial amount of p21CIP1/WAF1 was redistributed to cyclin B in the absence of cyclin A.
We noted that knockdown of cyclin A was typically associated with gel mobility shifts of CDC2, indicative of inhibitory phosphorylation (e.g., Figure 1F). Because these shifts were capriciously dependent on gel-running conditions, inhibitory phosphorylation was also detected using specific antibodies against phospho-CDC2Tyr15. We found that knockdown of cyclin A (but not cyclin B or both cyclins together) enhanced CDC2Tyr15 phosphorylation (Figures 2B and 4A), suggesting that cyclin B-CDC2 was inactivated by inhibitory phosphorylation in the absence of cyclin A. In support of this hypothesis, CDC2 isolated from cyclin A shRNA-expressing cells could be dephosphorylated and reactivated in vitro with recombinant CDC25A (Figure 3C). To further test this hypothesis, we coexpressed a nonphosphorylatable version of CDC2 (CDC2AF) with cyclin A shRNA and found that the abolition of histone H3Ser10 phosphorylation by cyclin A shRNA was reversed (Figure 3D, compare lanes 4 and 12). In contrast, wild-type CDC2 as well as CDK2 or CDK2AF were less effective in this assay (despite their higher expression than CDC2AF). In addition to stimulating histone H3Ser10 phosphorylation, CDC2AF also increased the kinase activity associated with cyclin B (Figure 3E). Finally, the mitotic index was restored by CDC2AF, but not by other CDKs, even in the absence of cyclin A (Figure 3F). Collectively, these data indicate that mitosis is abolished in the absence of cyclin A because cyclin B-CDC2 complexes are inhibited by phosphorylation.
Figure 4.
MPF can override the G2 arrest induced by cyclin A shRNA. (A) Overexpression of cyclin B increases histone H3 phosphorylation after cyclin A knockdown. Control or cyclin A shRNA was coexpressed with empty vector or FLAG-tagged cyclins as indicated. The cyclin A construct contained silence mutations that rendered resistance to the shRNA. The cells were trapped in mitosis with nocodazole for 12 h before harvested. Cell-free extracts were prepared and the expression of the indicated proteins was detected by immunoblotting. The recombinant cyclins were detected with an antibody against FLAG. (B) Overexpression of cyclin B restores mitosis in cyclin A–ablated cells. Control or cyclin A shRNA was coexpressed with empty vector or FLAG-tagged cyclins as indicated. The mitotic index was measured as described in Figure 2A. (C) The kinase activity of cyclin B-CDC2 in cyclin A-ablated cells can be increased by expression of cyclin B. Control or cyclin A shRNA was coexpressed with empty vector or FLAG-tagged cyclins as indicated. Cell lysates were prepared, cyclin B was immunoprecipitated, and the associated histone H1 kinase activities were assayed.
Down-Regulation of Cyclin A Does Not Activate the DNA Integrity Checkpoints
Because checkpoints induced by DNA damage or stalled replication forks are also characterized by an accumulation of Tyr15-phosphorylated CDC2 (reviewed in Bartek et al., 2004), it is conceivable that the G2 arrest triggered by cyclin A shRNA was due to an activation of these checkpoints. To test this hypothesis, the activation of critical checkpoint proteins was examined. As we have shown previously (Ho et al., 2005), both Adriamycin and hydroxyurea can induce CHK1 activation (as indicated by gel mobility shifts). In contrast, no mobility shift of CHK1 was observed after the expression of cyclin A shRNA (Supplementary Figure S8A). We also found that knockdown of cyclin A or cyclin B did not significantly induce the phosphorylation of ATMSer1981, CHK1Ser345, or CHK2Thr68 (Supplementary Figure S8B). As controls, phosphorylation of these checkpoint kinases was robustly triggered by ionizing radiation.
Caffeine can inhibit ATM and ATR, thereby bypassing both the DNA damage checkpoint and the replication checkpoint. As we have shown before (Siu et al., 2004), caffeine restored histone H3Ser10 phosphorylation after DNA damage (Supplementary Figure S8C, compare lanes 4 and 10). In marked contrast, histone H3Ser10 phosphorylation was not restored by caffeine after the knockdown of cyclin A or cyclin B (lanes 11 and 12).
Finally, expression of cyclin A shRNA did not activate the stress-induced p38 MAP kinase (Supplementary Figure S9A). As a positive control, the activating phosphorylation of p38Thr180/Thr182 was induced after prolonged treatment with nocodazole. Consistent with this, treatment with a p38 inhibitor was not able to uncouple the cyclin A shRNA-induced arrest (Supplementary Figure S9B). Taken together, these data indicate that the G2 arrest induced by cyclin A removal was not caused by an activation of the DNA integrity checkpoints.
Cyclin A Cannot Replace the M Phase–promoting Functions of Cyclin B, but Overexpression of Cyclin B Can Drive Mitosis in the Absence of Cyclin A
We next investigated if cyclin A and cyclin B have unique functions during mitosis. Different cyclins were overexpressed in the cyclin A knockdown background to see if mitosis could be restored. As expected, histone H3Ser10 phosphorylation was restored by cyclin A expression (Figure 4A, compare lanes 4 and 6). In agreement with this, CDC2Tyr15 phosphorylation was also reduced after cyclin A expression. Interestingly, cyclin B expression could also rescue mitotic entry in the absence of cyclin A (lane 8). In contrast, neither cyclin E nor cyclin F could trigger histone H3Ser10 phosphorylation. This also indicates that the cell cycle arrest was unlikely to be due to the down-regulation of cyclin E and cyclin F upon cyclin A ablation (see Figure 1F). Likewise, either cyclin A or cyclin B could restore the mitotic index (Figure 4B) and the kinase activity of cyclin B-CDC2 (Figure 4C) caused by the cyclin A knockdown.
