Abstract
Transcription factors of the Snail family are key regulators of epithelial-mesenchymal transition (EMT). In many processes during development or disease, cells do not acquire all the characteristics associated with EMT, leading to what we refer to as partial EMT (p-EMT). However, little is known of the implications of the Snail transcription factors in processes that only involve a p-EMT. To assess this, we used the hepatocyte growth factor (HGF)-induced Madin-Darby canine kidney tubulogenesis system, which provides a three-dimensional culture model of a morphogenetic process including a p-EMT. We found that although Slug (Snail2) is highly and transitory up-regulated during the p-EMT phase of tubulogenesis, it is not a repressor of E-cadherin during this process. Using inducible knockdown of Slug, we demonstrate that Slug is not an inducer of cell movement and instead is required for survival during p-EMT. We conclude that in epithelial cells, promoting cell survival can be a primary function of Slug, rather than being acquired concomitantly with EMT.
INTRODUCTION
Epithelial-Mesenchymal transition (EMT) is a process during which nonmotile epithelial cells characterized by a stable apico-basal polarity become migratory mesenchymal cells. Characteristics of this process include complete loss of apico-basal epithelial polarity, loss of epithelial markers such as the adherens junction molecule E-cadherin and acquisition of mesenchymal markers such as changes in the repertoire of cytokeratins. EMT occurs during many developmental processes such as gastrulation, neural crest migration, and heart formation and also is implicated in pathological processes, such as fibrosis and metastasis (Hay, 1995; Birchmeier et al., 1996; Thiery, 2002; Kalluri and Neilson, 2003). In addition to processes involving complete EMT, many processes occurring during development and in adult organisms involve only a transient loss of epithelial polarity without full acquisition of mesenchymal characteristics. Examples of these processes, which exhibit a spectrum of changes in epithelial plasticity that we have defined as partial EMT (p-EMT), include some types of tubulogenesis (e.g., in mammary gland development) and epithelial wound healing. A variety of epithelial plasticity changes are also associated with pathologies such as chronic inflammation. These findings are consistent with the idea that complete EMT may be the result of a sequential multistep program, and there is a spectrum of p-EMT processes in which cells undergo only some of these steps, often for only a transient period (Grunert et al., 2003; Huber et al., 2005). Partial EMT has been suggested to occur in some metastatic cancers, where distant metastasis can retain much of the epithelial differentiation of the tissue of origin of the cancer (Debnath and Brugge, 2005).
Although the molecular mechanisms controlling EMT have only recently begun to emerge, key roles have been identified for the zinc finger transcription factors Snail (Snail1) and Slug (Snail2; Savagner, 2001; Huber et al., 2005). Slug was discovered in the chick as a key regulator of mesoderm formation and neural crest migration, two developmental processes involving EMT (Nieto et al., 1994). In the mouse, Snail fulfills some of the functions that Slug has during chick development (Sefton et al., 1998; Nieto, 2002). In cell culture experimental overexpression of Slug or Snail is sufficient to induce epithelial cells to undergo EMT (Savagner et al., 1997; Batlle et al., 2000; Cano et al., 2000; Bolos et al., 2003). Importantly, their expression is up-regulated in various epithelial cells (including Madin-Darby canine kidney [MDCK]) by growth factors that typically induce EMT such as transforming growth factor (TGF) β and fibroblast growth factor (FGF; Savagner et al., 1997; Romano and Runyan, 2000; Peinado et al., 2003). Slug and Snail can directly repress the transcription of the E-cadherin gene by binding to E-Box sequences within its promoter (Batlle et al., 2000; Cano et al., 2000; Bolos et al., 2003). Their role as inducers of EMT is partly due to the direct repression of E-cadherin expression that destabilizes cell–cell adhesion, a phenomenon necessary for cell migration. This role is supported by the correlation observed in various invasive carcinomas between Slug or Snail expression and loss of E-cadherin transcription (Blanco et al., 2002; Shih et al., 2005; Uchikado et al., 2005). Slug and Snail have additional functions associated with their role as triggers of EMT. Concomitantly with the induction of EMT, Snail expression can confer survival properties to the cells (Valdes et al., 2002; Vega et al., 2004; Robson et al., 2006). Slug has a critical role in re-epithelialization of cutaneous wounds in mice and has been proposed to be required for cell extension movement (Savagner et al., 2005). However, the process of re-epithelialization involves migration but does not require a full EMT.
Although the role of Slug and Snail as inducers of EMT start to be well understood, their implication in processes that do not require a complete EMT has been far less studied. One experimentally tractable model of how p-EMT is used during epithelial morphogenesis is the formation of tubules by MDCK epithelial cells. When MDCK cells are cultured in a three-dimensional (3D) gel of extracellular matrix (ECM), they form hollow spheres or cysts consisting of a monolayer of fully polarized cells, with their apical surfaces facing a central lumen and their basal surfaces contacting the ECM. Stimulation of these cysts with hepatocyte growth factor (HGF) causes the cysts to elaborate branching tubules, recapitulating some aspects of tubulogenesis in vivo (Montesano et al., 1991; Pollack et al., 1998; O'Brien et al., 2002; O'Brien et al., 2004). The first phase of MDCK tubulogenesis involves p-EMT. In the initial substep of this p-EMT phase, a subset of the fully polarized cells produce extensions of their basal surface, which radiate outward from the cyst. Although this involves cell movement, the epithelial cells at this point still retain some features of apico-basal polarity. In the second substep of the p-EMT phase, some of these cells migrate entirely away from the cyst wall, completely losing their apical surface and thus apico-basal polarity. These cells form chains of cells that retain some cell–cell junctions and do not acquire full mesenchymal characteristics at any point. In the second phase of tubulogenesis, the cells redifferentiate into fully polarized epithelial cells and form tubules with lumens.
Here we have used the 3D MDCK tubulogenesis system to examine the expression and function of endogenous Slug in a process involving a p-EMT. Although the studies of Slug and Snail obtained either from embryonic systems or from classic 2D culture models have provided important insight into Slug and Snail, this 3D model provides a physiologically more relevant cell environment than classic 2D culture, while permitting the analysis of cellular and molecular mechanisms with much greater spatial and temporal resolution than is typically attainable in vivo.
We report that Slug is highly and transiently up-regulated by HGF during the p-EMT phase of MDCK tubulogenesis. However, HGF-induced Slug expression is not necessary for initiation of cell movement during tubulogenesis. Rather, the first detectable role of Slug is that it is required for the cells to survive during the p-EMT phase of tubulogenesis. Our work reveals that Slug's survival function in epithelial cells can precede EMT rather than being acquired concomitantly to complete EMT and is the primary function of Slug during p-EMT. Our data also show that MDCK tubulogenesis requires a specific factor promoting cell survival, even though cell death was previously not known to play a major role in this epithelial remodeling and morphogenetic process.
