Abstract
Troyer syndrome is an autosomal recessive hereditary spastic paraplegia caused by mutation in the spartin (SPG20) gene, which encodes a widely expressed protein of unknown function. This mutation results in premature protein truncation and thus might signify a loss-of-function disease mechanism. In this study, we have found that spartin is mono-ubiquitinated and functions in degradation of the epidermal growth factor receptor (EGFR). Upon EGF stimulation, spartin translocates from the cytoplasm to the plasma membrane and colocalizes with internalized EGF-Alexa. Knockdown of spartin by small interfering RNA decreases the rate of EGFR degradation and also affects EGFR internalization, recycling, or both. Furthermore, overexpression of spartin results in a prominent decrease in EGFR degradation. Taken together, our data suggest that spartin is involved in the intracellular trafficking of EGFR and that impaired endocytosis may underlie the pathogenesis of Troyer syndrome.
INTRODUCTION
The hereditary spastic paraplegias (HSPs) comprise a cluster of inherited neurological disorders characterized by progressive spasticity and muscle weakness in the lower limbs (Crosby and Proukakis, 2002; Reid, 2003; Fink, 2006; Soderblom and Blackstone, 2006). Classically, the HSPs have been divided into two forms: “pure” when lower extremity spasticity and paraparesis are the only features and “complicated” when additional symptoms are present (Harding, 1983). More than 30 genetic loci (SPG1-33) and 14 gene products have been identified, yielding new insights into the molecular pathways involved in the pathogenesis of the HSPs. Troyer syndrome (SPG20), an autosomal recessive, complicated HSP that manifests in early childhood, is characterized by dysarthria, mental retardation, shortness of stature, and distal muscle wasting in addition to spasticity and weakness of the lower limbs (Cross and McKusick, 1967; Proukakis et al., 2004). To date, the only known mutation involved in Troyer syndrome is a single base deletion in the spartin gene, resulting in a 29-amino acid substitution at the C-terminus and premature truncation of the 666-amino acid protein by 268 residues (Patel et al., 2002; Proukakis et al., 2004).
Although the cellular functions of spartin are not known, it harbors two conserved domains, an MIT (contained within microtubule-interacting and trafficking molecules) domain at the N-terminus and a plant-related region at the C-terminus. Although the only known function of the latter domain is in senescence (Panavas et al., 1999), the MIT domain is found in several other proteins, including Vps4-A and -B, sortin 15, and spastin (Ciccarelli et al., 2003), which are involved in vesicle trafficking and binding of microtubules. Interestingly, spastin, a microtubule-severing ATPase (Errico et al., 2002; Trotta et al., 2004; Evans et al., 2005), is mutated in the most common form of autosomal dominant HSP, SPG4 (Crosby and Proukakis, 2002; Reid, 2003; Fink, 2006; Soderblom and Blackstone, 2006). Although a region adjacent to the MIT domain is involved in the interaction of spastin with microtubules (Errico et al., 2004), spastin's MIT domain interacts with charged multivesicular body protein 1B (CHMP1B; Reid et al., 2005), a member of the endosomal sorting complex required for transport (ESCRT)-III complex, which is critical for the sorting of membrane cargoes to the multivesicular bodies (Babst et al., 2002). Structural studies of the MIT domain of Vps4-A indicate that it also interacts with the charged multivesicular body protein family of ESCRT-III proteins (Scott et al., 2005).
Recent studies have localized endogenous spartin to the trans-Golgi network, neuritic processes, and nucleus (Robay et al., 2006) and overexpressed spartin to mitochondria (Lu et al., 2006). We previously showed that spartin is both a cytosolic and membrane-associated protein that interacts with Eps15 and Eps15R (Bakowska et al., 2005). Eps15 is an accessory protein involved in the internalization of epidermal growth factor (EGF) and transferrin receptors (Carbone et al., 1997; Benmerah et al., 1998; Huang et al., 2004), and it localizes to necks of clathrin-coated pits as well as to early and late endosomes (Tebar et al., 1996; Torrisi et al., 1999). Eps15 harbors several interaction domains and binds to proteins, such as epsin, intersectin, and synaptojanin, that are also engaged in endocytosis (Haffner et al., 1997; Chen et al., 1999; Slepnev and De Camilli, 2000).
Although the function of spartin has not been established, its interaction with Eps15 prompted us to examine whether it has a role in endocytosis. In this study, we analyzed the subcellular distribution of spartin, its posttranslational modification, and its function in trafficking of the epidermal growth factor receptor (EGFR).
