Abstract
The intracellular trafficking of Arn1, a ferrichrome transporter in Saccharomyces cerevisiae, is controlled in part by the binding of ferrichrome to the transporter. In the absence of ferrichrome, Arn1 is sorted directly from the Golgi to endosomes. Ferrichrome binding triggers the redistribution of Arn1 to the plasma membrane, whereas ferrichrome transport is associated with the cycling of Arn1 between the plasma membrane and endosomes. Here, we report that the clathrin adaptor Gga2 and ubiquitination by the Rsp5 ubiquitin ligase are required for trafficking of Arn1. Gga2 was required for Golgi-to-endosomal trafficking of Arn1, which was sorted from endosomes to the vacuole for degradation. Trafficking into the vacuolar lumen was dependent on ubiquitination by Rsp5, but ubiquitination was not required for plasma membrane accumulation of Arn1 in the presence of ferrichrome. Retrograde trafficking via the retromer complex or Snx4 was also not required for plasma membrane accumulation. High concentrations of ferrichrome led to higher levels of ubiquitination of Arn1, but they did not induce degradation. Without this ubiquitination, Arn1 remained on the plasma membrane, where it was active for transport. Arn1 was preferentially modified with polyubiquitin chains on a cluster of lysine residues at the amino terminus of the transporter.
INTRODUCTION
The transition metals iron, copper, zinc, and manganese are essential nutrients for all eukaryotes, because they are required for the activity of a variety of proteins involved in many cellular processes. Each of these metals is toxic when present in excess, and cells are protected from metal toxicity by precise regulation of the uptake, efflux, and use of nutrient metals. The primary locus of homeostatic control is at the level of the metal transporters. The activities of these proteins that move metals across membranes are controlled by a combination of transcriptional, translational, and posttranslational mechanisms. In mammals, many metal transporters are regulated at the transcriptional level by the abundance of their cognate metal. Transcript levels of the ZnT family of zinc efflux proteins and the ZIP family of zinc importers are reciprocally regulated by zinc (Liuzzi et al., 2004). DMT1, an iron importer, and ferroportin, an iron exporter, exhibit coordinated transcriptional regulation by iron in cultured cells, animal models, and human intestinal tissues (Zoller et al., 2001, 2002; Dupic et al., 2002). Some mRNA transcripts for DMT1 and ferroportin contain iron-responsive elements, which are RNA structures that permit binding of iron-regulatory proteins, and thus they raise the possibility of translational/posttranscriptional control by iron (Gruenheid et al., 1995; McKie et al., 2000).
Metal transport processes in eukaryotes are also regulated posttranslationally by alterations in the intracellular trafficking of the transporter proteins. In humans, copper is exported from cells by the Cu-ATPases 7A and 7B, which encode the transporters mutated in Menkes and Wilson diseases, respectively (Suzuki and Gitlin, 1999; Lutsenko and Petris, 2003). Both transporters undergo relocalization in the late secretory pathway when cells are exposed to copper. The Menkes transporter moves from the trans-Golgi network (TGN) to the basolateral plasma membrane, and the Wilson transporter moves from the TGN to secretory vesicular membranes located near bile canaliculi in the liver. Ferroportin, the vertebrate iron exporter, undergoes endocytosis and degradation when it binds hepcidin, the iron regulatory hormone (Nemeth et al., 2004).
In yeast, high-affinity transporters of nutrient metals are homeostatically regulated by their cognate metals at the transcriptional level (Rutherford and Bird, 2004). Genes encoding transporters that facilitate the uptake of iron, copper, and zinc are all activated by transcription factors that specifically respond to the depletion of the cognate metal. Supplementation with the cognate metal rapidly switches off this transcriptional activation. In addition to this transcriptional regulation, metal supplementation also triggers the rapid degradation of some of these transporter proteins. The manganese transporter Smf1p, the zinc transporter Zrt1p, and the iron transporter complex Fet3p/Ftr1p are removed from the plasma membrane and transferred to the vacuole for degradation in response to increased levels of their respective metal substrates (Liu and Culotta, 1999; Gitan and Eide, 2000; Felice et al., 2005). Increased uptake of their cognate metal ions also triggers the ubiquitination of these transporters, and the attachment of the ubiquitin (Ub) moieties is thought to trigger endocytosis and trafficking of the transporters to the vacuole (Eguez et al., 2004). Ubiquitination of cytosolic proteins frequently leads to their degradation in the proteasome, whereas ubiquitination of integral membrane proteins of the late secretory pathway leads to changes in intracellular trafficking (Hicke and Dunn, 2003). Ubiquitination of Zrt1p and Smf1p is dependent on the Ub ligase Rsp5p (Gitan and Eide, 2000; Hettema et al., 2004). RSP5 is an essential gene and the only member of the Rsp5/Nedd4 family of HECT domain Ub ligases in yeast (Dupre et al., 2004). To date, Rsp5 is the only Ub ligase that is known to be involved in the ubiquitination of plasma membrane proteins in yeast.
In addition to the high-affinity ferrous iron transporter Fet3p/Ftr1p, yeast express another group of iron transporters that undergo changes in intracellular trafficking in response to their metal substrates (Philpott, 2006). The trafficking of Arn1p, the yeast ferrichrome (FC) transporter, differs from the other metal transporters in that the substrate does not trigger the degradation of the transporter, but rather the substrate triggers the recruitment of the transporter to the plasma membrane (Kim et al., 2002). Arn1p also differs from the other metal transporters in that it transports iron in the form of a siderophore–iron chelate rather than as a free ion (Heymann et al., 2000; Yun et al., 2000b; Moore et al., 2003). Siderophores are small organic molecules that bind ferric iron with exceptionally high affinity and specificity (Neilands, 1995). They are synthesized and secreted in their iron-free form by many species of bacteria and fungi as well as some species of plants. The secreted siderophores bind ferric iron, and the iron–siderophore chelates can be taken up by specific cellular transport systems. Although Saccharomyces cerevisiae does not synthesize or secrete siderophores, it can take up the siderophores secreted by a variety of species of bacteria and fungi. A family of four homologous transporters of the major facilitator superfamily is expressed in S. cerevisiae in response to iron deprivation (Yun et al., 2000a), and each transporter exhibits specificity for different classes of siderophores. FC, a prototypical hydroxamate siderophore secreted by many species of fungi, is taken up through Arn1p and Arn3p/Sit1p (Lesuisse et al., 1998; Heymann et al., 2000; Yun et al., 2000a,b). FC–iron chelates accumulate in the cytosol after uptake, and these chelates constitute a stable form of iron storage in S. cerevisiae, as they do in other species of fungi (Oberegger et al., 2001; Moore et al., 2003).
FC also directs the intracellular trafficking of Arn1p and Arn3p. When Arn1p is expressed in the absence of FC, the transporter is sorted directly from the TGN to late endosomes (Kim et al., 2002). When cells are exposed to low concentrations of FC, the siderophore is thought to gain access to the endosomal compartment via fluid phase endocytosis, where it binds to a high-affinity receptor domain of Arn1p. FC binding at the receptor site is associated with a redistribution of Arn1p to the plasma membrane, and mutations in extracytosolic loops of Arn1p that impair high-affinity binding also inhibit relocalization to the plasma membrane (Kim et al., 2004). At higher FC concentrations, Arn1p efficiently transports FC into the cytosol, and this transport activity is associated with the cycling of Arn1p between the plasma membrane and endosomal compartments. Ubiquitinated forms of Arn1p can be detected, and, in the presence of high concentrations of FC, this modification is dependent on Rsp5p. Here, we show that ubiquitination is required for multiple steps in the trafficking of Arn1p and that Arn1p is modified by poly-Ub chains attached at multiple sites on the protein.
MATERIALS AND METHODS
Yeast Strains, Plasmids, and Media
Strains were derived from YPH499 (MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1) and BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0). FET3 was deleted in LHY23 (MATa ura3 leu2 trp1 bar1 GAL rsp5-1) as described previously (Philpott et al., 1998), and the resulting strain was mated with the RSP5+ parent strain LHY299. The resulting diploid strain was sporulated and the following congenic haploid strains were isolated: YKY102 (MATa ura3 leu2 trp1 ade2 bar1), YKY103 (MATa ura3 leu2 trp1 ade2 bar1 rsp5-1), YKY104 (MATa ura3 leu2 trp1 ade2 bar1 fet3::URA3), and YKY105 (MATa ura3 leu2 trp1 ade2 bar1 rsp5-1 fet3::URA3). The strains pep4Δ, gga2Δ, vps5Δ, vps35Δ, snx4Δ, tul1Δ, npr1Δ, and bsd2Δ are congenic to BY4742 and were obtained from the complete yeast deletion collection (American Type Culture Collection, Manassas, VA). The strain expressing Arn1-green fluorescent protein (GFP) was obtained from the yeast GFP clone collection (Invitrogen, Carlsbad, CA). Arn1–GFP fusion proteins have been shown to be functional in Candida albicans (Hu et al., 2002), Fusarium graminearum (Park et al., 2006), and S. cerevisiae (C. W. Yun, personal communication). GGA2 was deleted in other strains by transforming then with the KanMX cassette amplified from the strain gga2Δ. The strain bul1Δbul2Δ was derived from YPH499 and was a kind gift from F. Abe (Abe and Iida, 2003).
The plasmids pArn1-hemagglurinin (HA) and pMetArn1-HA were described previously (Yun et al., 2000a,b). Plasmids containing lysine-to-arginine substitutions of Arn1p were constructed in pMetArn1-HA as described previously (Kim et al., 2004) by using the oligonucleotides listed in Table 1, and mutations were confirmed by sequencing. The plasmid pArn1-HA(ADE) was constructed by replacing the URA3 gene of pArn1-HA with the ADE2 gene amplified from the plasmid YIpDCE1 (Stearman et al., 1998). The plasmid YEp96 contains a synthetic yeast Ub open reading frame under the control of the copper-inducible CUP1 promoter (Ecker et al., 1987). Plasmids containing mutant Ub were derived from YEp96 and were a generous gift of J. M. Galan (Galan and Haguenauer-Tsapis, 1997).
Table 1.
Oligonucleotides used to construct Arn1p lysine substitution mutants
| Mutation | Oligonucleotide sequencea |
|---|---|
| K11R | CACTCTCGCGATCCTGTTAGGGAGGAAAAGAAACATGTC |
| K14R | GATCCTGTTAAGGAGGAAAGGAAACATGTCTTCATGGGA |
| K15R | CCTGTTAAGGAGGAAAAGAGACATGTCTTCATGGGAATG |
| K259R | ATGCAATGGAGGGCCAGAAGGACTCCTGAATGGCACGCT |
| K267R | CCTGAATGGCACGCTTTGAGGGGCCAGAAGTCCTATTAT |
| K270R | CACGCTTTGAAGGGCCAGAGGTCCTATTATCAAGAACAT |
| 3NKR | CACTCTCGCGATCCTGTTAGGGAGGAAAGGAGACATGTCTTCATGGGAATGGAA |
| 3CKR | ATTTTGGATTGGTTTGAAAGACTTCCATCAAGATTCACTTTTAGACGGGAAGAACAAAAGCTG |
a Underlined sequences indicate site of arginine substitution.