The above data suggest that overexpression of cyclin B can overcome the requirement of cyclin A during mitosis. We next performed the reciprocal experiments by expressing cyclin A after cyclin B knockdown. Figure 5A shows that overexpression of cyclin A could not compensate for the loss of cyclin B: CDC2Tyr15 remained hyperphosphorylated and histone H3Ser10 hypophosphorylated (lane 6). Similarly, neither cyclin E nor cyclin F could compensate for the loss of cyclin B. In marked contrast, the control reaction of expressing cyclin B (a shRNA-resistant construct) restored histone H3Ser10 phosphorylation (Figure 5B, lane 5).
In contrast to the experiments with cyclin A knockdown (Figure 3D), none of the wild type or AF mutants of CDC2 and CDK2 could reactivate histone H3Ser10 phosphorylation after cyclin B knockdown (Figure 5C). Likewise, the mitotic index and the CDC2 kinase activity were rescued by cyclin B, but not by other cyclins or CDKs (Figure 5D). These data indicate that even when ectopically expressed, other cyclin-CDK pairs cannot compensate the mitotic functions of cyclin B-CDC2.
One possible explanation that cyclin A could not drive cyclin B knockdown cells into mitosis is that the activity of cyclin A-CDK was somehow suppressed. To ensure that the expressed cyclin A was highly active, cells were cotransfected with CDC2AF- and cyclin A-expressing plasmids. Figure 5E shows that although cyclin A-CDC2AF was active, histone H3Ser10 phosphorylation was still absent in cyclin B knockdown cells.
Collectively, our results suggest that while expression of cyclin B can drive mitosis without cyclin A, cyclin A cannot promote mitosis in the absence of cyclin B: i.e., cyclin A cannot form MPF independent of cyclin B.
Down-Regulation of Cyclin A Correlates with the Inactivation of CDC25C and PLK1 and the Activation of WEE1 Pathways
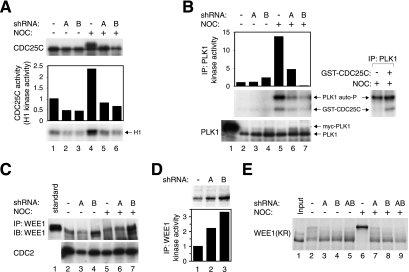
We next investigated the effects of cyclin A on the upstream regulators of cyclin B-CDC2. Knockdown of either cyclin A or cyclin B abolished the phosphorylation shifts of CDC25C (Figure 6A). Moreover, the phosphatase activity of CDC25C (measured in vitro toward cyclin B-CDC2) was reduced in the absence of cyclin A or cyclin B. To a lesser extent, the activities of CDC25A and CDC25B were also reduced after cyclin A knockdown (Supplementary Figure S10). PLK1 is an upstream regulator of CDC25C. Although the amount of PLK1 remained constant, the kinase activity of PLK1 (using purified GST-CDC25C as a substrate) decreased in the absence of cyclin A or cyclin B (Figure 6B).
Figure 6.
Down-regulation of cyclin A correlated with the inactivation of CDC25C and PLK1 and the activation of WEE1 pathways. (A) Activation of CDC25C requires cyclin A. Cells were transfected with control vector or plasmids expressing shRNA against cyclin A or cyclin B. The cells were treated with buffer or nocodazole for 16 h before harvested. Cell-free extracts were prepared, and the expression of CDC25C was detected by immunoblotting (top panel). The phosphatase activity of CDC25C was detected by the activation of WEE1-treated cyclin B-CDC2 as described in Materials and Methods. (B) Cyclin A is required for PLK1 activity. Cells expressing control vector, cyclin A shRNA, or cyclin B shRNA were treated with buffer or nocodazole for 16 h before harvested. The kinase activity of PLK1 was assayed using GST-CDC25CCΔ272 (containing the regulatory domain of CDC25C) as a substrate. The CDC25C substrate was either added or excluded in the reactions in the right-hand panel to indicate the positions of the PLK1 (autophosphorylated) and the CDC25C substrate. The phosphorylation incorporated into the CDC25C bands was quantified with a PhosphorImager. The expression of PLK1 in total cell lysates was detected by immunoblotting (bottom panel). A myc-tagged PLK1 was loaded as a positive control (lane 1). (C) WEE1 does not accumulate in the absence of cyclin A. Cells expressing control vector, cyclin A shRNA, or cyclin B shRNA were treated with buffer or nocodazole for 16 h before harvested. Cell-free extracts were prepared, and the expression of WEE1 was detected by immunoprecipitation followed by immunoblotting. Total CDC2 was detected to assess the loading of the lysates. (D) The activity of WEE1 is elevated in the absence of cyclin A. Cells were transfected with empty vector, cyclin A shRNA, or cyclin B shRNA plasmids. Cell lysates were prepared, and the kinase activity of WEE1 was assayed using GST-CDK2K33R as a substrate. (E) Expression of cyclin A is required for the hyperphosphorylation of WEE1. Rabbit reticulocyte lysates expressing 35S-labeled kinase-inactive WEE1K328R was mixed with extracts prepared from cells expressing control vector, cyclin A shRNA, or cyclin B shRNA (with or without nocodazole treatment for 16 h). The samples were applied onto SDS-PAGE and the 35S-labeled proteins were detected by phosphorimagery.