MATERIALS AND METHODS
Antibodies and Reagents
Primary antibodies used were chicken anti-Slug (raised as described in Supplemental Experimental Procedures), mouse anti-E-cadherin (BD Biosciences, San Jose, CA), rabbit anti-Cleaved Caspase-3 (Cell Signaling, Beverly, MA), mouse anti-Glyceraldehyde-3-Phosphate Dehydrogenase (Gapdh; Chemicon, Temecula, CA). Secondary antibodies used for immunoblotting were peroxidase-conjugated rabbit anti-chicken and rabbit anti-mouse (Jackson ImmunoResearch, West Grove, PA) and for confocal microscopy: goat anti-chicken Alexa Fluor 488, donkey anti-mouse Alexa Fluor 647 and goat anti-rabbit Alexa Fluor 555 (Molecular Probes, Eugene, OR). Actin filaments were stained with Alexa Fluor 546 or 633 phalloidin (Molecular Probes). Nuclei were stained with Hoechst 33342 (Molecular Probes). HGF was a gift of Ralph Schwall (Genentech, South San Francisco, CA).
Cell Culture
MDCK cells were maintained in minimal essential medium (MEM) Eagle's with Earle's balanced salt solution (Mediatech Cellgro, Herndon, VA) supplemented with 5% fetal bovine serum (FBS; Hyclone, Logan, UT), 100 U/ml penicillin, and 100 mg/ml streptomycin in 5% CO2, 95% air. MDCK T23T.1 cells were obtained from T. S. Jou (National Taiwan University, Tapei) and were maintained as described for MDCK with addition of 150 μg/ml zeocin.
Growth of cysts in 3D collagen type I gels was performed as described previously (Pollack et al., 1998) with the following modifications. Briefly, cells were trypsinized into a single-cell suspension, centrifuged, and mixed to a type I collagen solution containing 66% Vitrogen 100 (3 mg/ml; Cohesion, Palo Alto, CA). Cells (n = 2000–4000) mixed with 150 μl of collagen solution were plated on the top of a cell-free gelled collagen layer onto anopore membrane filters with a 10-mm diameter and 0.2-μm pore size (Nunc). The collagen mixture was allowed to gel at 37°C, and then medium was added. Cells were fed every 2–3 d and grown for 9–10 d until cysts with lumen were formed. To induce tubulogenesis, MDCK cysts were treated by addition of HGF to the medium at the indicated concentration during the indicated time.
RNA Extraction and Relative and Quantitative RT-PCR Analysis
Cysts embedded in collagen were rinsed with phosphate-buffered saline (PBS) and homogenized in lysis buffer (Qiagen, Chatsworth, CA) using a homogenizer. The homogenate was treated with 200 μg/ml Proteinase K (Qiagen) for 30 min at 55°C before RNA extraction. Total RNA were extracted on columns using the RNeasy Mini kit (Qiagen). DNase I treatment was done directly on the column. 0.5–1 μg of total RNA were reverse-transcribed as previously described (Leroy et al., 2004). Relative RT-PCR was done as previously described with the internal standard and the specific gene studied amplified in the same tube (Leroy et al., 2004). The yield of cDNA was normalized using the expression of the gene coding for Gapdh as an internal standard. Linear amplification ranges were tested and experiments were done with 28 cycles for Slug and 23 cycles for Gapdh. The volume of each cDNA sample was adjusted to give the same PCR signal strength for Gapdh after 22–24 cycles, i.e., in its linear amplification range. The expected fragment (358 base pairs for Slug and 271 base pairs for Gapdh) were visualized on a 2% agarose gel. Quantitative RT-PCR was done using SYBR green PCR master mix (Applied Biosystems, Foster City, CA) and the Mx4000 multiplex quantitative PCR system and software (Stratagene, La Jolla, CA).
Annealing was at 59°C and primers used were as follows: for Slug: Forward primer: 5′ AGCAGTTGCACTGTGATGCC 3′, Reverse primer: 5′ ACACAGCAGCCAGATTCCTC 3′; for E-cadherin : Forward primer 5′ GAGAGCGGTGGTCAAAGAGC 3′, Reverse primer: 5′ GAGGAGTTCAGGGAGCTCAG 3′, for Snail: Forward primer: 5′ TGCCCTCAAGATGCACATCC 3′, Reverse primer: 5′ TGACATCTGAGTGGGTCTGC 3′; for p53 : Forward primer 5′ TGTGGTGGTGCCTTATGAGC 3′, Reverse primer: 5′ ATGGCGAGAGGTAGATTGCC 3′; for Bid : Forward primer 5′ AGCTACTTCCTGGATGGTGG 3′, Reverse primer: 5′ CATAGGTGAGCAGGTTCTGG 3′; for Gapdh : Forward primer 5′ CAGTTGTGGATCTGACCTGC 3′, Reverse primer: 5′ CCTTGGAGGCCATGTAGACC 3′.
Generation of Stable MDCK Transfectants
The cloning and sequencing of the canine Slug cDNA is described in supplemental experimental procedures. Slug antisense construct was generated by inserting a 5′XbaI-3′BamHI fragment corresponding to the full-length Slug CDS excised from a pBluescript-Slug construct in the pIND(SP1) ecdysone-inducible vector (Invitrogen, Carlsbad, CA). MDCK T23T.1 cells (Lai et al., 2003) were cotransfected with this construct and a blasticidin-resistant gene using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). Stable transfectants were isolated after 2–3 wk of culture in the selective medium. Different transfectant clones were selected that show a knockdown of Slug transcripts specifically when the inducer ponasterone A (Invitrogen) is added.
Immunoblotting
Cysts were isolated from collagen by treatment with 4000 U/ml collagenase type IV (Worthington, Lakewood, NJ). Isolated cysts were rinsed with PBS, lysed with 1% sodium dodecyl sulfate (SDS) solution, boiled and shaken for 5 min. The protein concentration in the lysates was determined using a BCA protein assay (Pierce). Samples were run on SDS-page gels, electrotransferred to immobilon-P membrane (Millipore, Bedford, MA). Blots were blocked and hybridized in 0.05% tween in PBS (PBST) containing 5% milk, washed with PBST, and revealed with detection reagent (Amersham Biosciences, Piscataway, NJ). Gapdh was used as an internal marker to normalize protein amounts between samples. Quantification was done using Quantity one software (Bio-Rad, Richmond, CA).
Immunofluorescence Staining
Immunofluorescence staining of cysts cultured in collagen gel was done as previously described (Pollack et al., 1998; O'Brien et al., 2001) with some modifications. Briefly, samples were rinsed with PBS before treatment with collagenase type VII (Sigma, St. Louis, MO) at 37°C for 10 min and fixed with 4% paraformaldehyde in PBS for 30 min at 4°C. Samples were rinsed with PBS, permeabilized with 0.2% Triton X-100 in PBS for 10 min, and permeabilized and blocked with 0.7% fish gelatin (Sigma) and 0.025% saponin in PBS for 30 min. Samples were then incubated in primary antibodies at 4°C overnight and extensively washed. Samples were incubated at RT for a minimum of 4 h with the corresponding Alexa Fluor–conjugated secondary antibody at a dilution of 1:300 and Alexa Fluor phalloidin and Hoechst (1:1000 each) then washed extensively. All incubations and washing were done with the blocking solution. Samples were rinsed with PBS before be mounted using ProLong gold (Invitrogen). MDCK monolayer on filter were similarly processed but with shorter times of incubation and lower concentration of antibodies or dyes (Alexa Fluor–conjugated secondary antibody at a dilution of 1:600 and Alexa Fluor phalloidin and Hoechst; 1:3000 each).