MATERIALS AND METHODS
Mammalian Expression Vectors and Small Interfering RNA Oligonucleotides
The full-length human spartin cDNA in the pGW1-Myc mammalian expression vector has been described previously (Bakowska et al., 2005). To generate the hemagglutinin (HA)-tagged spartin construct, the spartin cDNA was cloned into the pGW1-HA mammalian expression vector. Deletion fragments of human spartin (residues 1–206 and 210–666) were PCR amplified using PfuTurbo (Stratagene, La Jolla, CA) and cloned in-frame into the EcoRI site of the pGW1-Myc vector. Spartin(1–408) was amplified by PCR and cloned into the AscI site of pGW1-Myc. Wild-type GFP-Vps4-A and mutant forms GFP-Vps4-AE228Q and GFP-Vps4-AK178Q were provided by Dr. W. Sundquist (Garrus et al., 2001), and GFP-EGFR was from Dr. A. Sorkin (Carter and Sorkin, 1998). To generate spartin 8K→R, site-directed mutagenesis of eight Lys residues (Lys287, Lys362, Lys370, Lys377, Lys382, Lys384, Lys387, Lys390) to Arg in pGW1-Myc-spartin was performed using the QuikChange protocol (Stratagene). The resulting construct served as a template to generate spartin(1–408) 8K→R and spartin(210–666) 8K→R by PCR. To generate the yellow fluorescence protein (YFP)-tagged spartin construct, the full-length spartin cDNA was amplified by PCR and cloned in-frame into the HindIII site of the YFP-N1 expression vector (BD Biosciences Clontech, Mountain View, CA). For knockdown experiments we used small interfering RNA (siRNAs; Invitrogen, Carlsbad, CA) that target the following spartin sequences: 5′-GGCAAGGAUUGGAAUGUGCAGCUAA-3′ (sequence 1), and 5′-CAAAUACGGAUAUAAUGCAGGAGAA-3′ (sequence 2). Control siRNA duplexes and siRNAs that target human Eps15 sequences [5′-GCUGAAUUAACUAGUCAGGtt-3′ (sequence 1) and 5′-GCCAACAAUAGCAGUAUUAtt-3′ (sequence 2)] were purchased from Ambion (Austin, TX).
Cell Lines and Transfection
HeLa, MN-1, SH-SY5Y, and 293 cells were maintained and transfected with mammalian expression vectors using Lipofectamine (Invitrogen) as described previously (Bakowska et al., 2005). Transfections of HeLa cells with siRNA oligonucleotide duplexes were performed twice for 72 h using Oligofectamine (Invitrogen) according to the manufacturer's instructions.
Antibodies and Reagents
To generate polyclonal antibodies against human spartin, we used a pGEX-6P-1 spartin(108–367) construct (Bakowska et al., 2005) to express the glutathione S-transferase (GST) fusion protein in bacteria. This was purified, digested with PreScission Protease (GE Healthcare Life Sciences, Piscataway, NJ), eluted, and then injected into rabbits for production of polyclonal antisera (Veritas Labs, Rockville, MD). The anti-spartin IgG fraction was purified using protein A-Sepharose (Sigma-Aldrich, St. Louis, MO). The following antibodies were obtained commercially: rabbit polyclonal anti-HA-epitope and anti-Eps15 (Abcam, Cambridge, MA), rabbit polyclonal anti-STAM1 (Chemicon, Temecula, CA), rabbit polyclonal anti-Myc-epitope (Sigma-Aldrich), mouse monoclonal anti-Myc-epitope conjugated to agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA), and mouse monoclonal anti-ubiquitin (BioMol, Plymouth Meeting, PA).
Ubiquitin “Pulldown” Assays and Immunoprecipitations
An ubiquitin cDNA (residues 1–76) was cloned into the EcoRI site of pGEX-6P-1 vector (GE Healthcare Life Sciences), and the GST fusion protein was expressed in bacteria and purified. The ubiquitin pull-down assay was performed as described by Puertollano and Bonifacino (2004). For immunoprecipitations, HeLa cells were washed twice with ice-cold phosphate-buffered saline (pH 7.4; PBS) and then lysed with PBS containing 1.0% Triton X-100, 5 mM EDTA, and protease inhibitors. Cell lysates were clarified by centrifugation (13,000 × g, 30 min) and then incubated with anti-Myc antibody beads or anti-spartin IgG bound to protein A-Sepharose for 3 h, washed three times with lysis buffer containing 0.5% Triton X-100 and then once with PBS, and finally analyzed by immunoblotting.
Confocal Microscopy and Photobleaching
All images were obtained using a Zeiss LSM510 laser scanning microscope (Thornwood, NY) with a 63× 1.4 NA Plan Apochromat oil immersion objective. Excitation wavelengths were 488, 543, and 633 nm for Alexa-Fluor 488, 555, and 633 antibodies, respectively. Single sections were acquired at 0.8-μm thickness.
For fluorescence recovery after photobleaching (FRAP) experiments, transfected HeLa cells were cultured in imaging medium (DMEM without phenol red, 10% fetal bovine serum, and 20 mM HEPES; Invitrogen), placed at 4°C for 10 min, and then treated with 10 μg/ml nocodazole for 5 min at 37°C. YFP was excited at λ = 514 nm and detected at λ = 527 nm. The digital zoom was set at 2.0×, and the image area acquired before and after bleaching was set at 150 × 150 pixels to minimize the time needed to obtain images. An area of 29 × 29 pixels was photobleached with four iterations at 65% laser power and 100% transmission. The recovery of fluorescence intensity in the bleached area was recorded at the same laser power, but using 0.1% transmission, and the images were obtained every second for 100 s. Fluorescence intensities of the prebleached, postbleached, background areas were measured with LSM510 Software and results were analyzed using Microsoft Excel software (Redmond, WA). For images acquired using differential interference contrast, we applied flat-field correction.