WEE1 is normally degraded during mitosis (Watanabe et al., 2005). As expected, inhibition of cyclin B lead to an accumulation of WEE1 (Figure 6C). Interestingly, inhibition of cyclin A did not result in WEE1 accumulation. Nevertheless, the kinase activity of WEE1 (using a kinase-dead GST-CDK2K33R as a substrate) was significantly higher in the absence of cyclin A (Figure 6D).
WEE1 is also negatively regulated by phosphorylation (Harvey et al., 2005). To see if the phosphorylation control of WEE1 was impaired after cyclin A knockdown, reticulocyte lysates-produced WEE1 was mixed with extracts expressing cyclin A and/or cyclin B shRNA. A kinase-dead version (WEE1K328R) was used in these experiments to ensure that the phosphorylation (mobility shifts) were not due to autophosphorylation. Figure 6E shows that knockdown of either cyclin A or cyclin B inhibited the phosphorylation of WEE1K328R. Taken together, these data indicate that the PLK1-CDC25C pathway is inactivated and WEE1 is activated in the absence of cyclin A.
Deregulation of WEE1 But Not the PLK1-CDC25 Pathway Bypasses the Requirement of Cyclin A for Mitosis
The above results suggest that cyclin A may regulate cyclin B-CDC2 indirectly through PLK1, CDC25, or WEE1. A major caveat is that it is not clear whether the changes in the activities of these enzymes are directly due to the lack of cyclin A or indirectly through the inactivation of cyclin B-CDC2. To shed light on this issue, the CDC2 regulators were deregulated in the cyclin A knockdown background to see if this could tip the balance of cyclin B-CDC2 activation.
As we have previously shown, ectopic expression of CDC25A and CDC25B can stimulate histone H3Ser10 phosphorylation after DNA damage (Chow et al., 2003b; Supplementary Figure S12A). In marked contrast, neither CDC25A nor CDC25B could efficiently restore histone H3Ser10 phosphorylation after cyclin A knockdown (Supplementary Figure S11A). Not surprisingly, CDC25A and CDC25B could not promote mitosis in cells lacking cyclin B. Similar experiments were performed with CDC25C. Problems associated with the recombinant CDC25C was its low expression level (Supplementary Figure S11B) as well as its potential inactivation by 14-3-3 binding. To circumvent these, a S216A mutant (which accumulated to higher levels and cannot be inactivated by 14-3-3 binding) was also used. Supplementary Figure S11B shows that neither CDC25C nor CDC25CS216A was very effective in restoring the histone H3Ser10 phosphorylation after the knockdown of the cyclins. In contrast, CDC25CS216A was highly effective in uncoupling histone H3Ser10 phosphorylation after DNA damage or replication block (Supplementary Figure S12B).
Similar to the experiments with CDC25, ectopic expression of PLK1 did not overcome the arrest associated with cyclin A or cyclin B knockdown (Supplementary Figure S11C). A mutation (T210D) was introduced into the activation loop of PLK1 to mimic the activated state (van de Weerdt et al., 2005), but this was also ineffective in restoring mitosis after the knockdown of the cyclins.
To disrupt the other arm of CDC2Tyr15 regulation, an shRNA against WEE1 was generated and coexpressed with shRNA targeting cyclin A or cyclin B (Figure 7A). We found that down-regulation of WEE1 promoted CDC2Tyr15 hypophosphorylation and histone H3Ser10 hyperphosphorylation in the absence of cyclin A (Figure 7B, lanes 8 and 10). Similarly, the kinase activity of CDC2 (Figure 7C) and the mitotic index (Figure 7D) after cyclin A knockdown were restored when WEE1 was concurrently down-regulated. Not surprisingly, none of the mitotic markers was restored by WEE1 shRNA in the absence of cyclin B.
Figure 7.
Down-regulation of WEE1 allows mitosis even in the absence of cyclin A. (A) Down-regulation of endogenous WEE1 with shRNA. Cells were transfected with empty vector or constructs expressing shRNA against cyclin A, cyclin B, or WEE1 as indicated. The expression of WEE1 was detected by immunoprecipitation followed by immunoblotting. Total CDC2 was detected to assess the loading of the lysates. (B) Expression of WEE1 shRNA allows mitosis even in the absence of cyclin A. Control, cyclin A shRNA, or cyclin B shRNA was coexpressed with empty vector or WEE1 shRNA as indicated. The cells were mock-treated or treated with nocodazole for 12 h before cell-free extracts were prepared. The expression of the indicated proteins was then detected by immunoblotting. (C) Down-regulation of WEE1 restores CDC2 activity after cyclin A knockdown. Coexpression of shRNA against cyclin A, cyclin B, and WEE1 was performed as in A. CDC2 was immunoprecipitated, and the histone H1 kinase activity was assayed. (D) Down-regulation of WEE1 restores the mitotic index after cyclin A knockdown. Coexpression of shRNA against cyclin A, cyclin B, and WEE1 was performed as in A, except that nocodazole was not added. The mitotic index was measured as described in Figure 2A. (E) Model of the regulation of M phase entry by cyclin A. A bistable system consists of cyclin B-CDC2, CDC25, and WEE1 is regulated by inputs from cyclin A-CDK, PLK1, C-TAK1, and checkpoint kinases (CHK1 and CHK2). See text for details.