Synthetic complete (SC) medium and iron-poor medium containing 10 μM ferrous ammonium citrate and 100 μM ferrozine were prepared as described previously (Sherman, 1991; Philpott et al., 1998). FC was added at the indicated concentrations as the ferric chelate. Copper sulfate was added at 100 μM for the induction of CUP1-Ub expression.
Fluorescence Microscopy
Strains were transformed with pArn1-HA and grown in iron-poor medium to mid-log phase at 30°C. Where indicated, strains were grown at 25°C and shifted to 37°C for 2 h before fixation and preparation for fluorescence microscopy (Stearman et al., 1996; Yun et al., 2000a). Where indicated, epifluorescence images were obtained as a vertical series by using IPLabs software and a charge-coupled device camera (Hamamatsu, Bridgewater, NJ). Images were then analyzed by digital deconvolution (Huygens Essential; Scientific Volume Imaging, Hilversum, The Netherlands), and individual deconvolved images are presented.
Half-Life Determination, Immunoprecipitation, and Western Blotting
For half-life determinations, cycloheximide and methionine at 5 μg/ml culture were added to cells in mid-log phase, aliquots of the culture were removed at intervals, 20 mM NaN3 and 20 mM KF were added, and cells were collected. For immunoprecipitations, cells in mid-log phase were treated with 20 mM NaN3 and 20 mM KF for 10 min before harvesting. Immunoprecipitations and Western blotting were performed as described previously (Philpott et al., 1998), with the following modifications. Cells were disrupted by glass bead lysis in 50 mM Tris, pH 7.5, 0.3 M NaCl, 5 mM EDTA, 1 mM dithiothreitol, and protease inhibitors. Unbroken cells and cellular debris were cleared by centrifugation at 500 × g for 5 min. Membranes were collected from the 500 × g supernatant by centrifugation at 18,000 × g for 60 min at 4°C. Membranes were solubilized on ice in lysis buffer containing 1% dodecylmaltoside (Anatrace, Maumee, OH) for 30 min. Membrane proteins were quantitated and used directly for Western blotting. Alternatively, solubilized membrane proteins were used for immunoprecipitations with 1 μl of anti-HA antibody and protein G-Sepharose. Western blots were performed using anti-HA antibody (Covance, Princeton, VA) or anti-Ub antibody P4D1 (Covance) at 1:5000 dilution.
Ferrichrome Uptake and Binding Assays
Cells were grown to mid-log phase in iron-poor medium at 25°C, and then cultures were divided. One set was shifted to 37°C for 1 h. FC uptake assays were performed as described using [55Fe]-FC at a final concentration of 4 μM (Yun et al., 2000a). Uptake was allowed to proceed at 25°C or 37°C for 30 min. Duplicate samples were incubated at 0°C to determine surface binding of FC, and these values were subtracted from those at the reaction temperature. Surface binding of [55Fe]-FC was performed as described previously (Kim et al., 2002) at 0°C in the presence of 20 mM NaN3 and KF and 100 nM [55Fe]-FC. For experiments using pMET-Arn1-HA, cells were grown in SC medium containing methionine at 10 mg/l.
RESULTS
Sorting of Arn1p to Vacuole for Degradation in the Absence of Ferrichrome
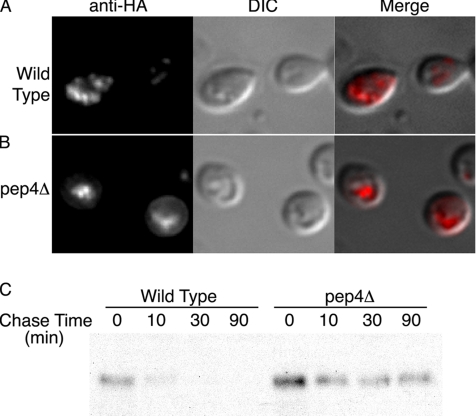
We investigated whether endosomal Arn1p underwent further trafficking to the vacuole in the absence of FC. Integral membrane proteins that are destined for degradation in the vacuole are sorted into intraluminal vesicles that accumulate within endosomes before fusion with the vacuolar membrane. These specialized organelles of the late endosomal compartment are known as the multivesicular body (MVB) (Katzmann et al., 2002). A series of three high-molecular-weight, multiprotein complexes, known as the ESCRT complexes, function in the sorting of ubiquitinated membrane proteins into the luminal vesicles of the MVB (Hurley and Emr, 2006). VPS4 encodes an AAA-ATPase that is required for the dissociation of the ESCRT complexes from the surface of the MVB. We previously demonstrated that Arn1p was detected in the exaggerated MVB that accumulates in the class E vacuolar sorting protein (vps) mutant strain vps4-ts (Babst et al., 1997; Kim et al., 2002). This observation suggested that Arn1p might also be sorted, via the MVB pathway, to the vacuole for degradation. We investigated this possibility by examining the localization of HA-tagged Arn1p by indirect immunofluorescence in a strain made deficient in the degradation of vacuolar proteins by deletion of the vacuolar protease Pep4p. Congenic wild-type and pep4Δ strains were transformed with a low-copy-number plasmid containing ARN1-HA under the control of the endogenous promoter. Strains were grown in iron-poor medium to mid-log phase, fixed, and prepared for indirect immunofluorescence microscopy. In a wild-type strain, Arn1p was detected in punctate intracellular vesicles that were previously demonstrated to be late endosomes (Figure 1A). In the pep4Δ strain, however, Arn1p was primarily detected within one or two large structures that colocalized with the vacuole (Figure 1B), indicating that Arn1p was targeted to the lumen of the vacuole for degradation. In this case, deletion of PEP4 would be predicted to prolong the half-life of Arn1p. To examine the rate of degradation of Arn1p, we transformed the congenic wild-type and pep4Δ strains with a low-copy-number plasmid containing ARN1-HA under the control of the methionine-regulatable MET3 promoter (pMET-Arn1-HA), grew transformants in methionine-free medium to mid-log phase, and halted new protein synthesis with the addition of cycloheximide and methionine. Cells were collected at intervals, and lysates were subjected to Western blotting (Figure 1C). We found that in the wild-type strain, Arn1p was rapidly degraded with a half-life of ∼10 min. In the pep4Δ strain, Arn1p was present at higher levels and exhibited a much longer half-life. These data indicated that in the absence of ferrichrome, Arn1p was rapidly sorted directly from the TGN to the vacuole for degradation, and it only transiently accumulated in the late endosome.
Figure 1.
Vacuolar sorting and degradation of Arn1p. (A) Accumulation of Arn1p in the vacuolar lumen of pep4Δ strain. BY4742 (Wild Type) and pep4Δ strains were transformed with pArn1-HA and grown in iron-poor medium to mid-log phase before fixation and preparation for indirect immunofluorescence. Mouse monoclonal HA.11 was the primary antibody and Cy-3–conjugated donkey anti-mouse was the secondary antibody. Images are in sets of three: fluorescence on the left, differential interference contrast (DIC) in the center, and the merged image on the right. (B) Increased stability of Arn1 in pep4Δ strain. BY4742 (Wild Type) and pep4Δ strains were transformed with pMetArn1-HA and grown in SC medium without methionine to mid-log phase. Cycloheximide and methionine were added, and aliquots of cells removed at the indicated times. Cells were lysed and equivalent amounts of cellular proteins were analyzed by SDS-PAGE and immunoblotting with anti-HA antibody.
Requirement of Ubiquitination for Vacuolar Degradation
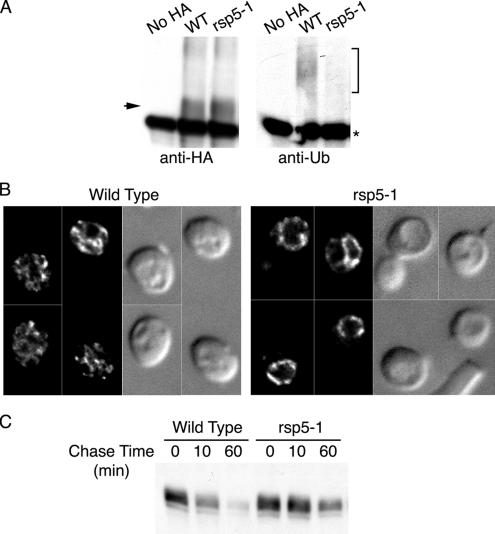
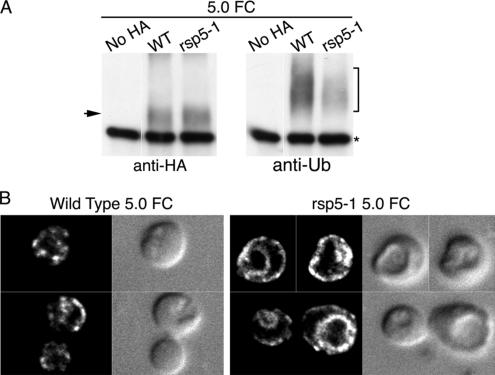
The covalent attachment of mono- or poly-Ub peptides to transmembrane proteins is required for their interaction with the ESCRT machinery and subsequent sorting into the MVB (Hurley and Emr, 2006). Ubiquitination is also proposed to regulate the sorting of the Gap1p amino acid permease at the TGN, with conditions that favor ubiquitination resulting in routing to the vacuole rather than routing to the plasma membrane (Magasanik and Kaiser, 2002). We therefore examined the roles of Ub and the Rsp5p Ub ligase in the trafficking of Arn1p to the vacuole. The ubiquitination of Arn1p was directly examined in congenic strains bearing the wild-type RSP5 allele or a temperature-sensitive mutant allele of RSP5, rsp5-1. This strain exhibits inactivation of Rsp5p and very low levels of ubiquitination of integral membrane proteins at the restrictive temperature (Dunn and Hicke, 2001). These strains also expressed Ub, under the control of the CUP1 promoter, and Arn1p-HA, under the control of the MET3 promoter. Cells were grown in Ub-inducing conditions at the permissive temperature (25°C) and then shifted to the restrictive temperature (37°C) in methionine-free medium for 2 h. Arn1p-HA was immunoprecipitated from cellular membranes and the precipitated Arn1p-HA was probed with either anti-HA or anti-Ub antibodies (Figure 2A). Higher-molecular-weight forms of Arn1p were readily detected in immunoblots from the wild-type strain probed for Ub, and these higher-molecular-weight forms were not detected in the rsp5-1 strain (Figure 2A, right). Comparable amounts of unmodified Arn1p were detected in both the wild type and rsp5-1 strain (Figure 2A, left). Ubiquitinated proteins were not detected in a strain that did not express HA-tagged Arn1p, confirming that the higher-molecular-weight species corresponded to modified Arn1p-HA. These data indicate that Arn1p was modified by ubiquitination and that this ubiquitination was dependent on Rsp5p.
Figure 2.