EGFR Degradation Assay
HeLa cells transfected with spartin siRNA or control siRNA oligonucleotide duplexes were split, serum-starved for 16 h, and treated with EGF (100 ng/ml) in the presence of cyclohexamide (10 μg/ml). At the indicated times, cells were washed with cold PBS, lysed with Laemmli sample buffer, resolved on SDS-PAGE gels, and immunoblotted. To quantify the degradation of EGFR, we measured the intensity of EGFR immunoreactive bands at each time point using Adobe Photoshop 7.0 software (San Jose, CA). The amount of EGFR is expressed as a percentage of remaining EGFR compared with unstimulated cells.
Kinetics of EGF and Transferrin Internalization
To examine the rate of 125I-EGF internalization, siRNA-treated HeLa cells were split to 12-well plates and serum-starved in binding medium (DMEM, 20 mM HEPES, 0.5% bovine serum albumin) for 4 h. Cells were then incubated with 1.5 ng/ml 125I-EGF in binding medium at 37°C for 2, 4, 6, and 8 min. Nonspecific binding was determined at each time point in the presence of 300 ng/ml unlabeled EGF. At the indicated times, surface-bound and internalized 125I-EGF were quantified as previously described (Sorkin et al., 1996; Haglund et al., 2003). Rate constants for internalization were expressed as the ratio of internalized to surface 125I-EGF for each time point, after subtracting the values for nonspecific binding. Rates of 125I-transferrin internalization were determined as described by Huang et al. (2004).
RESULTS
Overexpressed Spartin Colocalizes with Eps15
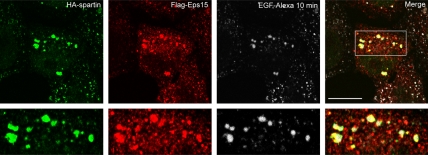
We previously demonstrated that spartin is a cytosolic and membrane-associated protein that interacts with Eps15 in in vitro pulldown assays and yeast two-hybrid tests (Bakowska et al., 2005). Here, we examined the distribution of overexpressed spartin and Eps15 in HeLa cells at steady state and after treatment with EGF-Alexa, and we observed colocalization of HA-spartin with Flag-Eps15 in punctuate structures in unstimulated cells (data not shown). Importantly, in HeLa cells treated with EGF-Alexa, both HA-spartin and Flag-Eps15 robustly colocalized with endosomes containing fluorescent EGF-Alexa after a 10-min chase (Figure 1). This colocalization was not observed at 35 min or longer times after stimulation with EGF, indicating that the association of these two proteins is transient in cells. Lastly, we found partial colocalization of HA-spartin puncta with early and late endosomal markers (data not shown).
Figure 1.
Colocalization of HA-spartin and Flag-Eps15 with internalized EGF-Alexa. HeLa cells cotransfected with HA-spartin and Flag-Eps15 were serum-starved and then incubated with EGF-Alexa 555 for 30 min at 4°C. Next, cells were warmed to 37°C for 10 min, fixed, and immunostained with anti-HA (green) and anti-Flag (red) antibodies using confocal fluorescence microscopy. EGF-Alexa labeling is shown in white. The merged image is shown in the far right panel, and the boxed area is enlarged in the lower panels. Scale bar, 15 μm.
Overexpressed Spartin is Recruited to Enlarged Endosomes Induced by Dominant-Negative Vps4-A
Spartin colocalizes with Eps15 on vesicular structures (Figure 1), and Eps15 accumulates on enlarged endosomes that have been induced by a dominant-negative form of Vps4-A (Bache et al., 2003). Vps4-A is an ATPase important for cargo sorting to the multivesicular bodies, and its ATPase cycle couples the dissociation/association of Vps4-A to endosomal membranes (Babst et al., 1998; Bishop and Woodman, 2000). Although ATP hydrolysis results in the release of Vps4-A and other ESCRT proteins from membranes to the cytosol, a mutation (E228Q) affecting the ATP hydrolysis of Vps4-A results in enlarged endosomes (Babst et al., 1998; Bishop and Woodman, 2000).