Collectively, these data indicate that inactivation of WEE1 allowed cyclin B-CDC2 activation even in the absence of cyclin A. Forced activation of the PLK1-CDC25 axis could not achieve the same effect, suggesting that WEE1 may be a more critical component in the pathway that triggered the activation of cyclin B-CDC2 by cyclin A-CDK.
DISCUSSION
Can Cyclin A Serve as a Component of MPF in Mammalian Cells?
Our point of departure was that knockdown of cyclin A delayed entry into mitosis (Figure 1). Mitotic entry was assessed by multiple approaches in this work, including phosphorylation of histone H3Ser10, mitotic index, and CDC2 kinase activity. One hypothesis is that cyclin A itself is a component of MPF; the alternative is that cyclin A is part of the machinery that activates MPF. The foundation of the former hypothesis stems from the fact that cyclin A is related in sequence to cyclin B, binds CDC2, and the complex is active at the similar time as cyclin B-CDC2. Moreover, microinjection of cyclin A-CDK2 into G2 cells can accelerate mitotic entry (Furuno et al., 1999). However, there is no formal support that cyclin A possess MPF functions in the absence of the classic MPF (cyclin B-CDC2).
An important question is whether cyclin A can replace cyclin B to form MPF. Of the three mammalian cyclin B (B1, B2, and B3), cyclin B3 is restricted only to developing germ cells and adult testis (Nguyen et al., 2002), and cyclin B2 does not appear to have an essential function in mice (Brandeis et al., 1998). Cyclin B1 is the major mitotic cyclin, and mice lacking the cyclin B1 gene die in utero (Brandeis et al., 1998). Accordingly, we found that mitosis is interrupted after down-regulation of cyclin B1 with shRNA (Figures 2, A and B, and 3B). Mitosis could be restored with a shRNA-resistant cyclin B (Figure 5B), but not by cyclin A, cyclin E, or cyclin F (Figure 5, A and D). In the absence of cyclin B, the mitotic index of the population was reduced and the cells could not be trapped with high histone H3Ser10 phosphorylation by nocodazole. Interestingly, some cyclin B shRNA-expressing cells could undergo chromosome condensation but then failed to properly execute mitosis or be trapped by nocodazole (our unpublished data). Collectively, our data show that cyclin A-CDK alone cannot promote mitosis in the absence of cyclin B-CDC2.
Perhaps due to the effect of cell cycle blockade, the expression of cyclin E also diminished after cyclin A knockdown (Figure 1F). However, it is unlikely that cyclin E is involved in cyclin B-CDC2 activation, because expression of cyclin E (Figure 4) or CDK2AF (Figure 3, D–F) could not bypass the cyclin A shRNA-induced arrest. Cyclin E also could not drive mitosis in the absence of cyclin B (Figure 5A). Similarly, we have no evidence that cyclin F could compensate the functions of cyclin A or cyclin B (Figures 4 and 5).
Given that the endogenous cyclin B-CDC2 was inactive without cyclin A, it is hence surprising that overexpression of cyclin B could overcome the block imposed by cyclin A shRNA (Figure 4). A noteworthy point is that the expression of cyclin B was certainly many times highly than the endogenous levels (Figure 4A). As low but significant CDK activity can be stimulated by cyclins even in the absence of any phosphorylation control, we think that this overexpression of cyclin B was sufficient to overcome the WEE1/CDC25 control system. We speculate that the seeded (ectopically expressed) cyclin B served as a trigger and tipped the balance of this bistable system toward activation (Pomerening et al., 2003).
Cyclin A as an Activator of MPF
Knockdown of cyclin A resulted in an inactive cyclin B-CDC2 complex (Figure 3E) and an inhibition of mitosis (Figures 1F and 2A). Knockdown of cyclin B, however, disrupted mitosis (Figures 2A and 2B) while keeping cyclin A-CDK active (Figure 3A). These results unequivocally show that the relationship between cyclin A and cyclin B is linear and not reciprocal. This causal relationship also fits in with the fact that cyclin A is active ahead of cyclin B during the cell cycle.
The hypothesis that cyclin A is part of the machinery that activates MPF has been supported by evidence including those that demonstrated the requirement of cyclin A and CDK2 for CDC2 activity (Guadagno and Newport, 1996; Hu et al., 2001; Mitra and Enders, 2004). Interestingly, cell cycle progression does not absolutely require CDK2 (Berthet et al., 2003; Ortega et al., 2003; Tetsu and McCormick, 2003), although a compensation by CDC2 is likely to be the underlying mechanism (Aleem et al., 2005). In this work, we have not deliberately differentiate the functions of cyclin A-CDC2 and cyclin A-CDK2, as endogenous and expressed cyclin A bind to both CDC2 and CDK2 (our unpublished data). This may not be a critical issue as the prevailing view is that the substrate recognition site resides on the cyclin subunit (reviewed in Miller and Cross, 2001), but we cannot rule out that CDC2 and CDK2 complexes are regulated differently (for example, the susceptibility to Tyr15 phosphorylation).