Image Analysis
Cysts were analyzed for immunofluorescence using a Zeiss 510 LSM confocal microscope (Thornwood, NY). Samples were viewed using an argon laser (488 line) and helium-neon lasers (543 and 633 lines) and a 2-photon 780 in conjunction with a Zeiss 510 confocal laser scan head attached to a Zeiss Axiovert 200M microscope. Digital images of optical sections were collected with a Zeiss Plan Apo 63× 1.40 NA objective. Images were analyzed using LSM 510 software and resized with Adobe Photoshop 8 software (San Jose, CA).
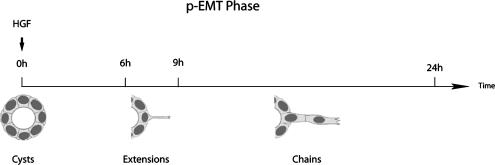
Slug mRNA Levels Are Transiently Up-regulated in MDCK Cysts by HGF during the p-EMT Phase of Tubulogenesis
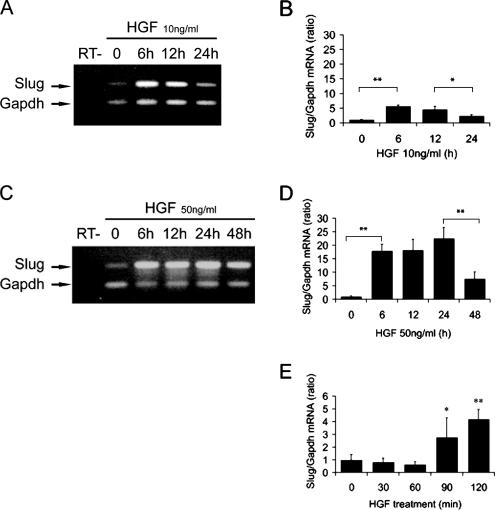
In 2D culture, Slug and Snail have been shown to be up-regulated at the transcriptional level in different cell types by EMT-inducing growth factors such as FGF and TGFβ. To determine if these genes are up-regulated during the p-EMT phase of HGF-induced MDCK tubulogenesis, we analyzed their mRNA expression during a time course of HGF stimulation of cysts. As described previously 10 ng/ml HGF is the optimal concentration to induce MDCK tubulogenesis (O'Brien et al., 2004). Under these conditions extensions are first seen after ∼6 h of HGF treatment; chains appear at the earliest after 9 h, but most of the chains start to form by 12–16 h (Figure 1). By 24 h of treatment the p-EMT phase has ended, and the redifferentiation phase of tubulogenesis begins. Although a certain degree of asynchrony is observed, the majority of the cysts follow the time frame presented in Figure 1. Using both relative and quantitative RT-PCR analysis, we found that Slug mRNA levels are highly up-regulated following HGF stimulation of cysts (Figure 2, A and B). We also observed an induction of Snail expression, which is much more modest than Slug as shown by quantitative RT-PCR analysis (Supplementary Figure S1) and confirmed this by Northern blot analysis (not shown). We therefore concentrated on Slug. Slug mRNA levels increase as much as five times within 6 h of HGF treatment as measured by quantitative RT-PCR, and this increase is maintained at 12 h. Notably, this up-regulation of mRNA is transient, with a significant decrease observed after 24 h of HGF exposure (Figure 2B).
Figure 1.
Time course of the partial EMT of MDCK tubulogenesis.
Figure 2.
Slug mRNA levels are transiently up-regulated during the HGF-induced p-EMT phase. MDCK cysts grown in collagen were treated with HGF to induce tubulogenesis, and total RNA was extracted at indicated times. Cysts were treated with either 10 ng/ml HGF (A and B) or 50 ng/ml HGF (C–E). Gapdh was used to normalize the cDNA amounts between samples. (A and C) Slug mRNA levels were analyzed by relative RT-PCR using Gapdh as an internal marker in each reaction. Amplification products were analyzed on an agarose gel. RT− is a control without reverse transcriptase. (B, D, and E) Quantitative RT-PCR analysis of Slug mRNA levels. Results are presented as the ratio of Slug to Gapdh. Data shown represent the mean ± SD, with n > 3. Differences in values are significant at ** p < 0.01 or * p < 0.05 as indicated.
We also observed that HGF-induced Slug up-regulation is dose dependent, with higher concentrations of HGF, such as 50 ng/ml, up-regulating Slug mRNA as high as 25 times that of nontreated cysts (Figure 2, C and D). However, increasing the concentration of HGF to 50 ng/ml delays Slug down-regulation after its induction with high levels of mRNA maintained at 24 h and instead decreased only at 48 h. HGF, 50 ng/ml, increases the overall number of extensions and chains per cysts but impairs the redifferentiation process (data not shown). Interestingly, Slug up-regulation starts as early as 90 min after treatment (Figure 2E), i.e., several hours before the cells in the cyst walls start to form extensions.
Together these results show that Slug is highly and transiently up-regulated after HGF stimulation of MDCK cysts specifically during the time when cells undergo the p-EMT phase of tubulogenesis. This up-regulation occurs as an early response to HGF treatment and is HGF dose-dependent. However, we cannot conclude from our results that Slug mRNA levels are increasing by regulation at the transcriptional level, or alternatively by stabilization of the mRNA. HGF is involved in cancer and metastasis and is usually described as an inducer of EMT (Birchmeier et al., 2003) and blocking Slug expression has been previously shown to interfere with the HGF pathway in NBTII bladder cells (Savagner et al., 1997). However, regulation of Slug by HGF has not been reported previously, as far as we know.
Slug Protein Levels Are Specifically Up-regulated in the Nuclei of the Cells Forming Chains
To determine the expression of Slug protein in the treated cysts, we raised an anti-canine Slug antibody that recognizes Slug protein with high specificity both on Western blot and by immunofluorescence in 3D structures (described in Material and Methods and Supplementary Figure S2).
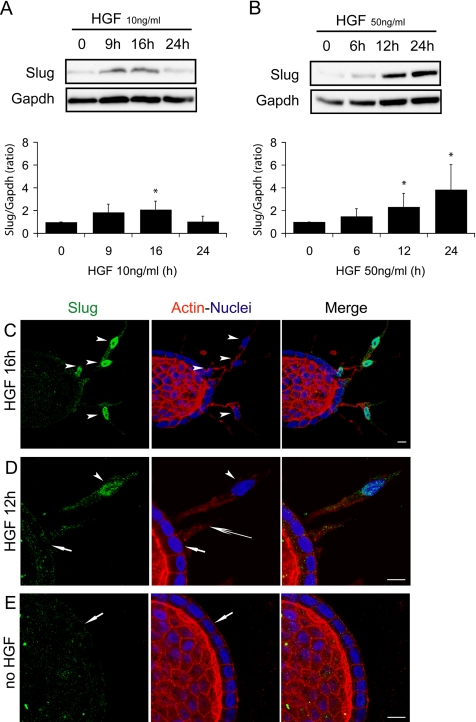
Slug protein levels were analyzed on total lysates extracted from MDCK cysts after different times of HGF treatment. After 6 h of treatment with 10 ng/ml HGF no significant increase in Slug protein is detectable (data not shown), and after 9 h a modest increase can be observed but not in all experiments performed. Slug protein levels are significantly increased after 16 h of treatment and return to baseline levels after 24 h (Figure 3A). With 50 ng/ml HGF, the increase in protein levels is higher, reaching as much as four times over Slug protein levels baseline, and still sustained at 24 h in accordance with the mRNA levels observed for the same high concentration of HGF (Figure 3B). The overall fold increase of the mRNA levels is clearly higher than for the protein levels with as much as 10× difference when 50 ng/ml HGF is used (Figure 2D). Furthermore, comparison of the kinetics of mRNA and protein induction reveals that the up-regulation of the mRNA precedes by several hours the appearance of the first extensions, whereas the detectable up-regulation of protein levels is only observed when formation of the first chains occurs (Figures 2, B and D, and 3, A and B).