Requirement of Rsp5-dependent ubiquitination for vacuolar degradation of Arn1p. (A) Loss of Arn1p ubiquitination in the rsp5-1 strain. Strains YKY102 (Wild Type) and YKY103 (rsp5-1) were transformed with either pMetArn1-HA or the empty vector pRS316 (no HA) and YEp96. YEp96 expresses untagged ubiquitin from the CUP1 promoter. Transformed strains were grown at 25°C in SC medium without methionine and with 100 μM copper sulfate to induce expression of Arn1-HA and ubiquitin, respectively. Cultures were shifted to 37°C for 2 h, and then an equivalent number of cells was lysed, and the membranes were purified, detergent solubilized, and subjected to immunoprecipitation with anti-HA antibody. Immunoprecipitates were then subjected to Western blotting using an anti-HA antibody to detect Arn1-HA (left) or an anti-ubiquitin antibody to detect ubiquitinated Arn1-HA (right). The arrow indicates unmodified Arn1-HA and the bracket indicates ubiquitinated forms of Arn1. The asterisk indicates immunoglobulin heavy chain. (B) Accumulation of Arn1p at vacuolar membrane in the rsp5-1 strain. Strains YKY102 (Wild Type) and YKY103 (rsp5-1) were transformed with pArn1-HA and grown at 25°C in iron-poor medium to mid-log phase. Cultures were shifted to 37°C for 2 h before fixation and preparation for indirect immunofluorescence microscopy. Images were collected as a vertical series and analyzed by digital deconvolution. Images are in pairs with deconvolved fluorescence on the left and DIC on the right. (C) Increased stability of Arn1p in rsp5-1 strain. Strains YKY102 (Wild Type) and YKY103 (rsp5-1) were transformed with pMetArn1-HA and grown in methionine-free medium to mid-log phase at 25°C. Cultures were shifted to 37°C for 2 h before addition of cycloheximide and methionine, and aliquots of cells were removed at the indicated times. Cells were lysed and membranes were isolated and solubilized. Equivalent amounts of membrane protein were subjected to SDS-PAGE and immunoblotting with anti-HA antibody.
We next questioned whether a defect in Arn1p ubiquitination would alter the trafficking of Arn1p. Wild-type and rsp5-1 strains were transformed with pMETArn1-HA and incubated in methionine-free medium at the restrictive temperature before preparation for fluorescence microscopy (Figure 2B). Again, Arn1p was detected in endosomes in wild-type cells, but in the rsp5-1 strain, Arn1p was detected primarily in the limiting membrane of the vacuole, with a small amount detected in intracellular vesicles, and none detected at the surface of the cell. Membrane proteins destined for the vacuole that fail to be sorted into MVBs are delivered to the limiting membrane of the vacuole, rather than the vacuolar lumen, and they are resistant to degradation by vacuolar proteases. To examine the degradation of Arn1p in the rsp5-1 strain, the transformed strains were grown overnight in methionine-free medium at 25°C and then shifted to 37°C for 1 h before the addition of methionine and cycloheximide as described above (Figure 2C). Again, in wild-type cells, Arn1p exhibited a short half-life of ∼10 min, whereas in the rsp5-1 strain, the half-life of Arn1p was greatly prolonged. These data indicated that Rsp5p-dependent ubiquitination was required for the sorting of Arn1p into intraluminal vesicles of the MVB but that ubiquitination was not required for sorting to the vacuole and that Ub was not the signal that diverts Arn1p from secretory vesicles destined for the plasma membrane to the vps pathway.
Sorting of Arn1p to the Cell Surface without Ubiquitination
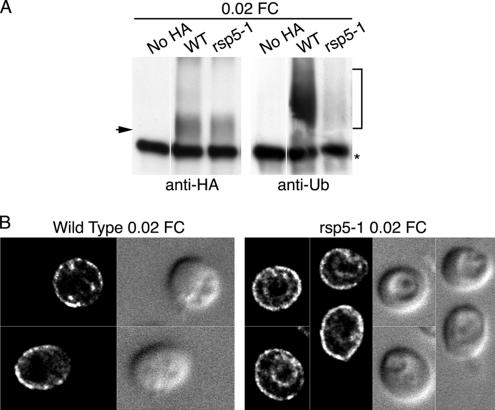
When cells expressing Arn1p are exposed to low concentrations of FC, that is, concentrations too low to permit uptake of FC through this transporter, Arn1p traffics to the plasma membrane. The binding of FC to a high-affinity receptor domain of Arn1p is thought to be a requirement for this trafficking, and phenylalanine residues in the carboxy terminus of Arn1p are also required for relocalization to the plasma membrane. We questioned whether ubiquitination was required for diversion to the plasma membrane by again examining the ubiquitination and localization of Arn1p in the rsp5-1 strain after treatment with low amounts of FC. Wild-type and rsp5-1 strains expressing ubiquitin and Arn1p-HA as in Figure 2A were incubated at 37°C in methionine-free medium containing 20 nM FC for 2 h. Western blotting of immunoprecipitated Arn1p indicated that similar amounts of Arn1p were present in both strains (Figure 3A, left) and that although ubiquitinated forms of Arn1p were readily detected in wild-type cells incubated in 20 nM FC, very little ubiquitinated Arn1p was detected in the rsp5-1 strain (Figure 3A, right). Immunofluorescence microscopy of the wild-type and rsp5-1 strains incubated in the presence of 20 nM FC demonstrated that Arn1p-HA localized predominately to the cell surface in both strains, with a small amount of signal in intracellular vesicles in the wild-type strain and in the vacuolar membrane in the rsp5-1 strain (Figure 3B). These data indicated that ubiquitination of Arn1p was not required for the FC-induced trafficking to the plasma membrane. These data also suggested that the FC-induced relocalization was not 100% efficient, as a small fraction of Arn1p accumulated at the vacuolar membrane rather than the plasma membrane in the rsp5-1 strain.
Figure 3.
Plasma membrane trafficking of Arn1p independent of ubiquitination. (A) Loss of ubiquitination in the rsp5-1 strain. Strains YKY102 (Wild Type) and YKY103 (rsp5-1) were transformed and grown at 25°C as in Figure 2A. Cultures were shifted to 37°C with the addition of 20 nM FC for 2 h, and cells were harvested and subjected to immunoprecipitation and SDS-PAGE as in Figure 2A. (B) Ubiquitin-independent relocalization of Arn1p to the plasma membrane. Strains YKY102 (Wild Type) and YKY103 (rsp5-1) were transformed and grown as in Figure 2B except that 20 nM FC was added for 2 h before preparation for fluorescence microscopy imaging.
Role of Gga2p in Sorting of Arn1p at the Late Golgi
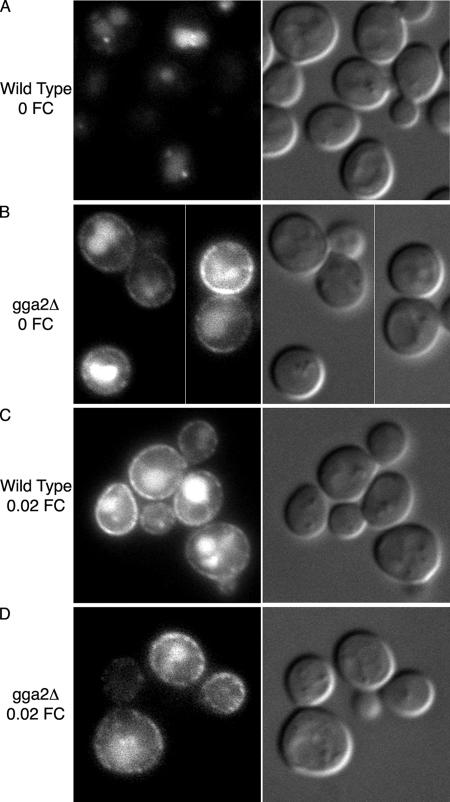
The clathrin adaptor proteins Gga1p and Gga2p mediate the trafficking of some transmembrane proteins from the TGN to endosomes via clathrin-coated vesicles (Bonifacino, 2004). Gga1p and 2p interact with multiple protein partners, including clathrin, ADP-ribosylation factors, and the yeast epsins Ent3 and Ent5, and recent reports suggest that Gga2p can act as a Ub receptor at the TGN by binding ubiquitinated cargo proteins and directing them into clathrin-coated vesicles (Bilodeau et al., 2004; Scott et al., 2004). We tested the role of Gga2p in the TGN to endosome sorting of Arn1p by examining the localization of an Arn1p–GFP fusion protein in strains bearing wild-type or deletion alleles of GGA2 (Figure 4). Strains containing GFP sequences integrated at the carboxy terminus of the chromosomal ARN1 open reading frame were grown in iron-poor medium to induce the expression of Arn1-GFP. Cultures were divided and 20 nM FC was added to one aliquot during the last 2 h of growth. In wild-type cells without FC, Arn1-GFP was primarily detected in vacuoles, with only a small amount of signal detected in endosomes (Figure 4A). The paucity of endosomal signal is likely due to the slow folding of the GFP domain (>30 min) (Heim et al., 1995) coupled with the very short half-life of Arn1p (∼10 min), which results in the degradation of Arn1p-GFP before the GFP domain is fully folded. The vacuolar signal is due to the protease resistance of the GFP domain. In contrast to the wild-type strain without FC, Arn1p-GFP was detected at both the plasma membrane and in vacuoles in the gga2Δ strain without FC (Figure 4B), indicating that in the absence of Gga2p, a fraction of Arn1p is mislocalized to the plasma membrane and that Gga2p is involved in the sorting of Arn1p from the TGN to endosomes. The addition of 20 nM FC to either the wild-type or gga2Δ strains resulted in the detection of Arn1-GFP at the plasma membrane and in the vacuole in a pattern that was very similar in the two strains (Figure 4, C and D), confirming that GFP-tagged Arn1p exhibited the expected trafficking in response to FC.
Figure 4.
Mislocalization of Arn1-GFP in a gga2Δ strain. Wild type (A and C) and congenic gga2Δ (B and D) strains containing GFP coding sequences integrated at the carboxy terminus of ARN1 were grown overnight in iron-poor medium with either no FC (A and B) or 0.02 μM FC (C and D) added during the final two hours of growth. Epifluorescent images are on the left, DIC images on the right.