Knockdown of cyclin A stimulated the inhibitory phosphorylation of CDC2 (Figures 2B and 4A). Neither cyclin B-CDC2 complex formation (Figure 3C) nor CDC2Thr161 phosphorylation (Figure 2B) was impaired after cyclin A knockdown. In agreement with this, CDC2 isolated from cyclin A knockdown cells could be reactivated with recombinant CDC25 (Figure 3C). These results also support that the notion that the inactivation of cyclin B-CDC2 after cyclin A knockdown was not due to CDK inhibitors like p21CIP1/WAF1 (Supplementary Figure S7).
Because the DNA integrity checkpoints also promote the accumulation of phospho-CDC2Tyr15, one of our chief concerns is that the cyclin A knockdown may have activated checkpoints that monitor DNA integrity. This could either be due to general stress responses to shRNA or to specific replication stress caused by the lack of cyclin A. We think this was unlikely because the checkpoint kinases ATM, CHK1, CHK2, and p38 were not activated after the expression of cyclin A shRNA (Supplementary Figures S8, A and B, and S9). Furthermore, caffeine could uncouple the DNA damage checkpoint but was ineffective against cyclin A knockdown (Supplementary Figure S8C). Finally, although CDC25A, CDC25B (Supplementary Figure S12A), and CDC25CS216A (Supplementary Figure S12B) could override the DNA damage checkpoint and replication checkpoint, these proteins were ineffective in overcoming the cyclin A shRNA-induced cell cycle block (Supplementary Figure S11, A and B). Together, these data argue that the inhibition of CDC2 caused by cyclin A shRNA was not caused by checkpoint activation.
In marked contrast to the rescue experiments with CDC25 (Figures S11, A and B), expression of CDC2AF could restore mitosis after cyclin A depletion (Figure 3, D–F). We have previously shown that CDC2AF could likewise uncouple the DNA integrity checkpoints (Chow et al., 2003a). Our interpretation is that cyclin A is involved in the control of CDC2Tyr15 phosphorylation, but this regulatory pathway is distinct from the checkpoint pathways. Although CDC25 appears to be the pivotal target of the checkpoint pathway, our evidence suggest that WEE1 may be the critical target of the cyclin A pathway (Figures 6E and 7).
A model of the role of cyclin A-CDK in mitotic entry is depicted in Figure 7E. It is postulated that cyclin B-CDC2 is kept inactive during G2 phase by WEE1. The activity of CDC25 remains low during this period due to Ser216 phosphorylation or the lack of mitotic phosphorylation. The balance of CDC2Tyr15 phosphorylation may be tipped when some WEE1 is inactivated by cyclin A-CDK. This flash point triggers the activation of some molecules of cyclin B-CDC2, which in turn are sufficient to activate more cyclin B-CDC2 through autocatalytic feedback loops involving WEE1 and CDC25. We postulate that cyclin A-CDK serves as a trigger of cyclin B-CDC2 activation, but the bulk of the cyclin B-CDC2 complexes are activated by autocatalytic mechanisms. This essentially places cyclin A as the “transient trigger stimulus” that pushes the cyclin B-CDC2 bistable system into an irreversible state (Ferrell, 2002).
This model depends on several characteristics of cyclin A that are distinct from cyclin B. In contrast to cyclin B-CDC2, cyclin A-CDK is relatively resistant to the inhibitory phosphorylation pathway (at least in embryonic cells; Clarke et al., 1992; Devault et al., 1992). This would allow cyclin A-CDK to trigger WEE1 inactivation without itself first being inactivated. This places WEE1 as the enzyme in the feedback circuit of a general bistable system that “respond to its upstream regulator in an ‘ultrasensitive' manner” (Ferrell, 2002). Indeed, we found that the N-terminal regulatory domain of WEE1 can be phosphorylated in vitro by both recombinant cyclin A-CDK2 and cyclin B-CDC2 expressed in baculoviruses (our unpublished data). It would be interesting to see how cyclin A– and cyclin B–mediated phosphorylation affects the activity and stability of WEE1. Another trait of cyclin A distinct from cyclin B is its subcellular localization. Although both inactive cyclin B-CDC2 and CDC25C are in the cytoplasm during G2, both cyclin A and WEE1 reside in the nucleus. Thus cyclin A is in an excellent position to inactivate WEE1, which in turn reduces the phosphorylation of cyclin B-CDC2 that is continually being shuttled into the nucleus. The activated cyclin B-CDC2 is then shuttled out to the cytoplasm and triggers the activation of the CDC25C feedback loop. Also compatible with the model of cyclin A as an upstream activator is that it is activated ahead of cyclin B (reviewed in Fung and Poon, 2005). Finally, cyclin A activity should no longer be required once cyclin B-CDC2 is activated, as indeed is the case during the activation of the spindle-assembly checkpoint. Another critical point of the model is that cyclin A-CDK does not trigger the proteolytic pathways that degrade cyclin B (in contrast to cyclin B-CDC2; Lorca et al., 1992).