Figure 3.
Slug protein is only expressed in the nuclei of cells forming chains. HGF-stimulated MDCK cysts were analyzed for Slug expression at different time points during the p-EMT phase either by immunoblotting (A and B) or by confocal microscopy (C–E). (A and B) Total protein was extracted and analyzed with Slug antibody by immunoblotting. HGF concentrations used were (A) 10 ng/ml and (B) 50 ng/ml. Results shown are a representative blot and quantification from several blots using Gapdh to normalize the protein amounts. Data are presented as the mean ± SD, with n ≥ 3. Differences of values are significant at * p < 0.05 as indicated. (C–E) Confocal images of cysts treated with 10 ng/ml HGF, fixed, and immunostained as whole mounts at different time points of the p-EMT. The whole mounts were labeled simultaneously with Slug antibody (green), Phalloidin to reveal filamentous actin (red), and Hoechst as a marker of nuclei (blue). (C) Cyst representative of 16 h of HGF treatment. Arrowheads point to nuclei in chains of cells that have migrated away from the wall of the cyst. Quantification performed on 326 nuclei from 22 independent samples allowed to determine that 77.6 ± 13.0% of the nuclei in chains are strongly positive for Slug. (D) Cyst representative of 12 h of HGF treatment. Both extensions (long arrow) and chains (arrowhead) are present. Arrowhead points to the nucleus in the chain that is strongly positive for Slug. Nuclei remaining in the wall of the cyst (short arrow) are all negative for Slug. (E) Cyst not treated by HGF with the nuclei in the wall of the cyst are all negative for Slug (short arrow). Bars, 10 μm.
It was then important to determine the cellular localization of Slug protein at different time points after HGF treatment. For this, we performed immunofluorescence staining of whole mount MDCK cysts treated with HGF and fixed after different times of HGF treatment. Analysis by confocal microscopy shows that Slug protein is clearly detected in the nuclei of the cells that formed chains, i.e., when their nuclei start to migrate away from the wall of the cyst (Figure 3C). A large majority (77.6 ± 13.0%) of the nuclei in chains are clearly positive for Slug. A higher magnification of a cyst with a cell forming a nascent chain shows that its nuclei is strongly labeled for Slug, whereas some traces of Slug are detected in the cytoplasm (Figure 3D). In contrast, the nuclei of the cells remaining in the wall of the cyst are all negative for Slug; this includes cells that have started to form extensions. No notable difference was observed in Slug staining for cells in the wall of cysts whether treated or not with HGF (Figure 3, D and E). In addition, we observed that the further the nuclei have migrated from the wall of the cysts the more intense the staining of Slug in the nuclei became (Supplementary Figure S3).
Together these results show that both Slug mRNA and protein are up-regulated during the HGF-induced p-EMT phase of MDCK tubulogenesis. mRNA up-regulation is a very early event after HGF treatment. However, the nuclei of the cells in the cyst wall are all negative for Slug protein, whereas its expression is only detected in the nuclei of the cells that have started to form chains. Taken together our results suggests that Slug is not necessary for the induction of cell extensions, which is the initial substep of the p-EMT phase, but may have another specific function during this phase of tubulogenesis.
Slug Does Not Repress E-Cadherin Expression during the p-EMT Phase of Tubulogenesis
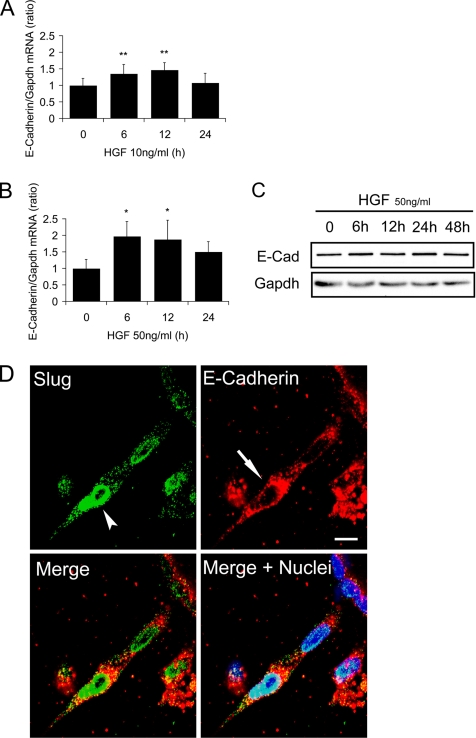
The well-established ability of the Snail family of transcription factors to directly repress the E-cadherin gene prompted us to determine if the up-regulation of Slug protein was accompanied by a concomitant repression of E-cadherin expression in our system. We first analyzed E-cadherin expression at the mRNA level using quantitative RT-PCR, and we surprisingly observed no decrease of E-cadherin mRNA for all times tested after treatment with 10 ng/ml HGF (Figure 4A). Indeed, on the contrary a small but statistically significant up-regulation of E-cadherin mRNA levels was observed after 12 h of HGF treatment even though Slug protein levels were increasing at this time (Figure 3A). E-cadherin up-regulation was transient and expression returned to initial levels at 24 h, i.e., when Slug protein levels also decreased. Furthermore with 50 ng/ml HGF, which induces higher expression of Slug protein, similar kinetics of up-regulation of E-cadherin mRNA were observed although with overall greater fold increases (Figure 4B). Western blot analysis of total lysates extracted from cysts treated under similar conditions shows that E-cadherin protein was not altered (Figure 4C).
Figure 4.
E-Cadherin expression is not repressed by Slug during the p-EMT phase. HGF-induced MDCK cysts were analyzed for the expression of E-cadherin at the mRNA level (A and B) or protein level (C and D) at different time points of p-EMT. (A and B) Quantitative RT-PCR of E-cadherin mRNA levels from cysts treated with (A) 10 ng/ml HGF or (B) 50 ng/ml HGF. Results are presented as the ratio of E-Cadherin to Gapdh. Data shown represent the mean ± SD, with n = 4. Differences of values are significant at ** p < 0.01 or * p < 0.05 as indicated. (C) Immunoblotting with E-cadherin antibody of total proteins extracted from cysts treated with 50 ng/ml HGF for different time points. Gapdh was used to normalize the protein amounts. (D) Confocal analysis of cysts treated for 24 h with 50 ng/ml HGF. Cysts were fixed as whole mounts and immunostained simultaneously with Slug antibody (green), E-cadherin antibody (red) or Hoechst as a marker of nuclei (blue). Confocal images of a representative chain emanating from a cyst with the arrowheads showing the nuclei in the chain strongly positive for Slug and the arrows showing that the cells are still strongly positive for E-cadherin. Bar, 10 μm.