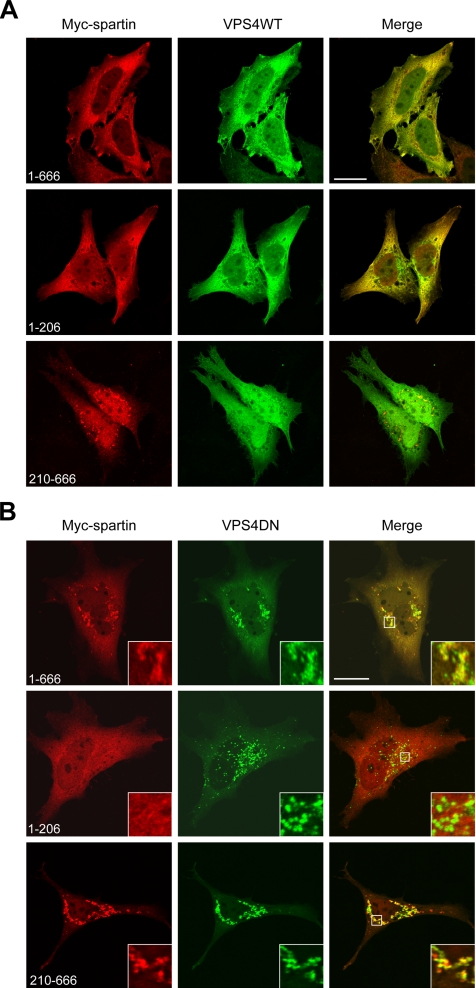
We examined the cellular localization of Myc-tagged full-length spartin as well as two deletion constructs, spartin(1–206) and spartin(210–666), in the presence of either wild-type GFP-Vps4-A or dominant-negative GFP-Vps4-AE228Q (Vps4DN). In transfected HeLa cells, wild-type Vps4-A was diffusely distributed throughout the cytoplasm, as previously reported (Bishop and Woodman, 2000). Although full-length spartin was localized predominantly in the cytoplasm with distinct vesicular staining, spartin(1–206) was localized exclusively within the cytoplasm (Figure 2A). In contrast, spartin(210–666) had a mostly vesicular distribution, with some diffuse cytoplasmic staining (Figure 2A). Overexpression of wild-type Vps4-A was not responsible for these differences, because the spartin fragments had similar cellular localizations when transfected alone (data not shown).
Figure 2.
Spartin is recruited to Vps4-AE228Q-induced enlarged endosomes. (A and B) HeLa cells were cotransfected with Myc-tagged full-length spartin(1–666) (top panels), spartin(1–206) (middle panels), spartin(210–666) (bottom panels) and either wild-type (WT) GFP-Vps4-A (A) or mutant (DN) GFP-Vps4-AE228Q (B). Cells were fixed and stained with anti-Myc epitope antibody to detect Myc-tagged spartin proteins and analyzed by confocal fluorescence microscopy. Colocalization of Myc-spartin (red) with wild-type or mutant Vps4-A (green) is indicated by yellow in the merged images. Boxed areas in B are enlarged in the insets. Scale bars, 20 μm.
We tested whether the cellular localizations of Myc-tagged full-length spartin and two deletion constructs of spartin (1–206 and 210–666) were altered upon expression of GFP-Vps4DN. Confocal microscopy analyses of transfected HeLa cells stained with anti-Myc antibody revealed that full-length spartin is robustly recruited to the Vps4DN-induced, enlarged endosomes in all overexpressing cells (Figure 2B). Cells overexpressing Myc-spartin(210–666) also showed vesicular staining that strongly colocalized with Vps4DN (Figure 2B). In contrast, Myc-spartin(1–206), which contains the MIT domain, showed only a diffuse cytoplasmic distribution and did not accumulate at enlarged endosomes in any examined cells (Figure 2B). We obtained similar results when Myc-tagged full-length spartin or the spartin fragments were coexpressed with another dominant-negative form of Vps4-A, GFP-Vps4-AK178Q, which does not bind ATP (data not shown). These results suggest that Vps4-A regulates membrane complexes harboring spartin and other proteins and that binding and/or hydrolysis of ATP is a critical step in this process.
Dynamic Association of Overexpressed Spartin with Internalized EGFR
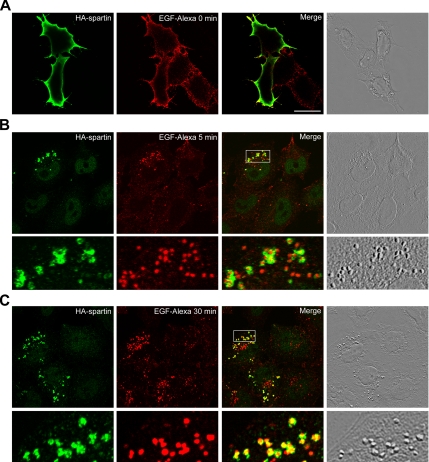
We used confocal analysis of immunofluorescence staining of HA-spartin in HeLa cells to demonstrate the colocalization of spartin with overexpressed Eps15 (Figure 1). In cells stimulated with EGF at 4°C, endogenous Eps15 translocates to the plasma membrane (Torrisi et al., 1999). In addition, endogenous Eps15 colocalizes with EGFR at 10 and 30 min after stimulation with EGF (Sorkina et al., 1999; Torrisi et al., 1999). These reports prompted us to examine further the distribution of HA-spartin upon stimulation with labeled EGF. Transfected HeLa cells were starved for 3 h, treated with EGF-Alexa 555 for 30 min at 4°C, chased in EGF-free medium for various periods of time (0, 5, 30, 60, and 90 min) at 37°C, and then fixed with paraformaldehyde. Using confocal immunofluorescence microscopy, we found that in cells stimulated with EGF-Alexa at time 0, HA-spartin was recruited from the cytoplasm to the plasma membrane (Figure 3A). In addition, HA-spartin colocalized with internalized EGF-containing endosomes 5 and 30 min after we triggered synchronous endocytosis by warming the cells to 37°C (Figure 3, B and C). Specifically, HA-spartin showed ring-like staining that surrounds the lumen containing EGF-Alexa. We observed no colocalization of HA-spartin with fluorescently labeled EGF after 60 min of warming the cells or at any later time points (data not shown). Importantly, HA-spartin colocalized in punctuate structures with GFP-EGFR after treatment with a low concentration of EGF (1 ng/ml) for 30 min at 4°C followed by a 1-min chase at 37°C (Supplementary Figure S1).