Because PLK1 activity was reduced in the absence of cyclin A (Figure 6B), it is possible that cyclin A-CDK also activates PLK1. However, this could be an indirect effect because PLK1 activity was also suppressed in the absence of cyclin B. Moreover, deregulation of the PLK1 system (by overexpression of PLK1 or PLK1T210D) could not reverse the arrest induced by cyclin A shRNA. In this connection, cyclin A may regulate MYT1 indirectly even though the two proteins are localized to distinct cellular compartments. We speculate that MYT1 may remain active after cyclin A knockdown because it has been reported that inactivation of MYT1 in Xenopus eggs involves PLK1 (Plx1; Inoue and Sagata, 2005). MYT1 is associated with the endoplasmic reticulum in the cytoplasm and may able to act on cyclin B-CDC2. It should be noted that the negative results from ectopic expression of CDC2Tyr15 upstream activators (CDC25s and PLK1) do not emphatically exclude their involvement in the regulation by cyclin A-CDK. It remains possible that multiple CDC2Tyr15 regulators are targets of cyclin A-CDK.
In conclusion, we found that the G2 arrest induced by the absence of cyclin A was caused by the inactivation of cyclin B-CDC2 through inhibitory phosphorylation. Deregulation of WEE1 can override this arrest, suggesting that cyclin A-CDK may tip the balance of cyclin B-CDC2 through initiating the inhibition of WEE1. We also found that even when ectopically expressed, cyclin A cannot drive cells into mitosis in the absence of cyclin B, indicating the specific role of cyclin B as a component of MPF.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. David Turner and Katsumi Yamashita for generous gifts of reagents, Tim Hunt for helpful discussions, and members of the Poon lab for their assistance. This work was supported in part by the Hong Kong University of Science and Technology Grant HIA03/04.SC01 and Research Grants Council Grants CUHK2/02C and HKUST6135/03M to R.Y.C.P.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-12-1092) on March 7, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Aleem E., Kiyokawa H., Kaldis P. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 2005;7:831–836. doi: 10.1038/ncb1284. [DOI] [PubMed] [Google Scholar]
- Arooz T., Yam C. H., Siu W. Y., Lau A., Li K. K., Poon R.Y.C. On the concentrations of cyclins and cyclin-dependent kinases in extracts of cultured human cells. Biochemistry. 2000;39:9494–9501. doi: 10.1021/bi0009643. [DOI] [PubMed] [Google Scholar]
- Ausubel F., Brent R., Kingston R., Moore D., Seidman J., Smith J., Struhl K. Current Protocols in Molecular Biology. New York: John Wiley and Sons; 1991. [Google Scholar]
- Ayad N. G., Rankin S., Murakami M., Jebanathirajah J., Gygi S., Kirschner M. W. Tome-1, a trigger of mitotic entry, is degraded during G1 via the APC. Cell. 2003;113:101–113. doi: 10.1016/s0092-8674(03)00232-0. [DOI] [PubMed] [Google Scholar]
- Bartek J., Lukas C., Lukas J. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell Biol. 2004;5:792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- Berthet C., Aleem E., Coppola V., Tessarollo L., Kaldis P. Cdk2 knockout mice are viable. Curr. Biol. 2003;13:1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Brandeis M., Rosewell I., Carrington M., Crompton T., Jacobs M. A., Kirk J., Gannon J., Hunt T. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc. Natl. Acad. Sci. USA. 1998;95:4344–4349. doi: 10.1073/pnas.95.8.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulavin D. V., et al. Dual phosphorylation controls Cdc25 phosphatases and mitotic entry. Nat. Cell Biol. 2003;5:545–551. doi: 10.1038/ncb994. [DOI] [PubMed] [Google Scholar]
- Chow J. P., Siu W. Y., Fung T. K., Chan W. M., Lau A., Arooz T., Ng C. P., Yamashita K., Poon R.Y.C. DNA damage during the spindle-assembly checkpoint degrades CDC25A, inhibits cyclin-CDC2 complexes, and reverses cells to interphase. Mol. Biol. Cell. 2003a;14:3989–4002. doi: 10.1091/mbc.E03-03-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J.P.H., Siu W. Y., Ho H.T.B., Ma K.H.T., Ho C. C., Poon R.Y.C. Differential contribution of inhibitory phosphorylation of CDC2 and CDK2 for unperturbed cell cycle control and DNA integrity checkpoints. J. Biol. Chem. 2003b;278:40815–40828. doi: 10.1074/jbc.M306683200. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Leiss D., Pagano M., Karsenti E. Cyclin A- and cyclin B-dependent protein kinases are regulated by different mechanisms in Xenopus egg extracts. EMBO J. 1992;11:1751–1761. doi: 10.1002/j.1460-2075.1992.tb05227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Fesquet D., Cavadore J. C., Garrigues A. M., Labbe J. C., Lorca T., Picard A., Philippe M., Doree M. Cyclin A potentiates maturation-promoting factor activation in the early Xenopus embryo via inhibition of the tyrosine kinase that phosphorylates cdc2. J. Cell Biol. 1992;118:1109–1120. doi: 10.1083/jcb.118.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doree M., Hunt T. From Cdc2 to Cdk1, when did the cell cycle kinase join its cyclin partner? J. Cell Sci. 2002;115:2461–2464. doi: 10.1242/jcs.115.12.2461. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- Fung T. K., Poon R.Y.C. A roller coaster ride with the mitotic cyclins. Semin. Cell Dev. Biol. 2005;16:335–342. doi: 10.1016/j.semcdb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Fung T. K., Siu W. Y., Yam C. H., Lau A., Poon R.Y.C. Cyclin F is degraded during G2-M by mechanisms fundamentally different from other cyclins. J. Biol. Chem. 2002;277:35140–35149. doi: 10.1074/jbc.M205503200. [DOI] [PubMed] [Google Scholar]