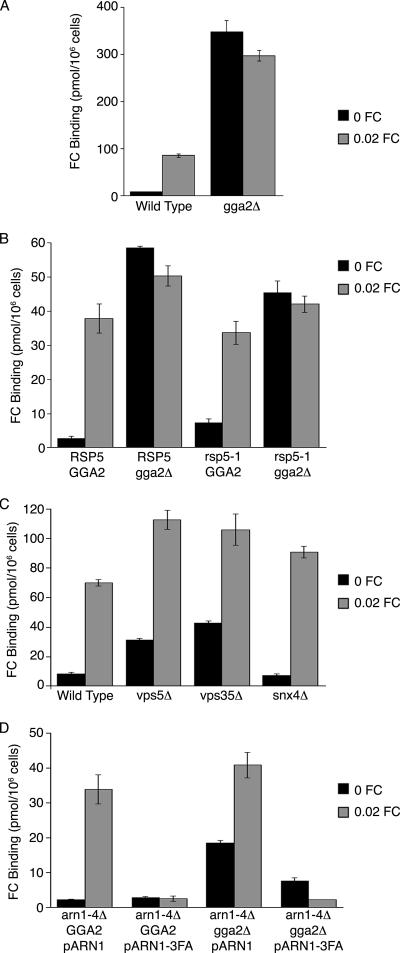
Plasma membrane localization of endogenous Arn1p can be assessed quantitatively by measuring the capacity of Arn1p to bind radiolabeled FC at the surface of intact cells (Kim et al., 2002). Wild-type cells grown in iron-poor medium without FC exhibited very low levels of FC binding that increased 10-fold when 20 nM FC was added to the growth medium in the final 2 h (Figure 5A), indicating the relocalization of Arn1p to the plasma membrane in the presence of FC. In contrast, the gga2Δ strain exhibited 38-fold greater FC binding in the absence of FC pretreatment than the congenic wild-type strain, confirming the mislocalization of Arn1p to the cell surface in the absence of FC in the gga2Δ strain. Pretreatment of the gga2Δ strain with 20 nM FC did not substantially alter the surface binding of FC, and the overall increase in surface-localized Arn1p suggested that the half-life was increased in the gga2Δ strain.
Figure 5.
Surface localization of Arn1p in wild-type, gga2Δ, rsp5-1, rsp5-1 gga2Δ, and retrograde trafficking-deficient strains. (A) Mislocalization of Arn1p to cell surface in the gga2Δ strain. Strain BY4742 and the congenic gga2Δ strain were grown in iron-poor medium overnight to induce expression of Arn proteins, cultures were divided, and 0.02 μM FC was added to one aliquot for the final 2 h of growth. Cells were treated with 20 mM NaN3 and KF, harvested, washed, and mixed with [55Fe-FC] at 100 nM at 0°C for 15 min. Cells were again washed, and retained [55Fe-FC] was measured. (B) Mislocalization of Arn1p to cell surface in rsp5-1 gga2Δ strain. Congenic strains of the indicated genotypes were grown in iron medium overnight at 25°C, and then they were divided and shifted to 37°C for two additional hours of growth. FC at 0.02 μM was added to one aliquot for the final 2 h. Cells were then treated as described in A. (C) Surface localization of Arn1p in the absence of retrograde trafficking. Congenic strains of the indicated genotype were treated as described in A. (D) Failure of the Arn1p-3FA mutant to traffic to the cell surface in the gga2Δ strain. Congenic arn1–4Δ and arn1–4Δgga2Δ strains were transformed with pArn1-HA or pArn1–3FA-HA and treated as described in A. All samples were prepared in triplicate, and binding assays were repeated three times with similar results. Data from a single representative experiment are shown, error bars represent average deviation.
The data presented in Figure 2 indicated that Rsp5-mediated ubiquitination was not required for Arn1p to be sorted from the TGN to the vacuolar membrane, whereas data presented in Figures 4 and 5A indicated that Gga2p was required for Arn1p to be sorted from the TGN to the vacuole. These results raised the possibility that ubiquitination of Arn1p was not required for Gga2p-mediated sorting. To determine the epistatic relationship between Gga2p and Rsp5p, we constructed congenic strains in which RSP5 and GGA2 were mutated individually and together. We then monitored the trafficking of endogenous Arn1p to the plasma membrane by measuring binding of FC at the surface of the cell after growth in iron-poor medium, with and without 20 nM FC pretreatment (Figure 5B). Again, the gga2Δ strain demonstrated high amounts of plasma membrane Arn1p in the absence of FC pretreatment. In contrast, the rsp5-1 strain exhibited relatively low amounts of plasma membrane Arn1p, although surface binding of FC was consistently higher than the wild type, suggesting a small amount of Arn1p was mislocalized to the surface in the rsp5-1 strain. Large amounts of Arn1p were detected on the surface of the rsp5-1 gga2Δ strain in a pattern that was very similar to that of the gga2Δ strain, suggesting that GGA2 is epistatic to RSP5 and acts earlier in the TGN to vacuole sorting of Arn1p.
Sorting of Arn1p to the Plasma Membrane Directly from the Late Golgi
We questioned whether retrograde trafficking from endosomes to the TGN was required for the plasma membrane relocalization of Arn1p in the presence of FC. At least two retrograde sorting pathways have been identified. One preferentially retrieves proteins from the early endosomal or “post-Golgi” compartment and is dependent on the sorting nexins Snx4p, Snx41p, and Snx42p, whereas a second preferentially acts at the late endosomal or prevacuolar compartment and is dependent on components of the retromer coat (Hettema et al., 2003). None of the strains bearing deletions of retromer components (Vps5p and Vps35p) or sorting nexins (Snx4p) exhibited defects in FC-induced plasma membrane localization of Arn1p (Figure 5C), suggesting that retrograde trafficking through these identified pathways was not required for Arn1p localization to the plasma membrane. Because Gga2p was required for TGN to endosomal sorting of Arn1p and Arn1p trafficked to the plasma membrane in the absence of Gga2, we considered whether FC-mediated sorting of newly synthesized Arn1p occurred at the TGN. A mutant form of Arn1p (Arn1p-3FA) in which alanine residues substitute for a cluster of phenylalanine residues at the cytosolic, carboxy terminus is stably expressed in cells, binds FC with wild-type affinity, yet fails to relocalize to the plasma membrane in the presence of FC (Kim et al., 2004), suggesting that at positive sorting signal is required for plasma membrane localization. The Arn1p-3FA mutant was expressed in cells deleted for ARN1, -2, -3, and -4 and surface binding of FC revealed that Arn1p-3FA failed to localize to the plasma membrane in both the GGA2 wild-type and deletion strains, in the presence and the absence of FC (Figure 5D). These data indicate that mutations in the carboxy terminal phenylalanine residues result in the loss of a plasma membrane sorting signal that is required for plasma membrane localization even in the absence of Gga2p-mediated TGN-to-endosome sorting.
Requirement of Ubiquitination for Arn1p Endocytosis
When cells expressing Arn1p are exposed to higher concentrations of FC, the siderophore interacts with the lower-affinity binding site of Arn1p, and FC is transported into the cell. Arn1p is localized to both the plasma membrane and intracellular vesicles at these concentrations of FC, and the transporter accumulates on the plasma membrane when endocytosis is inhibited. These observations indicate that higher concentrations of FC that are permissive for transport also are associated with the cycling of Arn1p on and off the plasma membrane. We tested whether ubiquitination was required for the endocytosis of Arn1p in the presence of FC. Wild-type and rsp5-1 strains were transformed and grown as in Figures 2A and 3A, except that 5 μM FC was added to the medium. Again, ubiquitinated forms of Arn1p were detected in wild-type, but not in the rsp5-1 strain (Figure 6A), when cells were grown in 5 μM FC. The localization of Arn1p in the wild-type and rsp5-1 strains was also markedly different when cells were grown in 5 μM FC (Figure 6B). Although Arn1p was predominately detected in intracellular vesicles in wild-type cells, the transporter was mainly localized to the plasma membrane and vacuolar membranes in the rsp5-1 strain. One interpretation of these data is that ubiquitination of Arn1p was required for the internalization of the transporter in the presence of 5 μM FC and that in the absence of ubiquitination Arn1p stably localized to the plasma membrane in the presence of FC. Alternatively, ubiquitination could delay the recycling of internalized Arn1p to the plasma membrane, and in the absence of ubiquitination, Arn1p was rapidly recycled to the plasma membrane. The vacuolar Arn1p detected in the rsp5-1 strain likely represented the small fraction of Arn1p that failed to relocalize to the plasma membrane in the presence of FC.
Figure 6.
Requirement of Rsp5-dependent ubiquitination for endocytosis of Arn1p. (A) Loss of ubiquitination in the rsp5-1 strain. Strains YKY102 (Wild Type) and YKY103 (rsp5-1) were transformed and grown at 25°C as in Figure 2A. Cultures were shifted to 37°C with the addition of 5 μM FC for 2 h, and cells were harvested and subjected to immunoprecipitation and SDS-PAGE as in Figure 2A. (B) Loss of FC-induced endocytosis in the rsp5-1 strain. Strains YKY102 (Wild Type) and YKY103 (rsp5-1) were transformed and grown as in Figure 2B except that 5 μM FC was added for 2 h before preparation for fluorescence microscopy imaging.
Ubiquitination Independent of Known Accessory Proteins
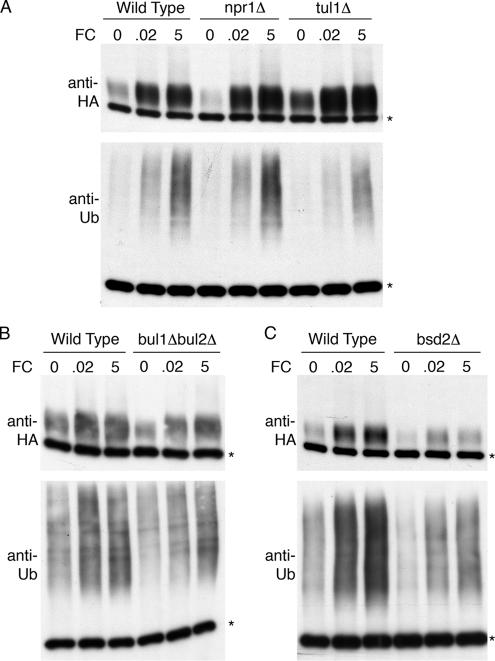
Several protein partners that facilitate the ubiquitination and degradation of yeast membrane proteins have been identified, and some of these proteins are involved in the regulated trafficking of Smf1p and the amino acid transporters Gap1p and Tat2p. We tested whether Npr1p, a serine/threonine kinase that regulates the degradation of Gap1p and Tat2p (Abe and Horikoshi, 2000; De Craene et al., 2001), or Tul1p, a ubiquitin ligase that ubiquitinates the transmembrane protein carboxypeptidase S (Reggiori and Pelham, 2002), was required for ubiquitination of Arn1p. Congenic wild-type, npr1Δ, and tul1Δ strains were transformed with a myc-tagged ubiquitin and pARN1-HA and grown to mid-log phase with the addition of no, 20 nM, or 5 μM FC to the medium. Arn1p-HA was immunoprecipitated and subjected to immunoblotting with anti-HA and anti-myc antibodies (Figure 7A). No reproducible differences were detected in the ubiquitination patterns of Arn1p from the wild-type and deletion mutant strains when grown in either the presence or absence of FC. Similarly, no difference was found in the half-life of Arn1p or in the localization of Arn1p in these strains (data not shown), suggesting that they are not required for the ubiquitination or trafficking of Arn1p. We also examined the roles of the Rsp5 binding proteins Bul1p/Bul2p and Bsd2p, which have been shown to be required for the Rsp5-dependent ubiquitination of Gap1p and Tat2p (Bul1p/Bul2p) and Smf1p (Bsd2p) (Liu and Culotta, 1999; Helliwell et al., 2001; Abe and Iida, 2003). Again, we detected no reproducible differences in the ubiquitination of Arn1p when it was immunoprecipitated from congenic wild-type, bul1Δbul2Δ, and bsd2Δ strains (Figure 7, B and C), although Arn1p was present at slightly lower levels in the bsd2Δ strain. These data suggest that other, unidentified accessory proteins may be involved in the recruitment of Rsp5p to Arn1p or that Rsp5p can directly interact with Arn1p without additional accessory proteins. The data presented in Figure 7 also indicated that exposure to 5 μM FC was associated with increased levels of ubiquitination of Arn1p and was not associated with lower levels of total Arn1p.