Figure 3.
Spartin colocalizes with EGF-Alexa. HeLa cells transfected with HA-spartin were serum-starved, incubated with EGF-Alexa 555 for 30 min at 4°C, and then warmed to 37°C for 0 min (A), 5 min (B), or 30 min (C). After fixing and immunostaining for the HA-epitope (green), cells were analyzed by confocal fluorescence microscopy. Colocalization of EGF-Alexa (red) and HA-spartin (green) is indicated by yellow in the merged images. Images acquired using differential interference contrast are shown in the far right panels. In B and C, boxed areas are enlarged in the bottom panels. Scale bar, 20 μm.
Endogenous Spartin Is Constitutively Mono-Ubiquitinated
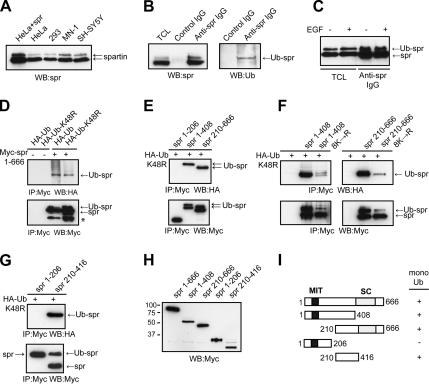
Many proteins that are associated with EGF-positive endosomes, including Hrs, Eps15, and STAM, are mono-ubiquitinated (Bishop et al., 2002; Klapisz et al., 2002; Bache et al., 2003), so we examined whether spartin undergoes this modification. When immunoblots of cell lysates from HeLa cells overexpressing spartin as well as from different untransfected cell lines were probed with spartin-specific antibodies, they revealed a distinct doublet: a prominent band at ∼85 kDa and a weaker band at ∼95 kDa (Figure 4A). We obtained similar results when HeLa cells were serum-starved overnight before harvesting the cells (data not shown). Both bands correspond to spartin, because neither band was detected after treatment of HeLa cells with spartin siRNA (Supplementary Figure S2A). We considered the possibility that the ∼10-kDa difference in mobility between the two spartin bands might indicate that spartin is mono-ubiquitinated. Thus, we immunoprecipitated endogenous spartin from cell lysates and subsequently performed immunoblotting with anti-spartin or anti-ubiquitin antibodies. As shown in Figure 4B, anti-spartin antibodies but not control IgG precipitated both species of spartin (nonubiquitinated and ubiquitinated) as well as a ubiquitin conjugate, which corresponded in size to the high-molecular-weight band of spartin (∼95 kDa). Densitometric measurements of the spartin doublet revealed that ∼30–35% of spartin exists in the ubiquitinated form (data not shown).
Figure 4.
Spartin is a mono-ubiquitinated protein. (A) Lysates from HeLa cells overexpressing spartin (5 μg protein/lane) or from untransfected cells (10 μg protein/lane) were immunoblotted (WB) using anti-spartin (spr) antibodies. Arrows indicate the ∼85- and ∼95-kDa spartin doublet. (B) HeLa cell lysates were immunoprecipitated with anti-spartin antibodies (Anti-spr IgG) or control rabbit IgG. Aliquots from total cell lysates (TCL), and immunoprecipitates were immunoblotted with anti-spartin (WB; left panel) or anti-ubiquitin (Ub; right panel) antibodies. (C) HeLa cells were serum starved for 16 h and either left unstimulated (−) or stimulated (+) with EGF for 10 min, and then lysates were immunoprecipitated with anti-spartin antibodies. Aliquots from total cell lysates and immunoprecipitates were then immunoblotted with anti-spartin antibodies. All samples had both mono-ubiquitinated spartin (Ub-spr) and nonubiquitinated spartin (spr). (D) Lysates from HeLa cells overexpressing either HA-Ub or HA-UbK48R alone or else together with full-length Myc-spartin(1–666) were immunoprecipitated (IP) with anti-Myc-antibody beads and analyzed by immunoblotting using antiHA (top panels) or anti-Myc antibodies (bottom panels). An asterisk (*) indicates a spartin degradation product. (E–G) Lysates from HeLa cells transfected with HA-UbK48R alone or together with the indicated deletion fragments of spartin were immunoprecipitated with Myc-antibody beads and analyzed by immunoblotting using anti-HA (top panels) or anti-Myc antibodies (bottom panels). Spartin fragments 210–666(8K→R) and 1–408(8K→R) contain mutations of all eight Lys (K) residues to Arg (R) within the region spanning residues 210–408. (H) Lysates of HeLa cells expressing the indicated Myc-tagged spartin deletion constructs were immunoblotted with anti-Myc antibody to verify expression. Sizes of protein standards are indicated to the left in kDa. (I) Schematic diagram of spartin deletion constructs used in this study; boundary amino acid residues are indicated. +, fragments that are mono-ubiquitinated; MIT, contained within microtubule-interacting and trafficking proteins; SC, senescence domain.