- Fung T. K., Yam C. H., Poon R.Y.C. The N-terminal regulatory domain of cyclin A contains redundant ubiquitination targeting sequences and acceptor sites. Cell Cycle. 2005;4:1411–1420. doi: 10.4161/cc.4.10.2046. [DOI] [PubMed] [Google Scholar]
- Furuno N., den Elzen N., Pines J. Human cyclin A is required for mitosis until mid prophase. J. Cell Biol. 1999;147:295–306. doi: 10.1083/jcb.147.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno T. M., Newport J. W. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2-cyclin B kinase activity. Cell. 1996;84:73–82. doi: 10.1016/s0092-8674(00)80994-0. [DOI] [PubMed] [Google Scholar]
- Harvey S. L., Charlet A., Haas W., Gygi S. P., Kellogg D. R. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell. 2005;122:407–420. doi: 10.1016/j.cell.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Hendzel M. J., Wei Y., Mancini M. A., Van Hooser A., Ranalli T., Brinkley B. R., Bazett-Jones D. P., Allis C. D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Ho C. C., Siu W. Y., Chow J. P., Lau A., Arooz T., Tong H. Y., Ng I. O., Poon R.Y.C. The relative contribution of CHK1 and CHK2 to Adriamycin-induced checkpoint. Exp. Cell Res. 2005;304:1–15. doi: 10.1016/j.yexcr.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Hu B., Mitra J., van den Heuvel S., Enders G. H. S and G2 phase roles for Cdk2 revealed by inducible expression of a dominant-negative mutant in human cells. Mol. Cell. Biol. 2001;21:2755–2766. doi: 10.1128/MCB.21.8.2755-2766.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins J. R., Clarke P. R. Many fingers on the mitotic trigger: post-translational regulation of the Cdc25C phosphatase. Cell Cycle. 2004;3:41–45. [PubMed] [Google Scholar]
- Inoue D., Sagata N. The Polo-like kinase Plx1 interacts with and inhibits Myt1 after fertilization of Xenopus eggs. EMBO J. 2005;24:1057–1067. doi: 10.1038/sj.emboj.7600567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K. K., Ng I. O., Fan S. T., Albrecht J. H., Yamashita K., Poon R.Y.C. Activation of cyclin-dependent kinases CDC2 and CDK2 in hepatocellular carcinoma. Liver. 2002;22:259–268. doi: 10.1046/j.0106-9543.2002.01629.x. [DOI] [PubMed] [Google Scholar]
- Liu D., Matzuk M. M., Sung W. K., Guo Q., Wang P., Wolgemuth D. J. Cyclin A1 is required for meiosis in the male mouse. Nat. Genet. 1998;20:377–380. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- Loog M., Morgan D. O. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature. 2005;434:104–108. doi: 10.1038/nature03329. [DOI] [PubMed] [Google Scholar]
- Lorca T., Labbe J. C., Devault A., Fesquet D., Strausfeld U., Nilsson J., Nygren P. A., Uhlen M., Cavadore J. C., Doree M. Cyclin A-cdc2 kinase does not trigger but delays cyclin degradation in interphase extracts of amphibian eggs. J. Cell Sci. 1992;102(Pt 1):55–62. doi: 10.1242/jcs.102.1.55. [DOI] [PubMed] [Google Scholar]
- Ma H. T., On K. F., Tsang Y. H., Poon R.Y.C. An inducible system for expression and validation of the specificity of short hairpin RNA in mammalian cells. Nucleic Acids Res. 2007 doi: 10.1093/nar/gkl1109. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. E., Cross F. R. Cyclin specificity: how many wheels do you need on a unicycle? J. Cell Sci. 2001;114:1811–1820. doi: 10.1242/jcs.114.10.1811. [DOI] [PubMed] [Google Scholar]
- Mitra J., Enders G. H. Cyclin A/Cdk2 complexes regulate activation of Cdk1 and Cdc25 phosphatases in human cells. Oncogene. 2004;23:3361–3367. doi: 10.1038/sj.onc.1207446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. D., Kirk J. A., Hunt T. Unmasking the S-phase-promoting potential of cyclin B1. Science. 2003;300:987–990. doi: 10.1126/science.1081418. [DOI] [PubMed] [Google Scholar]
- Murphy M., Stinnakre M. G., Senamaud-Beaufort C., Winston N. J., Sweeney C., Kubelka M., Carrington M., Brechot C., Sobczak-Thepot J. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat. Genet. 1997;15:83–86. doi: 10.1038/ng0197-83. [DOI] [PubMed] [Google Scholar]
- Nguyen T. B., Manova K., Capodieci P., Lindon C., Bottega S., Wang X. Y., Refik-Rogers J., Pines J., Wolgemuth D. J., Koff A. Characterization and expression of mammalian cyclin b3, a prepachytene meiotic cyclin. J. Biol. Chem. 2002;277:41960–41969. doi: 10.1074/jbc.M203951200. [DOI] [PubMed] [Google Scholar]