Because our quantitative RT-PCR and Western blot analysis was performed on bulk MDCK cyst cultures we wished to examine E-cadherin protein levels in individual cells. Confocal analysis on HGF-induced MDCK cysts expressing Slug and stained by immunofluorescence for both E-cadherin and Slug protein confirm that E-cadherin is still present in cells expressing high levels of Slug (Figure 4D and Supplementary Figure S4). We previously showed using this 3D model that during the p-EMT phase, E-cadherin is redistributed and no longer mostly restricted to the adherens junctions (Pollack et al., 1998). Our results show that this redistribution is not accompanied by a reduction in the levels of E-cadherin protein even in the cells with high concentrations of nuclear Slug.
These results suggest that Slug does not act as a repressor of E-cadherin expression during the p-EMT phase of MDCK tubulogenesis. Moreover, the similar kinetics of E-cadherin expression regardless of HGF concentration contrasts with the significant HGF-dose dependent expression of Slug and this further suggests that E-cadherin and Slug do not depend on each other at any detectable level of regulation.
Slug Is Necessary for the Survival of the Cells Forming Chains during the p-EMT of Tubulogenesis.
Our results show that during HGF-induced tubulogenesis Slug is specifically up-regulated in the nuclei of cells in chains, but does not repress E-cadherin in these cells. Furthermore, the fact that Slug is not detected in cells that have initiated p-EMT by forming extensions suggests that Slug does not act as an inducer of p-EMT. To further investigate the role of Slug in MDCK tubulogenesis, we sought to examine the consequences of loss of Slug expression during tubulogenesis. For this we needed to block Slug expression only after cysts were formed. As currently available inducible shRNA technology works poorly for MDCK cysts after 10–12 d culture period, we pursued an antisense strategy analogous to that successfully used for other transcription factors, including Slug (Savagner et al., 1997; Verrecchia et al., 2001; Boudreau and Varner, 2004; Kuphal et al., 2005). Inducible expression of the antisense construct was conferred by a promoter whose expression is induced by ponasterone, an analog of ecdysone (Lai et al., 2003).
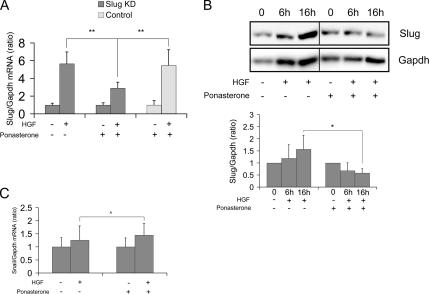
First, we validated the efficiency and specificity of our approach to inducibly knock down Slug. MDCK cells stably transfected with Slug antisense were seeded in collagen and grown to form cysts without addition of the inducer. The cysts were then treated with HGF and simultaneously with ponasterone to induce expression of the Slug antisense. By quantitative RT-PCR analysis, we found that addition of ponasterone to the cysts treated with HGF inhibited the up-regulation of Slug mRNA by ∼50% after 6 h of treatment with HGF when compared with the level of up-regulation normally observed (Figure 5A). This inhibition was not observed when ponasterone was added to control cells showing that the inhibition is not due to a toxic effect of ponasterone (Figure 5A). We then confirmed that the inhibition of the up-regulation of Slug mRNA was reflected at the protein level. Figure 5B shows that Slug protein is not anymore up-regulated when ponasterone is added in combination with HGF, demonstrating that Slug protein is successfully knocked down.
Figure 5.
Slug is specifically knocked down in inducible Slug antisense transfectants. Cysts formed with ponasterone inducible Slug knockdown cells were treated with HGF in the presence or absence of ponasterone and analyzed for the expression of Slug mRNA (A) or Slug protein (B) as well as for Snail mRNA (C). (A) Quantitative RT-PCR of Slug performed on cysts treated with 10 ng/ml HGF for 6 h or not treated and in the absence or presence of 10 μM ponasterone. Slug KD is Slug knockdown transfectant cells. Control is parental cells. Results are presented as the ratio of Slug to Gapdh. Data shown represent the mean ± SD, with n = 6. Differences of values are significant at ** p < 0.01 as indicated. (B) Immunoblotting of Slug. Slug knockdown cysts were treated with 10 ng/ml HGF in the absence or presence of 10 μM of ponasterone, and total protein was extracted at different time points. Results show a representative blot and quantification from several blots using Gapdh to normalize protein amounts. Data are presented as the mean ± SD with n = 3. Differences of values are significant at * p < 0.05 as indicated. (C) Quan-titative RT-PCR analysis of Snail performed on Slug knockdown cysts treated with 10 ng/ml HGF for 6 h or not treated and in the absence or presence of 10 μM ponasterone. Results are presented as the ratio of each gene to Gapdh. Data shown represent the mean ± SD, with n = 6. Differences of values are not significant at ‸ p > 0.05 as indicated.
To verify that Slug was specifically knocked down by induction of Slug antisense, we examined the expression of other genes. Of particular importance was the assessment of Snail expression, which we found is also up-regulated by HGF in our model with kinetics similar to that of Slug, although to much lower levels, as shown using quantitative RT-PCR (Supplementary Figure S1 and Figure 5C). HGF-induced up-regulation of Snail is not affected by addition of ponasterone and induction of Slug antisense (Figure 5C). The expression of E-cadherin is also not affected by addition of ponasterone (Supplementary Figure S5). These results confirm that ponasterone does not have a toxic effect and that with our approach, we do not induce a broad effect on transcription, but we are able to knockdown Slug protein in an inducible and specific manner.
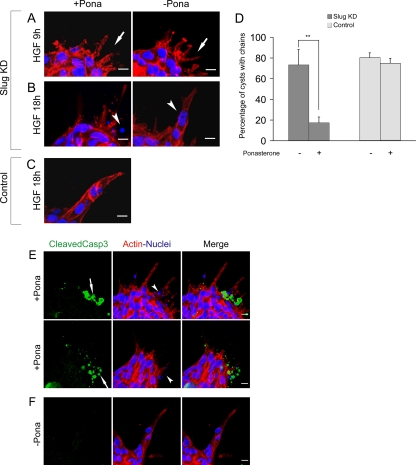
We next analyzed the phenotypic effect of Slug knockdown. Slug knockdown cysts treated with HGF in presence of ponasterone were fixed and stained for actin and nuclei at different time points of the p-EMT phase for analysis by confocal microscopy. Appearance of normal cellular extensions from cysts was observed after HGF treatment despite Slug being knocked down, confirming that Slug is not required for the initial cell movement associated with p-EMT (Figure 6A). However, observation of later time points revealed that as a consequence of Slug knockdown, a large majority of cysts were subsequently devoid of chains, and this defect was not observed in the absence of ponasterone. Control cysts were not affected by the presence of ponasterone, confirming that the effect is due to Slug knockdown and not to a toxic effect of ponasterone (Figure 6, B and C). Quantification of the effect confirmed a marked reduction of cysts displaying chain formation; 17.5 ± 5.4% of the cysts had chains when Slug was knocked down compared with 73.5 ± 14.7% when ponasterone was not added, whereas control cysts have a similar percentage of cysts displaying chain formation in the presence or absence of ponasterone (Figure 6D).
Figure 6.