Figure 7.
Ubiquitination independent of Npr1p, Tul1p, and Rsp5p accessory proteins. Congenic strains BY4742 (Wild Type), npr1Δ, tul1Δ, and bsd2Δ (A and C), and congenic strains YPH499 (Wild Type) and FAJ75 (bul1Δbul2Δ) (B) were transformed with pMetArn1-HA and Yep96. Transformed strains were grown to mid-log phase in SC without methionine and with 100 μM copper. FC (micromolar) was added at the indicated concentrations for the last 2 h. An equivalent number of cells were lysed, the membranes were purified and subjected to immunoprecipitation with anti-HA antibody. Immunoprecipitates were then subjected to Western blotting using anti-HA antibodies to detect Arn1-HA (top) or anti-ubiquitin antibodies (P4D1) to detect ubiquitinated Arn1 (bottom).
Ferrichrome Transport Activity in the Absence of Ubiquitination
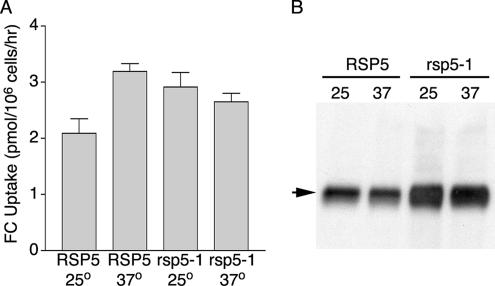
Previous studies have indicated that uptake of FC through Arn1p and Arn3p/Sit1p is reduced when endocytosis is inhibited, either through temperature-sensitive mutations in proteins required for endocytosis or by treatment with an actin-depolymerizing drug. This reduction in FC uptake occurs despite the accumulation of Arn1p and Arn3p/Sit1p on the plasma membrane under these conditions. We questioned whether the Arn1p (and presumably Arn3p) that accumulated on the surface of the rsp5-1 strain also exhibited reduced uptake activity. We constructed congenic RSP5+ and rsp5-1 strains in which the reductive iron uptake system was inactivated by deletion of FET3. Inactivation of reductive iron uptake ensured that uptake of FC could only occur via Arn1p- and Arn3p/Sit1p-mediated transport (Yun et al., 2000a). Strains were transformed with pArn1-HA and grown in iron-poor medium to induce the expression of Arn1p and Arn3p, and then uptake of [55Fe]-FC was measured at the permissive and restrictive temperatures in these strains (Figure 8A). Although the rsp5-1 strain failed to demonstrate the increase in FC uptake at 37°C that was typically seen in the RSP5+ strain, the overall FC uptake activity was comparable in the two strains, indicating that Arn1p and Arn3p transporters that failed to undergo endocytosis due to a block in ubiquitination were nevertheless competent for transport. Interpretation of these results was complicated by the prolonged half-life, and therefore increased levels, of Arn1p in the rsp5-1 strain. Aliquots of cells identical to those used in Figure 8A were subjected to Western blotting for Arn1p-HA (Figure 8B), which indicated that more than twofold greater amounts of Arn1p were present in the rsp5-1 strain compared with the RSP5+ strain. These data suggested two possible interpretations: either forms of Arn1p and Arn3p expressed in the rsp5-1 strain had reduced, but still significant transport activity, or there were two (or more) populations of Arn1p and Arn3p in the rsp5-1 strain, and one population had full activity and a second population had low activity. Because immunofluorescence studies indicated that a portion of Arn1p was localized to the vacuolar membrane in the rsp5-1 strain, where it was unlikely to function in FC uptake, we favored the latter interpretation. That is, the population of Arn1p on the plasma membrane in the rsp5-1 strain had near normal levels of transport activity, whereas the population on the vacuolar membrane did not interact with substrate and was not active for transport.
Figure 8.
Transport of ferrichrome in absence of ubiquitination. Congenic strains YKY104 (RSP5+ fet3Δ) and YKY105 (rsp5-1 fet3Δ) were transformed with pArn1-HA(ADE) and were grown at 25°C in iron-poor medium. Cultures were divided and shifted to 37°C for 1 h and divided again for the measurement of uptake of [55Fe] FC (A) and for Western blotting (B). (A) Uptake of FC in the rsp5-1 strain. Uptake assays were performed using four samples for each data point and were repeated three times; data from a representative experiment are shown. (B) Increased levels of Arn1p-HA in the rsp5-1 strain. Cells were lysed and membranes were isolated and solubilized. Equivalent amounts of membrane protein were subjected to SDS-PAGE and immunoblotting with anti-HA antibody. Arrow indicates Arn1p-HA.
Preferential Ubiquitination of Lysine Cluster in Amino Terminus of Arn1p
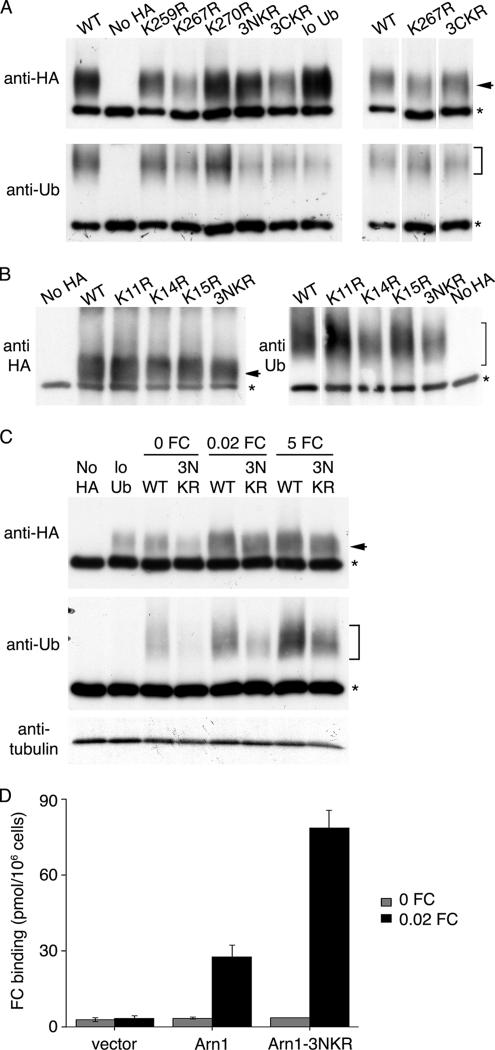
Lysine residues in the cytosolic domains of transmembrane proteins are required for the conjugation of Ub monomers (Hicke and Dunn, 2003), and frequently one or a small number of lysine residues are specifically targeted for ubi-quitination (Dupre et al., 2004). The predicted topology of Arn1p indicates that the transporter has 14 transmembrane domains and that both the amino and carboxy termini are oriented toward the cytoplasm. In total, 17 lysine residues are potentially exposed to the cytosol, and these residues cluster in the amino and carboxy termini and in a large cytosolic loop between the sixth and seventh transmembrane domains. To test the role of these lysine residues as targets for ubiquitin conjugation, we constructed mutant forms of pMetArn1p-HA in which arginine residues were substituted for these lysines, individually and in groups. The wild-type strain was transformed with plasmids containing mutated Arn1p and ubiquitin under the control of the CUP1 promoter. Transformants that did not express HA-tagged Arn1p (no HA) or did not overexpress ubiquitin (low Ub) were included as controls. Transformants were grown in methionine-free, copper-rich medium supplemented with 5 μM FC. Arn1p-HA was immunoprecipitated from cell lysates, and immune complexes were probed for either Arn1p-HA or ubiquitin (Figure 9A). Substitution of lysines 259 (K259R) and 270 (K270R) did not affect ubiquitination of Arn1p. Although substitution of lysine 267 (K267R) and lysines 617, 621, and 625 at the carboxy terminus (3CKR) resulted in the detection of less ubiquitinated Arn1p, there was a proportional decrease in the amount of total Arn1p-HA in these cells, which was apparent when the transformants carrying the mutant Arn1p-HAs were compared with a shorter exposure of the wild-type Arn1p-HA (Figure 9A, right). Substitution of lysines 11, 14, and 15 at the amino terminus (3NKR), however, resulted a marked decrease in ubiquitinated Arn1p-HA with no decrease in the unmodified form of Arn1p, suggesting that one or more of the lysines in this amino-terminal cluster were specific targets for Ub conjugation. We constructed individual arginine substitution mutations of lysines 11, 14, and 15 in Arn1p-HA and tested these mutants for ubiquitination (Figure 9B). Substitution of lysines 11 (K11R) and 15 (K15R) resulted in ubiquitination patterns similar to wild type, but the lysine 14 mutant (K14R) exhibited reduced ubiquitination, which was similar to that of the 3NKR mutant (Figure 9B, right), suggesting that lysine 14 was the primary site for Ub conjugation. Residual ubiquitination of both the K14R and the 3NKR mutants suggested that additional lysine residues could also serve as targets for ubiquitination.
Figure 9.
Identification of amino-terminal lysine residues as targets of ubiquitination. (A) Preferential ubiquitination of amino-terminal lysines. (B) Identification of lysine 14 of Arn1p as primary target of ubiquitination. (C) Targeting of amino-terminal lysines at all ferrichrome concentrations. YPH499 was transformed with YEp96 and a plasmid containing either wild-type Arn1-HA (pMetArn1-HA, WT) or mutant forms of Arn1-HA (K11R, K14R, K15R, K259R, K267R, K270R, 3NKR, or 3CKR). YPH499 transformed with pRS316 and YEp96 was used as the no-HA control. YPH499 integrated with pRS404 and transformed with pMetArn1-HA was used as the low-Ub control. Transformants were grown in SC without methionine and with 100 μM copper. FC was added at the last hour to the final concentration of 5 μM (A and B) or as indicated (C). Equivalent numbers of cells were harvested from each culture. Cells were lysed and membranes were purified, detergent solubilized, and subjected to immunoprecipitation with anti-HA antibody. Immunoprecipitates were then subjected to Western blotting using anti-HA antibodies to detect Arn1-HA (top) or anti-Ub antibodies to detect ubiquitinated Arn1 (bottom). Equivalent aliquots of the solubilized membranes used for immunoprecipitations in C were subjected to Western blotting with anti-tubulin antibody as a control. (D) Absence of surface mislocalization in Arn1-3NKR. The strain CWY101, from which all ARN transporters have been deleted, was transformed with pMetArn1-HA, pMetArn1-3NKR-HA, or the empty vector pRS316. Transformants were grown in SC medium, cultures were divided, and 0.02 μM FC was added to one aliquot for the final 2 h of growth. Cells were treated with 20 mM NaN3 and KF, harvested, washed, and mixed with [55Fe-FC] at 100 nM at 0°C for 15 min. Cells were again washed and retained [55Fe-FC] was measured. All samples were prepared in triplicate, and binding assays were repeated three times with similar results. Data from a single representative experiment are shown; error bars represent average deviation.