- Ortega S., Prieto I., Odajima J., Martin A., Dubus P., Sotillo R., Barbero J. L., Malumbres M., Barbacid M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- Pomerening J. R., Sontag E. D., Ferrell J. E., Jr Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat. Cell Biol. 2003;5:346–351. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- Poon R.Y.C., Chau M. S., Yamashita K., Hunter T. The role of Cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res. 1997;57:5168–5178. [PubMed] [Google Scholar]
- Poon R.Y.C., Hunter T. Dephosphorylation of Cdk2 Thr160 by the cyclin-dependent kinase-interacting phosphatase KAP in the absence of cyclin. Science. 1995;270:90–93. doi: 10.1126/science.270.5233.90. [DOI] [PubMed] [Google Scholar]
- Poon R.Y.C., Hunter T. Expression of a novel form of p21Cip1/Waf1 in UV-irradiated and transformed cells. Oncogene. 1998;16:1333–1343. doi: 10.1038/sj.onc.1201897. [DOI] [PubMed] [Google Scholar]
- Poon R.Y.C., Toyoshima H., Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin. CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol. Biol. Cell. 1995;6:1197–1213. doi: 10.1091/mbc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon R.Y.C., Yamashita K., Adamczewski J. P., Hunt T., Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993;12:3123–3132. doi: 10.1002/j.1460-2075.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu W. Y., Lau A., Arooz T., Chow J. P., Ho H. T., Poon R.Y.C. Topoisomerase poisons differentially activate DNA damage checkpoints through ataxia-telangiectasia mutated-dependent and -independent mechanisms. Mol. Cancer Ther. 2004;3:621–632. [PubMed] [Google Scholar]
- Tetsu O., McCormick F. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell. 2003;3:233–245. doi: 10.1016/s1535-6108(03)00053-9. [DOI] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F., Taniguchi E., Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002;3:341–348. doi: 10.1093/embo-reports/kvf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F., Taniguchi E., Shinya N., Iwamatsu A., Nishida E. Polo–like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature. 2001;410:215–220. doi: 10.1038/35065617. [DOI] [PubMed] [Google Scholar]
- van de Weerdt B. C., van Vugt M. A., Lindon C., Kauw J. J., Rozendaal M. J., Klompmaker R., Wolthuis R. M., Medema R. H. Uncoupling anaphase-promoting complex/cyclosome activity from spindle assembly checkpoint control by deregulating polo-like kinase 1. Mol. Cell. Biol. 2005;25:2031–2044. doi: 10.1128/MCB.25.5.2031-2044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt M. A., Medema R. H. Checkpoint adaptation and recovery: back with Polo after the break. Cell Cycle. 2004;3:1383–1386. doi: 10.4161/cc.3.11.1248. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Arai H., Iwasaki J., Shiina M., Ogata K., Hunter T., Osada H. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc. Natl. Acad. Sci. USA. 2005;102:11663–11668. doi: 10.1073/pnas.0500410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Arai H., Nishihara Y., Taniguchi M., Hunter T., Osada H. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc. Natl. Acad. Sci. USA. 2004;101:4419–4424. doi: 10.1073/pnas.0307700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo R. A., Poon R.Y.C. Cyclin-dependent kinases and S phase control in mammalian cells. Cell Cycle. 2003;2:316–324. [PubMed] [Google Scholar]
- Yam C. H., Fung T. K., Poon R.Y.C. Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 2002;59:1317–1326. doi: 10.1007/s00018-002-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam C. H., Ng R. W., Siu W. Y., Lau A. W., Poon R.Y.C. Regulation of cyclin A-Cdk2 by SCF component Skp1 and F-box protein Skp2. Mol. Cell. Biol. 1999a;19:635–645. doi: 10.1128/mcb.19.1.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam C. H., Siu W. Y., Arooz T., Chiu C. H., Lau A., Wang X. Q., Poon R.Y.C. MDM2 and MDMX inhibit the transcriptional activity of ectopically expressed SMAD proteins. Cancer Res. 1999b;59:5075–5078. [PubMed] [Google Scholar]
- Yam C. H., Siu W. Y., Kaganovich D., Ruderman J. V., Poon R.Y.C. Cleavage of cyclin A at R70/R71 by the bacterial protease OmpT. Proc. Natl. Acad. Sci. USA. 2001;98:497–501. doi: 10.1073/pnas.240461397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam C. H., Siu W. Y., Lau A., Poon R.Y.C. Degradation of cyclin A does not require its phosphorylation by CDC2 and cyclin-dependent kinase 2. J. Biol. Chem. 2000;275:3158–3167. doi: 10.1074/jbc.275.5.3158. [DOI] [PubMed] [Google Scholar]
- Yu J. Y., DeRuiter S. L., Turner D. L. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc. Natl. Acad. Sci. USA. 2002;99:6047–6052. doi: 10.1073/pnas.092143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Eckerdt F., Bereiter-Hahn J., Kurunci-Csacsko E., Kaufmann M., Strebhardt K. Cooperative phosphorylation including the activity of polo-like kinase 1 regulates the subcellular localization of cyclin B1. Oncogene. 2002;21:8282–8292. doi: 10.1038/sj.onc.1206011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.