Hrs, epsin1, and epsin2 proteins are mono-ubiquitinated in response to EGF treatment (Polo et al., 2002). However, spartin exhibited similar levels of ubiquitination in serum-starved and EGF-stimulated HeLa cells (Figure 4C), suggesting that it is constitutively mono-ubiquitinated. We confirmed that spartin is mono-ubiquitinated by coexpressing full-length Myc-spartin with either HA-ubiquitin or HA-ubiquitinK48R (mutated ubiquitin that cannot form a polyubiquitin chain destined for degradation via the proteasome) and then immunoprecipitating Myc-spartin; anti-Myc antibodies precipitated both species of spartin as well as HA-ubiquitin or HA-ubiquitinK48R. On immunoblots, the higher-molecular-weight band of Myc-spartin corresponded in size to an HA-tagged ubiquitin band in all cases (Figure 4D), indicating that HA-ubiquitin is covalently linked to spartin. Because spartin immunoprecipitation with HA-ubiquitinK48R resulted in a less prominent “smear” after immunoblotting with HA antibody than with HA-ubiquitin, it is feasible that spartin, in addition to being mono-ubiquitinated, is also polyubiquitinated via Lys48.
To identify the region where spartin is ubiquitinated, we expressed three different Myc-tagged fragments of spartin (comprising residues 1–206, 1–408, and 210–666) together with HA-ubiquitinK48R in HeLa cells, immunoprecipitated these fragments with anti-Myc antibodies, and then immunoblotted the precipitates with anti-Myc or anti-HA antibodies. Spartin(1–206), which contains the MIT domain, is not mono-ubiquitinated (Figure 4E), as indicated by the lack of an HA-immunoreactive band. However, immunoprecipitation of spartin(1–408) and spartin(210–666) with anti-Myc antibodies revealed two distinct Myc-spartin bands and one HA-ubiquitin band (Figure 4E), demonstrating that each of these fragments undergoes mono-ubiquitination. To further identify the region of mono-ubiquitination within spartin, we mutated the eight Lys residues that are present within amino acids 210–408 to Arg residues; this segment comprises the overlapping region of fragments 1–408 and 210–666, both of which are mono-ubiquitinated. The resulting mutated spartin fragments 1–408 (8K→R) and 210–666(8K→R) and the nonmutated fragments 1–408 and 210–666 were overexpressed together with HA-ubiquitinK48R and then immunoprecipitated with anti-Myc antibodies. In both spartin fragments, the mutations resulted in a marked decrease in the level of mono-ubiquitination (Figure 4F), suggesting that ubiquitination of spartin occurs within the region consisting of residues 210–408. Importantly, immunoprecipitation of extracts from cells overexpressing Myc-spartin(210–416) together with HA-ubiquitinK48R revealed that residues 210–416 are sufficient for mono-ubiquitination of spartin (Figure 4G). However, the full-length spartin with eight Lys mutated to Arg within the region 210–408 was still ubiquitinated to a significant extent, suggesting that ubiquitination at other lysines is more prevalent within the full-length protein (data not shown). All deletion constructs of spartin were expressed to similar levels upon transfection (Figure 4H). Taken together, these results demonstrate that endogenous spartin is mono-ubiquitinated and that a region sufficient for ubiquitination is located between amino acid residues 210–408 (Figure 4I).
Spartin Binds to Ubiquitin
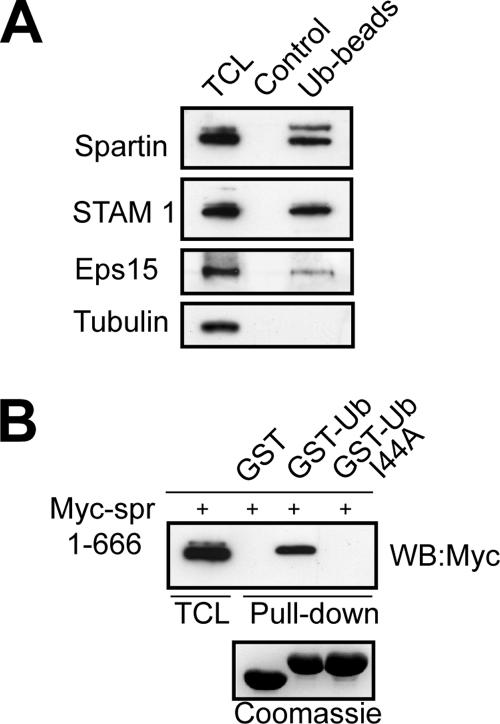
It is well documented that proteins that can undergo mono-ubiquitination have an intrinsic propensity to bind to ubiquitin (Oldham et al., 2002; Hicke et al., 2005; Hoeller et al., 2006). Because spartin is mono-ubiquitinated, we predicted that it would also interact with ubiquitin. To test this hypothesis, we performed in vitro binding assays using ubiquitin-agarose beads and found that endogenous spartin from HeLa cell lysates bound to ubiquitin-agarose beads but not to agarose alone. These results are similar to those of known ubiquitin-binding proteins Eps15 and STAM1. Tubulin was used as a negative control, and it did not interact with ubiquitin (Figure 5A). Most proteins containing a specific ubiquitin-binding motif interact with ubiquitin via a hydrophobic patch centered on Ile44 (Hicke et al., 2005). We tested Myc-tagged spartin for binding to ubiquitin and ubiquitinI44A in an in vitro binding assay. Although full-length spartin bound to GST-ubiquitin, it did not interact with GST-ubiquitinI44A or with GST alone (Figure 5B). Our results demonstrate that spartin interacts with ubiquitin via the Ile44 hydrophobic pocket. A particularly interesting finding is that both nonubiquitinated spartin and ubiquitinated spartin bound to exogenous ubiquitin. This is in contrast to findings for ubiquitinated Sts1, Eps15, and Hrs, in which intramolecular interactions prohibit ubiquitin-binding motifs from binding intermolecularly to ubiquitin (Hoeller et al., 2006).