Slug knockdown results in apoptotic death during the formation of chains. Cysts formed with ponasterone inducible Slug knockdown cells were treated with 10 ng/ml HGF and analyzed by confocal microscopy at different time points of chain formation in the absence or presence of 10 μM of ponasterone. Slug KD is Slug knockdown transfectant cells. Control is parental cells. (A and B) Slug knockdown cysts after 9 h (A) or 18 h (B) of HGF treatment. (A) In the absence or presence of ponasterone, normal extensions are formed (arrows). (B) In the presence of ponasterone, disintegrated nuclei are seen (arrowhead) instead of normal nuclei in chains in the absence of ponasterone (arrowhead) (C) Control Cysts have chains in the presence of ponasterone. (D) Quantification of the percentage of cysts with chains for Slug knockdown and control in the absence or presence of 10 μM of ponasterone. For each experiment, three independent fields were counted and an average of 30 cysts were used to determine the percentage of cysts with normal chains. Results present the mean ± SD of the percentages with n = 6 for Slug knockdown and n = 4 for the control. Differences of values are significant at ** p < 0.01 as indicated in the figure. (E and F) Confocal analysis of the expression of cleaved caspase 3 on Slug knockdown cysts treated for 18 h with HGF in the presence of 10 μM of ponasterone (E) or absence of ponasterone (F). (E) Arrows show a strong staining for cleaved caspase 3 at the surface of 2 representative cysts. In the same area, disintegrated nuclei are also seen (arrowheads). Quantification from five independent experiments and an average of 50 cysts for each experiment show that only an average of 2.9 ± 2.0% of Slug knockdown cysts are not presenting any staining for cleaved caspase 3, whereas 52.3 ± 15.1% are strongly positive for this marker. The remainingcysts are presenting some staining and various degree of abnormal chain formation. (F) For the majority of the cysts in the absence of ponasterone, chains are observed and cleaved caspase3 staining is not detected.
Cysts that did not have chains because of Slug knockdown displayed small disintegrated nuclei around their external surface, which were reminiscent of nuclei of apoptotic cells (Figure 6B). We thus stained these cysts using an antibody against the cleaved form of caspase 3, a marker of apoptosis. Figure 6E shows that these cysts had at their surface strong labeling for cleaved caspase 3 intermixed with some extensions. This suggests that cells die by apoptosis instead of forming chains upon Slug knockdown. For Slug knockdown cysts only an average of 2.9 ± 2.0% were not presenting any staining for cleaved caspase 3 while 52.3 ± 15.1% were strongly positive for this marker. The remaining cysts were presenting some staining and various degrees of abnormal chain formation. In contrast, when ponasterone was not added and then Slug expression was not blocked, only 10.9 ± 5.8% cysts presented cleaved caspase 3 staining associated with abnormal chain formation and the majority of the cysts had normal chains and no cleaved caspase 3 staining (Figure 6F).
P53 mRNA Expression Is Down-regulated during HGF-induced p-EMT Phase
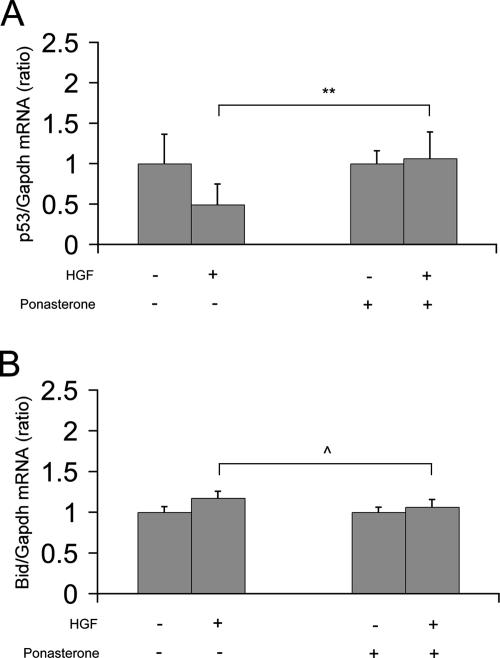
In epithelial cells, Slug expression promotes resistance to programmed cell death elicited by DNA damage by directly repressing the transcription of factors known to be involved in programmed cell death, in particular the proapoptotic factors p53 and Bid (Kajita et al., 2004). We then tested if in our model these factors were part of the downstream pathway of Slug and were repressed by Slug. Figure 7A shows that p53 mRNA levels were down-regulated after 16 h of HGF stimulation of cysts. In contrast, p53 mRNA levels were not changed when cysts were knockdown for Slug. However, Bid mRNA levels were not changed both in normal and Slug knockdown cysts (Figure 7B). Our results with p53 suggest that p53 is a direct target gene of Slug in our 3D system and that during the p-EMT of MDCK tubulogenesis, Slug acts as an anti-apoptotic factor by at least repressing p53 expression. In contrast to the work reported by Kajita et al. (2004), Bid mRNA levels were not observed to change in our system indicating that Bid is not a target gene of Slug in our system. This difference could reflect the fact that different downstream pathways are used, depending on the events that trigger programmed cell death.
Figure 7.
p53 mRNA expression is down-regulated during HGF-induced p-EMT phase. Cysts formed with ponasterone-inducible Slug knockdown cells were treated with 10 ng/ml HGF for 16 h or not treated, in the absence or presence of 10 μM ponasterone. Total RNA were extracted and analyzed for the expression of p53 mRNA (A) or Bid mRNA (B) by quantitative RT-PCR. Results are presented as the ratio of each gene to Gapdh. Data shown represent the mean ± SD, with n > 3. Differences of values are significant at ** p < 0.01 or not significant at ‸ p > 0.05 as indicated.
Our results confirm that Slug is not involved in the initial formation of the extensions, which mark the induction of the p-EMT phase during tubulogenesis. Rather, these findings demonstrate that Slug knockdown causes the cells to die during the process of chain formation, probably by apoptosis, as shown by cleaved caspase 3 staining and p53 expression. This implies that Slug is required for the survival of cells during chain formation and reveals an important role for Slug as a modulator of cell survival during epithelial morphogenesis.
DISCUSSION
HGF-induced MDCK tubulogenesis provides a model of an epithelial morphogenetic process involving cell migration without full EMT, which we refer as p-EMT. This model allowed us to monitor Slug expression and function while following the sequence of events of the p-EMT process. We show that although Slug is among the early HGF-induced genes, Slug protein is not expressed until several hours after the cells start to form extensions, a cell movement process which initiates the p-EMT phase of tubulogenesis. We also present evidence that in this system Slug does not act as a repressor of E-cadherin. By knocking down Slug, we demonstrate that Slug is not required for the initial cell extension movement associated with p-EMT and instead show that Slug is required for cell survival during p-EMT. Taken together our results show that Slug is required for cell survival rather than being an inducer of the cell movement associated with migration during epithelial morphogenesis. Furthermore, our results also reveal that the Slug survival function, rather than being only acquired concomitantly with full EMT, can be the primary function of Slug during a process involving p-EMT.