Our data suggested that Arn1p exhibited two distinct Ub-dependent trafficking events: sorting to the vacuolar lumen in the absence of ferrichrome and endocytosis during ferrichrome transport. These trafficking events differed depending on the concentration of FC, and, thus, the cellular location of the transporter. We questioned whether the amino-terminal lysine cluster was the primary site of ubiquitination in both of the Ub-dependent trafficking steps by examining the ubiquitination of the 3NKR mutant in cells grown in no, low, or high concentrations of FC (Figure 9C). Again, a marked decrease in the ubiquitinated forms of Arn1p-HA was observed in the 3NKR mutant, and this loss of ubiquitination was present when cells were grown in the absence or presence of FC. These data suggested that the same preferential site of ubiquitination was selected, regardless of whether the Arn1p was trafficking from the endosomes to the vacuole or was undergoing endocytosis at the plasma membrane. We compared the localization of the 3NKR mutant to wild-type Arn1p in the presence and absence of FC by immunofluorescence and found no obvious differences in localization (data not shown), suggesting that the observed residual ubiquitination of the lysine mutants was sufficient to permit normal trafficking. We also measured the surface localization of wild-type Arn1p and the 3NKR mutant in the presence and absence of FC (Figure 9D). A strain from which ARN1, ARN2, ARN3, and ARN4 have been sequentially deleted (Yun et al., 2000a) was transformed with plasmids expressing the wild-type and 3NKR mutant forms of Arn1p-HA, and the surface binding of radiolabeled FC was determined after growth in the presence or absence of 20 nM FC. In the absence of FC, neither the wild type nor the 3NKR mutant was detected at the surface of the cell. This result was consistent with the observation that ubiquitination was not required for TGN to endosome sorting of Arn1p. In cells grown in the presence of 20 nM FC, both wild type and 3NKR mutants were detected at the plasma membrane, with a relatively greater amount of the 3NKR mutant present at the cell surface. These data suggested that a loss of ubiquitination at the amino-terminal lysine residues could result in either enhanced sorting to or reduced internalization from the plasma membrane.
Polyubiquitination at Multiple Sites of Arn1p
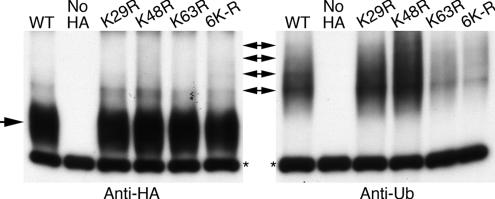
Covalent attachment of a single Ub peptide is sufficient to induce the endocytosis of some plasma membrane proteins in yeast (Hicke, 2001), and monoubiquitination at multiple lysine residues is sufficient for endocytosis of the maltose and galactose permeases (Dupre et al., 2004). However, the Fur4p uracil permease, and the Gap1p and Ztr1p transporters are modified by short chains of Ub residues, linked at the lysine 63 residue of Ub, which are attached to one, two, or more lysine residues on the transporters. Attachment of these poly-Ub chains is required for efficient internalization of Gap1p and Fur4p (Dupre et al., 2004). We investigated whether Arn1p was modified by mono- or poly-Ub chains by examining the ubiquitination patterns of Arn1p-HA in cells that overexpressed mutant forms of Ub and were grown in 5 μM FC (Figure 10). These Ubs carry arginine substitutions at lysine residues required for the formation of poly-Ub chains. In strains carrying wild-type Ub, ubiquitinated Arn1p was detected as four or more species, and expression of Ub mutants K29R and K48R did not lead to a loss of overall ubiquitination of Arn1p or to a change in the number of ubiquitinated species. Expression of the K63R mutant and the 6K-R mutant (in which arginine has been substituted for 6 lysines in Ub), however, led to a decrease in the overall amount of ubiquitinated Arn1p. These data indicated that Arn1p was modified by poly-Ub chains that were linked via lysine 63 of Ub. Ubiquitinated Arn1p was detected primarily as a single species in the K63R and 6K-R mutants, although additional higher-molecular-weight species were present in lesser amounts. These data are consistent with the data in Figure 9, which indicated that Arn1p was primarily ubiquitinated at a single lysine residue but that other lysine residues were also targets for Ub.
Figure 10.
Polyubiquitination of Arn1p at multiple sites in the presence of ferrichrome. YPH499 was transformed with pMetArn1-HA and a plasmid containing either wild-type ubiquitin (YEp96, WT) or mutant forms of ubiquitin (K29R, K48R, K63R, 6K-R). YPH499 transformed with pRS316 and Yep96 was used as the no-HA control. Transformed strains were grown in synthetic complete medium without methionine and with 100 μM copper. Ferrichrome at 5 μM was added in the last hour. Equivalent numbers of cells were harvested from each culture and treated as described in Figure 7 legend. Single arrow indicates unmodified Arn1p-HA. Double-headed arrows indicate bands corresponding to ubiquitinated species of Arn1p-HA.
DISCUSSION
Gga2 and Ubiquitin Control Some Trafficking of Arn1p
Here, we have shown that the yeast siderophore transporter Arn1p is ubiquitinated at its amino terminus and that in the absence of ubiquitination, Arn1p exhibits trafficking defects. Arn1p exhibits three separate trafficking patterns that are dependent on FC: 1) direct sorting from the TGN to the vacuole for degradation in the absence of FC, 2) relocalization from late endosomes to the plasma membrane in low concentrations of FC, and 3) cycling between the plasma membrane and endosomes during transport of FC. Our data indicated that steps 1 and 3 are dependent on ubiquitination and the Rsp5 Ub ligase and that step 1 is also dependent on Gga2p.
In the absence of FC, Rsp5p activity was required for delivery of Arn1p into the vacuolar lumen, and in the absence of ubiquitination, Arn1p accumulated on the limiting membrane of the vacuole. Integral membrane proteins destined for degradation in the vacuole are first sorted into luminal vesicles of the MVB. Membrane proteins of the MVB that are excluded from these luminal vesicles remain in the limiting membrane of the MVB and occur on the limiting membrane of the vacuole after fusion of the MVB with the vacuole (Hurley and Emr, 2006). The ubiquitination of Arn1p, which was required for its sorting into the luminal vesicles of the MVB, was consistent with recent studies that indicate Ub is specifically recognized by motifs within the Vps27/Hse1 complex and the ESCRT-I complex, which initiate the formation of vesicles within the MVB. The accumulation of Arn1p on the limiting membrane of the vacuole in the absence of ubiquitination also indicates that ubiquitination was not required for trafficking of Arn1p from the TGN to late endosomes. Furthermore, the trafficking of Arn1p to the plasma membrane in the presence of low concentrations of FC seems to occur completely independently of ubiquitination.
Gga2p was required for the trafficking of Arn1p from the TGN to endosomes, a process that did not require ubiquitination by Rsp5p. The yeast Gga proteins have been shown to bind Ub through a subdomain of the GAT (GGA and Target of Myb1) domain, and Ub binding by Gga is thought to be required for the sorting of ubiquitinated cargo proteins, such as Gap1p and Fur4p, at the TGN (Bilodeau et al., 2004; Scott et al., 2004). Because the Arn1p sorting defect of a gga2Δ rsp5-1 strain was identical to that of the gga2Δ strain, Gga2p seemed to function earlier in the sorting process than Rsp5p. These data suggested that ubiquitination was not required for Gga2p to mediate the TGN to endosome sorting of Arn1p and that a domain of Gga2p may interact with cargo proteins such as Arn1p in a ubiquitin-independent manner.
Arn1p trafficking to endosomes seemed to reflect the summation of competing trafficking signals recognized at the TGN. In the absence of FC, Gga2p (and possibly Gga1p) acted at the TGN to mediate Arn1p sorting to endosomes. When Gga2p was not present, a positive sorting signal in Arn1p (absent in Arn1p-3FA) directed it to the plasma membrane. Binding of FC to Arn1p in the TGN may “tip the balance” of these two competing processes to favor the plasma membrane pathway. Because identified retrograde sorting pathways were not required for FC to alter trafficking, Arn1p may not undergo trafficking from endosomes to the TGN for localization to the plasma membrane. Indeed, as the half-life of Arn1p in the late endosome is quite short, the bulk of surface Arn1p may come directly from the TGN.
When yeast cells are exposed to FC at concentrations high enough to stimulate transport, Arn1p cycles between the plasma membrane and endosomes. These higher levels of FC also stimulated increased ubiquitination of the transporter (Kim et al., 2004; Figure 7), and in the absence of Rsp5p-dependent ubiquitination, Arn1p did not occur in endosomes (Figure 6B). Ubiquitination by Rsp5 is required for the endocytosis of many plasma membrane proteins in yeast, including the high-affinity zinc and manganese transporters Zrt1p and Smf1p (Liu and Culotta, 1999; Gitan and Eide, 2000; Hettema et al., 2004) as well as other nutrient transporters (Dupre et al., 2004). Unlike these transporters, however, Arn1p was not degraded after endocytosis. Although the substrate-induced ubiquitination and internalization of the other metal and nutrient transporters results in their trafficking to the vacuole for degradation, levels of Arn1p did not decrease in the presence of higher concentrations of FC, indicating that the ubiquitination and internalization were not accompanied by trafficking to or degradation in the vacuole. How did ubiquitinated Arn1p escape trafficking to the vacuole after endocytosis? The cellular machinery involved in this process is unknown, but the cellular factors involved in recognizing Arn1p with FC bound at the high-affinity receptor site may also recognize Ub-Arn1p with bound FC after endocytosis. Similarly, the cluster of phenylalanine residues at the carboxy terminus of Arn1p, which are required for the initial relocalization of Arn1p to the plasma membrane, may also be involved in the retrieval of Arn1p from endosomes to the plasma membrane (Kim et al., 2004). Interestingly, a GTPase-containing complex recently identified as mediating the retrieval of Gap1p from endosomes also recognizes a peptide rich in aromatic amino acids in the carboxy terminus of Gap1p (Gao and Kaiser, 2006).