Figure 5.
Spartin binds to ubiquitin. (A) HeLa total cell lysates (TCL) were incubated with ubiquitin-agarose (Ub-beads) or protein A-agarose (Control), and aliquots from total cell lysate and bound proteins were analyzed by immunoblotting for the indicated proteins. Molecular sizes: Eps15, ∼145 kDa; STAM1, ∼62 kDa; tubulin, ∼52 kDa. (B) Lysates from HeLa cells transfected with Myc-spartin (Myc-spr) were incubated with GST, GST-Ub, or GST-UbI44A bound to glutathione-Sepharose. Total cell lysates (TCL) and bound proteins were analyzed by immunoblotting (WB) using an anti-Myc antibody. A Coomassie-stained gel (bottom panel) demonstrates that similar amounts of fusion proteins were used for the pulldowns.
Dynamics of YFP-Spartin in Cells
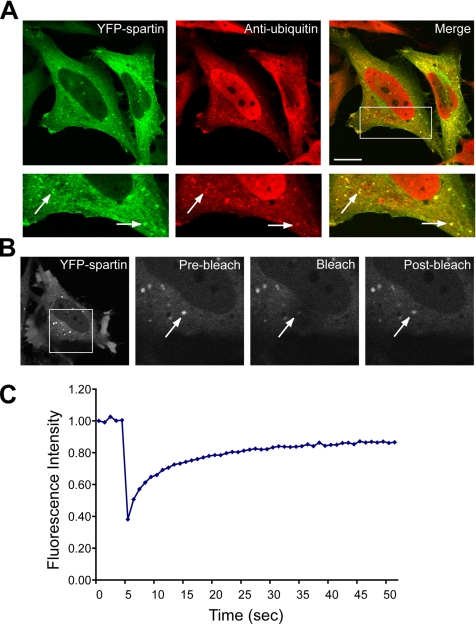
We generated a chimera of human spartin cDNA with the YFP for use in live cell imaging. We compared the localization of YFP-spartin to that of HA-spartin to ensure that the presence of the large fluorescence tag did not change the distribution of overexpressed protein. Transfected YFP-spartin showed a mostly cytoplasmic distribution but was also present in some discrete punctate structures that strongly colocalized with endogenous ubiquitin in all examined cells (Figure 6A). This cellular distribution corresponded to that of HA-spartin, and the punctate structures of both also colocalized with endogenous ubiquitin (data not shown).
Figure 6.
YPF-spartin puncta colocalize with endogenous ubiquitin and represent a highly mobile fraction. (A) HeLa cells were transfected with YFP-spartin, fixed, stained with anti-ubiquitin antibodies, and analyzed by confocal fluorescence microscopy. Bottom, enlargements of the boxed area. Colocalization of YFP-spartin (green) and anti-ubiquitin antibodies (red) is indicated by yellow in the merged images. Arrows indicate representative puncta exhibiting colocalization of YFP-spartin and ubiquitin. Scale bar, 15 μm. (B) HeLa cells were transfected with YFP-spartin and, after 18 h, treated with nocodazole as described in Materials and Methods. An area of 29 × 29 pixels was photobleached, and fluorescence recovery into the bleached region was measured by obtaining images every second. The boxed area in the left panel is enlarged in subsequent panels. Arrows indicate a representative YFP-spartin punctate structure subjected to FRAP analysis, while other puncta in the field were not bleached. The postbleach image was acquired after 18 s. (C) FRAP data from three experiments comprising a total of 16 puncta from different cells are presented graphically.