A large body of evidence points to Slug as a key regulator of EMT, both from its ability to induce EMT and from its ability to repress the adhesion molecule E-cadherin and desmosomal components in various epithelial cells (Savagner et al., 1997; Bolos et al., 2003). These results are reinforced by a correlation in various epithelial cancer cells between Slug expression and loss of E-cadherin expression, a hallmark of invasive cells (Shih et al., 2005; Uchikado et al., 2005). In our MDCK 3D system, E-cadherin is not lost but rather is redistributed during the p-EMT phase of MDCK tubulogenesis (Pollack et al., 1998). Our results show that this redistribution, which allows cells to migrate while remaining attached to their neighbors, is not accompanied by a decrease in E-cadherin levels despite high levels of Slug in the nuclei.
Slug has been recently reported to be involved in cell migration during re-epithelialization of adult keratinocytes, a process that also does not require full EMT. During this process, E-cadherin is not found repressed in cells expressing Slug but a functional relationship was observed between Slug expression and desmosome density. Although Slug is thought to repress the expression of some desmosomal components, there is no evidence that Slug is a direct repressor of desmosomal genes (Savagner et al., 1997, 2005). Dissociation of desmosomes is an initial and necessary step for these cells to start migrating. During extension formation in MDCK tubulogenesis, desmosomal components are also redistributed by internalization (Pollack et al., 1998). However, Slug expression is induced several hours after the first cellular extensions are formed and extensions still form when Slug is knocked down, precluding a role for Slug in the dissolution of desmosomes during MDCK tubulogenesis.
We show that during MDCK tubulogenesis Slug is not involved in the induction of p-EMT. We also found an HGF-induced up-regulation of Snail mRNA levels with kinetics similar to that of Slug although Snail expression increases at much lower levels than Slug (Figure 5C and Supplementary Figure S1). However, Snail has been reported to be a more potent inducer of EMT and repressor of E-cadherin than Slug (Batlle et al., 2000; Cano et al., 2000). In addition the analogous role of Slug in chick neural crest migration is instead fulfilled in mammals by Snail (Sefton et al., 1998). Because of the absence of available Snail antibodies that work in our system, we cannot rule out that Snail protein is expressed earlier than Slug protein and Snail may be the inducer of p-EMT. However, as E-cadherin expression is not repressed in our system, if Snail has a role in induction of p-EMT, it is also not as a repressor of E-cadherin.
Our work demonstrates a survival role for Slug during the p-EMT phase of MDCK tubulogenesis and suggests that Slug rescues the cells from apoptosis by repressing proapoptotic factors that otherwise would lead the cells to die during p-EMT. In our model, p53 is downstream of Slug, and this is in agreement with the work reported by Kajita et al. showing that in epithelial cells p53 is directly repressed by Slug rather than Slug being a downstream target of p53 as it has been demonstrated in mouse hematopoietic progenitor cells (Inoue et al., 2002; Wu et al., 2005). In addition, in our cells, we did not detect any expression of the proapoptotic factor Puma (data not shown), which is directly repressed by Slug in hematopoietic progenitor cells. Our results reinforce the differences already underlined by Kajita et al. between apoptotic pathways involved in epithelial cells and hematopoietic cells. We also cannot exclude that the factors involved in the apoptotic pathways are different also depending on what triggers apoptosis.
In epithelial cells, both Slug and Snail have a role as inducers of EMT but they have also been reported to confer survival properties to cells executing full EMT and suggested to be inducers of cell movement in some processes (Valdes et al., 2002; Vega et al., 2004; Savagner et al., 2005; Robson et al., 2006). Taken collectively, the data concerning the transcription factors of the Snail family in the animal kingdom lead to the interesting hypothesis that these factors could act primarily as survival factors and inducers of cell movement rather than as inducers of EMT (Barrallo-Gimeno and Nieto, 2005). Recently, Slug/Snail have been proposed to be involved in survival of premigratory neural crest during development (Cheung et al., 2005). Our work shows that Slug's primary function during MDCK tubulogenesis is cell survival, and we are able to dissociate this function from the initiation of cell movement. Slug knockout have been recently reported to block cell migration during re-epithelialization of keratinocytes (Savagner et al., 2005), and it will be of interest to test if in that system Slug is also primarily involved in survival rather than in cell movement per se.
The link between cell survival and cell migration is particularly interesting in the context of the ECM as a large body of evidence emphasizes the importance of the ECM during morphogenesis (Boudreau and Bissell, 1998; Bissell et al., 2002). 3D culture models that integrate the complexity of multicellular architecture of cells embedded in ECM have proved important in unraveling the mechanisms involving modifications of cell–cell and cell–ECM interactions during morphogenesis (O'Brien et al., 2002; Debnath and Brugge, 2005; Nelson and Bissell, 2005). In our 3D model, Slug protein is only expressed in cells forming chains, which correlates with the replacement of cell–cell contacts by cell–ECM interactions. In 2D cell culture, disruption of adherens junctions with an anti-E-cadherin antibody can lead to the induction of Slug expression showing that Slug expression can be a consequence of the disruption of cell–cell interactions (Conacci-Sorrell et al., 2003). Our 3D model reveals the importance of the control of cell survival during a process that involves migration. In contrast, Slug knock down does not result in apoptosis when tested in a scratch-wound healing assay (data not shown). This reinforces the idea that the Slug survival function may be required only in the context of migration within the 3D ECM environment; the MDCK tubulogenesis model provides a system to further investigate the relationship between Slug functions in survival and movement.
Our work also reveals that cell survival is the primary function of Slug in an EMT-related process. Slug or Snail confer survival properties that seems to be acquired by the cells concomitantly with full EMT (Valdes et al., 2002; Vega et al., 2004; Robson et al., 2006). Our work suggests that Slug's survival function temporally precedes its role in inducing full EMT. The great majority of the work done on Slug and Snail in cell culture has used cancer cells or cells experimentally overexpressing Slug or Snail. Although these studies continue to provide new insights, such overexpression conditions could mask the primary function of these genes. It has been suggested recently that EMT is a sequential multistep program and that the loss of E-cadherin transcription, a hallmark of invasive cancer cells, is among the late steps of the process (Grunert et al., 2003; Huber et al., 2005). The fact that we find that both Slug and Snail are part of the HGF-induced genes during MDCK tubulogenesis supports this idea. The 3D MDCK tubulogenesis model allows us to follow the early steps of loss of epithelial polarity as cells are fully polarized in the cysts wall before HGF treatment, and this model provides access to the early steps of the EMT program. However, in this model EMT is controlled and cells never reach a full EMT. Given the multistep nature of EMT, we suggest that Slug (and Snail) may have different functions in different steps of EMT. In particular, in the steps reached by the cells during p-EMT in MDCK tubulogenesis, Slug appears to have primarily a survival function. In other steps of EMT or in full EMT, Slug may have other functions, such as an inducer of cell movement or a repressor of E-cadherin. This view provides an interesting framework to fully understand the function of these transcription factors and also implies that the Slug and Snail target genes may be different depending on the stage of EMT and other contextual factors. For instance in contexts where Slug or Snail are overexpressed and/or ectopically expressed in culture, they can be repressors of E-cadherin, desmosomal components, or other cell–cell junction molecules and thus inducers of full EMT (Savagner et al., 1997, 2005; Batlle et al., 2000; Bolos et al., 2003; Cano et al., 2000; Ikenouchi et al., 2003, 2005). Understanding what leads Slug or Snail to change from survival factors to inducers of cell movement or repressors of E-cadherin and other cell–cell adherent molecules could be of major importance in understanding their role in pathologies such as cancer invasion and fibrosis.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the late Ralph Schwall (Genentech, South San Francisco) for HGF, Tzuu-Shuh Jou (National Taiwan University, Tapei) for MDCK T23T.1 cells, Pierre Savagner (INSERM EMI 229, Montpellier) for advice on raising Slug antibody. We thank Lucy O'Brien for critical reading of the manuscript and helpful discussion. We also acknowledge David Bryant, Elsa Ndiaye-Dulac, Naoki Tanimizu, Dennis Eastburn, and Fernando Martin-Belmonte for comments on the manuscript. P.L. was supported by the French National Center for Scientific Research (CNRS). This work was supported by National Institutes of Health grants to K.E.M.