Ubiquitination and Trafficking Machinery for Arn1p Differ from Those of Other Membrane Permeases
Although the patterns of trafficking observed for Arn1p differ significantly from those of other permeases, they are similar in some ways to that observed for Fur4p, the yeast uracil permease. Fur4p is normally expressed on the plasma membrane, but exposure to high levels of uracil will trigger the ubiquitination and endocytosis of the permease, resulting in its degradation in the vacuole (Galan et al., 1996). High levels of extracellular or intracellular uracil also promote the sorting of newly synthesized Fur4p directly from the TGN to the vacuolar lumen for degradation, and in the absence of ubiquitination by Rsp5p, Fur4p accumulates on the limiting membrane of the vacuole (Blondel et al., 2004). This vacuolar trafficking differs from the sorting of the general amino acid permease Gap1p. Gap1p is expressed on the plasma membrane when cells are grown in media that lack amino acids. However, when cells are grown in the presence of a rich nitrogen source, such as ammonium or glutamate, Gap1p is sorted to the vacuole for degradation, either directly from the TGN or by endocytosis from the plasma membrane (Magasanik and Kaiser, 2002). Both of these sorting steps require ubiquitination via Rsp5p, and in the absence of ubiquitination, newly synthesized Gap1p is sorted to the plasma membrane, even in the presence of glutamate, and it does not accumulate on the vacuolar membrane. Arn1p differs from both Gap1p and Fur4p in that its trafficking to the vacuole for degradation seems to be the “default” pathway, and FC, the transport substrate, diverts the transporter from this pathway.
Several proteins that assist in the trafficking of membrane proteins have been identified, but our data indicate that they do not have a role in the trafficking of Arn1p. Npr1p, a serine/threonine kinase that regulates the degradation of Gap1p (De Craene et al., 2001) and the high-affinity tryptophan permease, Tat2p (Abe and Horikoshi, 2000), did not affect the ubiquitination (Figure 7) or half-life of Arn1p. Consistent with these observations, we were unable to detect phosphorylated forms of Arn1p in the wild-type or rsp5-1 strains (data not shown). Ubiquitination of Gap1p is dependent on the Rsp5 accessory proteins Bul1p and Bul2p (Helliwell et al., 2001), and Tat2p shows similar requirements for vacuolar degradation (Abe and Iida, 2003). In contrast, trafficking of Fur4p is not affected by deletion of BUL1 and BUL2 (Dupre et al., 2004), and neither is the ubiquitination of the low-affinity tryptophan permease Tat1p (Abe and Iida, 2003). Similarly, whereas ubiquitination and degradation of Smf1p requires the Rsp5 accessory protein Bsd2p (Liu and Culotta, 1999) (and its binding partners Tre1p and Tre2p; Stimpson et al., 2006), Arn1p does not demonstrate this requirement.
Lysine 63-linked Poly-Ub Chains Attach to Lysine 14 in an Acidic Amino-terminal Peptide
Arn1p was found to be ubiquitinated preferentially at the amino terminus, particularly at lysine 14. The amino terminal lysines seemed to be the preferred ubiquitination site for both of the Rsp5-dependent trafficking steps of Arn1p. Lysine 14 is part of a cluster of lysyl and acidic residues with the sequence DPVKEEKKHVF. Previous attempts to identify ubiquitination sites in plasma membrane proteins have yielded sequences with both acidic residues and potential phosphorylation sites adjacent to the target lysine, with a consensus motif of D/EXKS/T (Dupre et al., 2004). Although the ubiquitination site of Arn1p carried the acidic residues, no serine or threonine residues were proximal in the primary, linear structure of Arn1p. A mass spectroscopy analysis of the ubiquitinated proteins in the yeast proteome identified several permeases, and the sites of ubiquitination in these proteins were distributed throughout their cytosolic domains. Sequences adjacent to the ubiquitinated lysines were rich in acidic residues, similar to the sequence surrounding lysine 14 of Arn1p (Peng et al., 2003). Of note, a similar cluster of lysyl and acidic residues is present in the amino terminus of Arn3p, with the sequence EKEVGAKVDVK, which could potentially serve as a ubiquitination site.
We demonstrated that Arn1p was primarily modified by poly-Ub chains conjugated at lysine 63 of Ub. Although mono-ubiquitination has been reported to be sufficient to mediate the internalization of the a-factor receptor and some modified forms of plasma membrane transporters, attachment of short chains of Ub at one or two specific lysine residues is required for full levels of endocytosis of Gap1p (Springael et al., 1999) and Fur4p (Galan and Haguenauer-Tsapis, 1997). Although poly-ubiquitination of cytosolic proteins destined for the proteasome occurs via lysine 48, both Gap1p and Fur4p are modified by poly-Ub chains linked at lysine 63. Recent work in mammalian cells indicates that poly-ubiquitination via lysine 63, rather than mono-ubiquitination, is necessary for the endocytosis of class I molecules that is stimulated by the Kaposi's sarcoma-associated herpes virus (Duncan et al., 2006). Poly-Ub chains are recognized by Ub-interacting motifs found in epsins, which are endocytic proteins involved in clathrin-mediated endocytosis (Barriere et al., 2006; Hawryluk et al., 2006). In yeast, the epsin homologues Ent1p and Ent2p are also required for endocytosis (Wendland et al., 1999) and may be involved in the recognition of poly-Ub chains on cell surface proteins.
Transport Activity of Plasma Membrane Arn1p
We measured the FC transport activity of Arn1p in the rsp5-1 mutant and determined that the protein had substantial transport activity (Figure 6), possibly equivalent to that measured in wild-type cells. This activity occurred in the absence of Arn1p ubiquitination and internalization. Previous studies by our laboratory indicated that FC uptake activity through Arn1p was diminished when Arn1p internalization was blocked in strains with defects in endocytosis. These endocytic defects occur downstream of the ubiquitination step, and they may explain the difference in FC transport activity. In the rsp5-1 strain, Arn1p is active for transport and trapped on the surface of the cell in a nonubiquitinated form. In the end4-1/sla2 and the ent1-ts ent2Δ strains, Arn1p is less active and accumulates on the surface in a polyubiquitinated form. One interpretation of these observations is that ubiquitination may decrease the transport activity of Arn1p, either directly, by inhibiting the conformational change that must occur during transport, or indirectly, by inducing trafficking to other lipid environments. The Tat2p tryptophan permease moves from bulk lipids on the cell surface to glycosphingolipid- and ergosterol- rich microdomains (“lipid rafts”) when ubiquitination is inhibited (Abe and Iida, 2003). Tat2p also exhibits reduced activity in cells depleted of ergosterol due to a mutation in Erg6 (Umebayashi and Nakano, 2003). An alternative explanation for the different activity of Arn1p in the ubiquitination-deficient versus the endocytosis-deficient strains is the recent observation that defects in endocytosis lead to alterations in the lipid composition of the plasma membrane. Deletion of END3 or END4/SLA2 is associated with the abnormal accumulation of phosphatidylserine and phosphatidylethanolamine on the outer leaflet of the plasma membrane and the loss of normal membrane asymmetry (Chen et al., 2006). This change in lipid environment could adversely affect the transport activity of Arn1p.
If endocytosis is not required for FC transport activity, what selective advantage does the cycling of Arn1p on and off the plasma membrane offer to cells? One possibility is that through Ub-mediated endocytosis, the cell would have an opportunity to clear Arn1p from the plasma membrane if extracellular FC levels were to fall. If FC levels were to fall to a level where binding to the receptor domain of Arn1p did not occur, then ubiquitinated and internalized Arn1p would lack the signal that allows it to be diverted from late endosomes to the plasma membrane, and the transporter could then traffic to the vacuole for degradation. The net effect of the FC-controlled trafficking of Arn1p seems to be to limit the appearance of the transporter on the plasma membrane to periods when FC is available for uptake, thereby minimizing the possibility that Arn1p will permit the low-specificity transport of a potentially toxic substrate. Further studies will indicate what cellular machinery participates in the delivery of Arn1p to the plasma membrane.
ACKNOWLEDGMENTS
We thank L. Hicke (Northwestern University), F. Abe (Japan Agency for Marine-Earth Science and Technology), and J. M. Galan (Institut Jacques Monod) for the generous gifts of strains and plasmids; J. Bonifacino and H. Watson for helpful discussions; and J. Hurvitz for technical assistance. These studies were supported by the Intramural Research Program of National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Abbreviations used:
- FC
ferrichrome
- MVB
multivesicular body
- TGN
trans-Golgi network
- Ub
ubiquitin.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0861) on March 7, 2007.