Abbreviations used:
- EMT
epithelial-mesenchymal transition
- HGF
hepatocyte growth factor
- p-EMT
partial epithelial-mesenchymal transition.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0823) on March 7, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Barrallo-Gimeno A., Nieto M. A. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- Batlle E., Sancho E., Franci C., Dominguez D., Monfar M., Baulida J., Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Birchmeier W., Brand-Saberi B. Epithelial-mesenchymal transitions in cancer progression. Acta Anat. (Basel) 1996;156:217–226. doi: 10.1159/000147848. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G. F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Radisky D. C., Rizki A., Weaver V. M., Petersen O. W. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco M. J., Moreno-Bueno G., Sarrio D., Locascio A., Cano A., Palacios J., Nieto M. A. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- Bolos V., Peinado H., Perez-Moreno M. A., Fraga M. F., Esteller M., Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Bissell M. J. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr. Opin. Cell Biol. 1998;10:640–646. doi: 10.1016/s0955-0674(98)80040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N. J., Varner J. A. The homeobox transcription factor Hox D3 promotes integrin alpha5beta1 expression and function during angiogenesis. J. Biol. Chem. 2004;279:4862–4868. doi: 10.1074/jbc.M305190200. [DOI] [PubMed] [Google Scholar]
- Cano A., Perez-Moreno M. A., Rodrigo I., Locascio A., Blanco M. J., del Barrio M. G., Portillo F., Nieto M. A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Cheung M., Chaboissier M. C., Mynett A., Hirst E., Schedl A., Briscoe. J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell. 2005;8:179–192. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M., Simcha I., Ben-Yedidia T., Blechman J., Savagner P., Ben-Ze'ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J. Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J., Brugge J. S. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- Grunert S., Jechlinger M., Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 2003;4:657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- Hay E. D. An overview of epithelio-mesenchymal transformation. Acta Anat. (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Huber M. A., Kraut N., Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J., Furuse M., Furuse K., Sasaki H., Tsukita S., Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J., Matsuda M., Furuse M., Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- Inoue A., et al. Slug, a highly conserved zinc finger transcriptional repressor, protects hematopoietic progenitor cells from radiation-induced apoptosis in vivo. Cancer Cell. 2002;2:279–288. doi: 10.1016/s1535-6108(02)00155-1. [DOI] [PubMed] [Google Scholar]
- Kajita M., McClinic K. N., Wade P. A. Aberrant expression of the transcription factors Snail and Slug alters the response to genotoxic stress. Mol. Cell. Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R., Neilson E. G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal S., Palm H. G., Poser I., Bosserhoff A. K. Snail-regulated genes in malignant melanoma. Melanoma Res. 2005;15:305–313. doi: 10.1097/00008390-200508000-00012. [DOI] [PubMed] [Google Scholar]
- Lai J. F., Juang S. H., Hung Y. M., Cheng H. Y., Cheng T. L., Mostov K. E., Jou T. S. An ecdysone and tetracycline dual regulatory expression system for studies on Rac1 small GTPase-mediated signaling. Am. J. Physiol. Cell Physiol. 2003;285:C711–C719. doi: 10.1152/ajpcell.00064.2003. [DOI] [PubMed] [Google Scholar]
- Leroy P., Berto F., Bourget I., Rossi B. Down-regulation of Hox A7 is required for cell adhesion and migration on fibronectin during early HL-60 monocytic differentiation. J. Leukoc. Biol. 2004;75:680–688. doi: 10.1189/jlb.0503246. [DOI] [PubMed] [Google Scholar]
- Montesano R., Schaller G., Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell. 1991;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- Nelson C. M., Bissell M. J. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin. Cancer Biol. 2005;15:342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M. A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Nieto M. A., Sargent M. G., Wilkinson D. G., Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Jou T. S., Pollack A. L., Zhang Q., Hansen S. H., Yurchenco P., Mostov K. E. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Tang K., Kats E. S., Schutz-Geschwender A., Lipschutz J. H., Mostov K. E. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev. Cell. 2004;7:21–32. doi: 10.1016/j.devcel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Zegers M. M., Mostov K. E. Opinion: building epithelial architecture: insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Peinado H., Quintanilla M., Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- Pollack A. L., Runyan R. B., Mostov K. E. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev. Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- Robson E. J., Khaled W. T., Abell K., Watson C. J. Epithelial-to-mesenchymal transition confers resistance to apoptosis in three murine mammary epithelial cell lines. Differentiation. 2006;74:254–264. doi: 10.1111/j.1432-0436.2006.00075.x. [DOI] [PubMed] [Google Scholar]
- Romano L. A., Runyan R. B. Slug is an essential target of TGFbeta2 signaling in the developing chicken heart. Dev. Biol. 2000;223:91–102. doi: 10.1006/dbio.2000.9750. [DOI] [PubMed] [Google Scholar]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- Savagner P., Kusewitt D. F., Carver E. A., Magnino F., Choi C., Gridley T., Hudson L. G. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J. Cell. Physiol. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- Savagner P., Yamada K. M., Thiery J. P. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J. Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton M., Sanchez S., Nieto M. A. Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development. 1998;125:3111–3121. doi: 10.1242/dev.125.16.3111. [DOI] [PubMed] [Google Scholar]
- Shih J. Y., et al. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin. Cancer Res. 2005;11:8070–8078. doi: 10.1158/1078-0432.CCR-05-0687. [DOI] [PubMed] [Google Scholar]
- Thiery J. P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Uchikado Y., Natsugoe S., Okumura H., Setoyama T., Matsumoto M., Ishigami S., Aikou T. Slug Expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin. Cancer Res. 2005;11:1174–1180. [PubMed] [Google Scholar]
- Valdes F., Alvarez A. M., Locascio A., Vega S., Herrera B., Fernandez M., Benito M., Nieto M. A., Fabregat I. The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor Beta in fetal rat hepatocytes. Mol. Cancer Res. 2002;1:68–78. [PubMed] [Google Scholar]
- Vega S., Morales A. V., Ocana O. H., Valdes F., Fabregat I., Nieto M. A. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F., Rossert J., Mauviel A. Blocking sp1 transcription factor broadly inhibits extracellular matrix gene expression in vitro and in vivo: implications for the treatment of tissue fibrosis. J. Invest. Dermatol. 2001;116:755–763. doi: 10.1046/j.1523-1747.2001.01326.x. [DOI] [PubMed] [Google Scholar]
- Wu W. S., Heinrichs S., Xu D., Garrison S. P., Zambetti G. P., Adams J. M., Look A. T. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.