REFERENCES
- Abe F., Horikoshi K. Tryptophan permease gene TAT2 confers high-pressure growth in Saccharomyces cerevisiae. Mol. Cell Biol. 2000;20:8093–8102. doi: 10.1128/mcb.20.21.8093-8102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe F., Iida H. Pressure-induced differential regulation of the two tryptophan permeases Tat1 and Tat2 by ubiquitin ligase Rsp5 and its binding proteins, Bul1 and Bul2. Mol. Cell Biol. 2003;23:7566–7584. doi: 10.1128/MCB.23.21.7566-7584.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M., Sato T. K., Banta L. M., Emr S. D. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriere H., Nemes C., Lechardeur D., Khan-Mohammad M., Fruh K., Lukacs G. L. Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in mammalian cells. Traffic. 2006;7:282–297. doi: 10.1111/j.1600-0854.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- Bilodeau P. S., Winistorfer S. C., Allaman M. M., Surendhran K., Kearney W. R., Robertson A. D., Piper R. C. The GAT domains of clathrin-associated GGA proteins have two ubiquitin binding motifs. J. Biol. Chem. 2004;279:54808–54816. doi: 10.1074/jbc.M406654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel M. O., Morvan J., Dupre S., Urban-Grimal D., Haguenauer-Tsapis R., Volland C. Direct sorting of the yeast uracil permease to the endosomal system is controlled by uracil binding and Rsp5p-dependent ubiquitylation. Mol. Biol. Cell. 2004;15:883–895. doi: 10.1091/mbc.E03-04-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S. The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell Biol. 2004;5:23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- Chen S., Wang J., Muthusamy B. P., Liu K., Zare S., Andersen R. J., Graham T. R. Roles for the Drs2p-Cdc50p complex in protein transport and phosphatidylserine asymmetry of the yeast plasma membrane. Traffic. 2006;7:1503–1517. doi: 10.1111/j.1600-0854.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- De Craene J. O., Soetens O., Andre B. The Npr1 kinase controls biosynthetic and endocytic sorting of the yeast Gap1 permease. J. Biol. Chem. 2001;276:43939–43948. doi: 10.1074/jbc.M102944200. [DOI] [PubMed] [Google Scholar]
- Duncan L. M., Piper S., Dodd R. B., Saville M. K., Sanderson C. M., Luzio J. P., Lehner P. J. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 2006;25:1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R., Hicke L. Domains of the Rsp5 ubiquitin-protein ligase required for receptor-mediated and fluid-phase endocytosis. Mol. Biol. Cell. 2001;12:421–435. doi: 10.1091/mbc.12.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupic F., Fruchon S., Bensaid M., Loreal O., Brissot P., Borot N., Roth M. P., Coppin H. Duodenal mRNA expression of iron related genes in response to iron loading and iron deficiency in four strains of mice. Gut. 2002;51:648–653. doi: 10.1136/gut.51.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre S., Urban-Grimal D., Haguenauer-Tsapis R. Ubiquitin and endocytic internalization in yeast and animal cells. Biochim. Biophys. Acta. 2004;1695:89–111. doi: 10.1016/j.bbamcr.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Ecker D. J., Khan M. I., Marsh J., Butt T. R., Crooke S. T. Chemical synthesis and expression of a cassette adapted ubiquitin gene. J. Biol. Chem. 1987;262:3524–3527. [PubMed] [Google Scholar]
- Eguez L., Chung Y. S., Kuchibhatla A., Paidhungat M., Garrett S. Yeast Mn2+ transporter, Smf1p, is regulated by ubiquitin-dependent vacuolar protein sorting. Genetics. 2004;167:107–117. doi: 10.1534/genetics.167.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice M. R., De Domenico I., Li L., Ward D. M., Bartok B., Musci G., Kaplan J. Post-transcriptional regulation of the yeast high affinity iron transport system. J. Biol. Chem. 2005;280:22181–22190. doi: 10.1074/jbc.M414663200. [DOI] [PubMed] [Google Scholar]
- Galan J. M., Haguenauer-Tsapis R. Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 1997;16:5847–5854. doi: 10.1093/emboj/16.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan J. M., Moreau V., Andre B., Volland C., Haguenauer-Tsapis R. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J. Biol. Chem. 1996;271:10946–10952. doi: 10.1074/jbc.271.18.10946. [DOI] [PubMed] [Google Scholar]
- Gao M., Kaiser C. A. A conserved GTPase-containing complex is required for intracellular sorting of the general amino-acid permease in yeast. Nat. Cell Biol. 2006;8:657–667. doi: 10.1038/ncb1419. [DOI] [PubMed] [Google Scholar]
- Gitan R. S., Eide D. J. Zinc-regulated ubiquitin conjugation signals endocytosis of the yeast ZRT1 zinc transporter. Biochem. J. 2000;346:329–336. [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S., Cellier M., Vidal S., Gros P. Identification and characterization of a second mouse Nramp gene. Genomics. 1995;25:514–525. doi: 10.1016/0888-7543(95)80053-o. [DOI] [PubMed] [Google Scholar]
- Hawryluk M. J., Keyel P. A., Mishra S. K., Watkins S. C., Heuser J. E., Traub L. M. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–281. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- Heim R., Cubitt A. B., Tsien R. Y. Improved green fluorescence. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Helliwell S. B., Losko S., Kaiser C. A. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol. 2001;153:649–662. doi: 10.1083/jcb.153.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema E. H., Lewis M. J., Black M. W., Pelham H. R. Retromer and the sorting nexins Snx4/41/42 mediate distinct retrieval pathways from yeast endosomes. EMBO J. 2003;22:548–557. doi: 10.1093/emboj/cdg062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema E. H., Valdez-Taubas J., Pelham H. R. Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J. 2004;23:1279–1288. doi: 10.1038/sj.emboj.7600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann P., Ernst J. F., Winkelmann G. Identification and substrate specificity of a ferrichrome-type siderophore transporter (Arn1p) in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2000;186:221–227. doi: 10.1111/j.1574-6968.2000.tb09108.x. [DOI] [PubMed] [Google Scholar]
- Hicke L. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- Hicke L., Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Hu C. J., Bai C., Zheng X. D., Wang Y. M., Wang Y. Characterization and functional analysis of the siderophore-iron transporter CaArn1p in Candida albicans. J. Biol. Chem. 2002;277:30598–30605. doi: 10.1074/jbc.M204545200. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Emr S. D. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu. Rev. Biophys. Biomol. Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann D. J., Odorizzi G., Emr S. D. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Kim Y., Yun C. W., Philpott C. C. Ferrichrome induces endosome to plasma membrane cycling of the ferrichrome transporter, Arn1p, in Saccharomyces cerevisiae. EMBO J. 2002;21:3632–3642. doi: 10.1093/emboj/cdf382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Lampert S. M., Philpott C. C. A receptor domain controls the intracellular sorting of the ferrichrome transporter, Arn1. EMBO J. 2005;24:952–962. doi: 10.1038/sj.emboj.7600579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuisse E., Simon-Casteras M., Labbe P. Siderophore-mediated iron uptake in Saccharomyces cerevisiae: the SIT1 gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily. Microbiology. 1998;144:3455–3462. doi: 10.1099/00221287-144-12-3455. [DOI] [PubMed] [Google Scholar]
- Liu X. F., Culotta V. C. Post-translation control of Nramp metal transport in yeast. Role of metal ions and the BSD2 gene. J. Biol. Chem. 1999;274:4863–4868. doi: 10.1074/jbc.274.8.4863. [DOI] [PubMed] [Google Scholar]
- Liuzzi J. P., Bobo J. A., Lichten L. A., Samuelson D. A., Cousins R. J. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc. Natl. Acad. Sci. USA. 2004;101:14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko S., Petris M. J. Function and regulation of the mammalian copper-transporting ATPases: insights from biochemical and cell biological approaches. J. Membr. Biol. 2003;191:1–12. doi: 10.1007/s00232-002-1040-6. [DOI] [PubMed] [Google Scholar]
- Magasanik B., Kaiser C. A. Nitrogen regulation in Saccharomyces cerevisiae. Gene. 2002;290:1–18. doi: 10.1016/s0378-1119(02)00558-9. [DOI] [PubMed] [Google Scholar]
- McKie A. T., et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- Moore R. E., Kim Y., Philpott C. C. The mechanism of ferrichrome transport through Arn1p and its metabolism in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2003;100:5664–5669. doi: 10.1073/pnas.1030323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Oberegger H., Schoeser M., Zadra I., Abt B., Haas H. SREA is involved in regulation of siderophore biosynthesis, utilization and uptake in Aspergillus nidulans. Mol. Microbiol. 2001;41:1077–1089. doi: 10.1046/j.1365-2958.2001.02586.x. [DOI] [PubMed] [Google Scholar]
- Park Y. S., Kim T. H., Chang H. I., Sung H. C., Yun C. W. Cellular iron utilization is regulated by putative siderophore transporter FgSit1 not by free iron transporter in Fusarium graminearum. Biochem. Biophys. Res. Commun. 2006;345:1634–1642. doi: 10.1016/j.bbrc.2006.05.071. [DOI] [PubMed] [Google Scholar]
- Peng J., Schwartz D., Elias J. E., Thoreen C. C., Cheng D., Marsischky G., Roelofs J., Finley D., Gygi S. P. A proteomics approach to understanding protein ubiquitination. Nat. Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- Philpott C. C. Iron uptake in fungi: a system for every source. Biochim. Biophys. Acta. 2006;1763:636–645. doi: 10.1016/j.bbamcr.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Philpott C. C., Rashford J., Yamaguchi-Iwai Y., Rouault T. A., Dancis A., Klausner R. D. Cell-cycle arrest and inhibition of G1 cyclin translation by iron in AFT1–1(up) yeast. EMBO J. 1998;17:5026–5036. doi: 10.1093/emboj/17.17.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Pelham H. R. A transmembrane ubiquitin ligase required to sort membrane proteins into multivesicular bodies. Nat. Cell Biol. 2002;4:117–123. doi: 10.1038/ncb743. [DOI] [PubMed] [Google Scholar]
- Rutherford J. C., Bird A. J. Metal-responsive transcription factors that regulate iron, zinc, and copper homeostasis in eukaryotic cells. Eukaryot. Cell. 2004;3:1–13. doi: 10.1128/EC.3.1.1-13.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. M., Bilodeau P. S., Zhdankina O., Winistorfer S. C., Hauglund M. J., Allaman M. M., Kearney W. R., Robertson A. D., Boman A. L., Piper R. C. GGA proteins bind ubiquitin to facilitate sorting at the trans-Golgi network. Nat. Cell Biol. 2004;6:252–259. doi: 10.1038/ncb1107. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. In: Guthrie C., Fink G., editors. Guide to Yeast Genetics and Molecular Biology. vol. 194. New York: Academic Press; 1991. pp. 3–20. [Google Scholar]
- Springael J. Y., Galan J. M., Haguenauer-Tsapis R., Andre B. NH4+-induced down-regulation of the Saccharomyces cerevisiae Gap1p permease involves its ubiquitination with lysine-63-linked chains. J. Cell Sci. 1999;112:1375–1383. doi: 10.1242/jcs.112.9.1375. [DOI] [PubMed] [Google Scholar]
- Stearman R., Dancis A., Klausner R. D. YIpDCE1–an integrating plasmid for dual constitutive expression in yeast. Gene. 1998;212:197–202. doi: 10.1016/s0378-1119(98)00179-6. [DOI] [PubMed] [Google Scholar]
- Stearman R., Yuan D. S., Yamaguchi-Iwai Y., Klausner R. D., Dancis A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science. 1996;271:1552–1557. doi: 10.1126/science.271.5255.1552. [DOI] [PubMed] [Google Scholar]
- Stimpson H. E., Lewis M. J., Pelham H. R. Transferrin receptor-like proteins control the degradation of a yeast metal transporter. EMBO J. 2006;25:662–672. doi: 10.1038/sj.emboj.7600984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Gitlin J. D. Intracellular localization of the Menkes and Wilson's disease proteins and their role in intracellular copper transport. Pediatr. Int. 1999;41:436–442. doi: 10.1046/j.1442-200x.1999.01090.x. [DOI] [PubMed] [Google Scholar]
- Umebayashi K., Nakano A. Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J. Cell Biol. 2003;161:1117–1131. doi: 10.1083/jcb.200303088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B., Steece K. E., Emr S. D. Yeast epsins contain an essential N-terminal ENTH domain, bind clathrin and are required for endocytosis. EMBO J. 1999;18:4383–4393. doi: 10.1093/emboj/18.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C. W., Ferea T., Rashford J., Ardon O., Brown P. O., Botstein D., Kaplan J., Philpott C. C. Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake. J. Biol. Chem. 2000a;275:10709–10715. doi: 10.1074/jbc.275.14.10709. [DOI] [PubMed] [Google Scholar]
- Yun C. W., Tiedeman J. S., Moore R. E., Philpott C. C. Siderophore-iron uptake in saccharomyces cerevisiae. Identification of ferrichrome and fusarinine transporters. J. Biol. Chem. 2000b;275:16354–16359. doi: 10.1074/jbc.M001456200. [DOI] [PubMed] [Google Scholar]
- Zoller H., Koch R. O., Theurl I., Obrist P., Pietrangelo A., Montosi G., Haile D. J., Vogel W., Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120:1412–1419. doi: 10.1053/gast.2001.24033. [DOI] [PubMed] [Google Scholar]
- Zoller H., Theurl I., Koch R., Kaser A., Weiss G. Mechanisms of iron mediated regulation of the duodenal iron transporters divalent metal transporter 1 and ferroportin 1. Blood Cells Mol. Dis. 2002;29:488–497. doi: 10.1006/bcmd.2002.0587. [DOI] [PubMed] [Google Scholar]