To examine the dynamics of YPF-spartin localized to the punctate structures in vivo, we applied the FRAP technique to transfected HeLa cells. To bleach individual vesicular structures and subsequently image those structures each second after photobleaching, we measured the FRAP of YFP-spartin in cells treated with nocodazole, which depolymerizes microtubules and prevents vesicles from moving, ensuring consistent FRAP measurements. We obtained images before and after photobleaching to monitor the recovery of fluorescence in the bleached area over time (Figure 6B). FRAP analyses of YFP-spartin showed that the bleached vesicular structures rapidly recovered their fluorescence within an average t1/2 = 5.0 ± 0.5 s. Moreover, YFP-spartin was a very mobile protein, as the Mf (mobile fraction) was ∼92%, and in some cells it approached 100% (Figure 6C). These data indicate that spartin rapidly cycles between the vesicular structures and cytoplasm and that this accounts for the fast recovery of fluorescence after photobleaching.
Depletion and Overexpression of Spartin Affects EGFR Trafficking
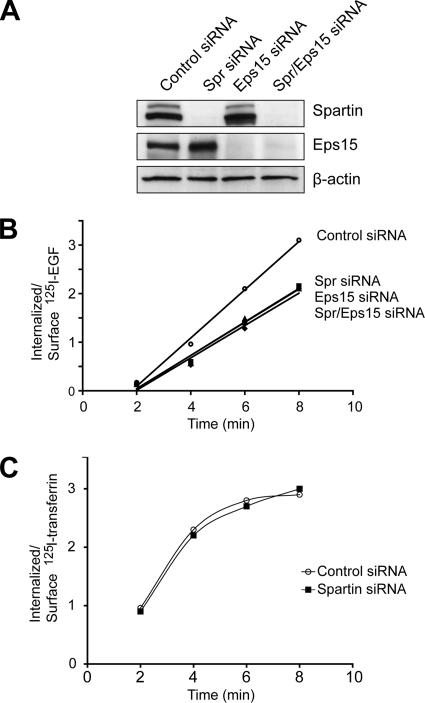
We demonstrated earlier that, upon activation of EGFR, spartin translocates from the cytoplasm to the plasma membrane (Figure 3A). This finding suggested that spartin might be involved in early events of EGFR internalization. We applied low concentrations of 125I-EGF, which preferentially uses the clathrin-dependent pathway for EGFR internalization, to test the effect of depleting spartin, Eps15, and spartin/Eps15 on the rate of EGF internalization in HeLa cells. All cells were treated with specific siRNAs to knock down protein expression, and then these cells were divided into two groups one day before the test, with half of the cells used for internalization experiments and half for immunoblotting to monitor the extent of protein depletion by the siRNAs. As shown in Figure 7A, there was nearly complete depletion of both spartin and Eps15 proteins when cells were treated simultaneously with specific spartin and Eps15 siRNAs, and there was a specific protein knockdown when cells were treated with each siRNA individually. We found that the rate of EGF uptake was decreased by ∼30% in cells depleted of spartin compared with cells treated with control siRNA. Importantly, knockdown of Eps15 decreased the rate of EGFR internalization to the same level as that in cells depleted of spartin. Simultaneous knockdown of both spartin and Eps15 resulted in diminished internalization of EGF, with kinetics indistinguishable from cells depleted of each protein alone (Figure 7B). This experiment was repeated with another set of spartin- and Eps15-specific siRNAs (Spr siRNA 2 and Eps15 siRNA 2, respectively; Supplementary Figure S2). The depletion of spartin, Eps15, or spartin/Eps15 resulted in ∼22% decrease of the rate of EGF uptake compared with cells treated with control siRNA (data not shown). In contrast, depletion of spartin using either Spr siRNA 1 or 2 had no effect on the rate of internalization of 125I-transferrin (Figure 7C).
Figure 7.
Effects of spartin depletion on rates of EGF and transferrin internalization. (A) HeLa cells were treated with the indicated siRNAs (sequence 1 for both spartin and Eps15), and cell lysates were analyzed by immunoblotting to evaluate expression levels of spartin and Eps15. β-Actin levels were monitored to confirm equal loading. (B) HeLa cells treated with the indicated siRNAs were incubated with 1.5 ng/ml 125I-EGF for 2–8 min, and rate constants for EGF internalization were calculated as described in Materials and Methods. Rate constants for cells treated with control, spartin, Eps15, and spartin/Eps15 siRNA are 0.49 min−1, 0.34 min−1, 0.34 min−1, and 0.33 min−1, respectively. ○, control siRNA; ■, spartin siRNA; ▴, Eps15 siRNA; ♦, both spartin and Eps15 siRNAs. Experiments were repeated twice, in triplicate, for each time point. Bars indicating the SDs are smaller than the symbols. (C) HeLa cells treated with control (○) or spartin-specific (■) siRNAs were incubated with 1.0 μg/ml 125I-transferrin for 2–8 min. Results of a representative experiment are shown; similar results were obtained in three additional trials.
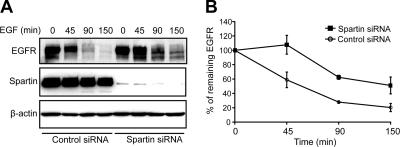
Overexpressed spartin was found in EGF-positive endosomes (Figure 3, A and B), suggesting that spartin might have a role in sorting and/or degradation of activated EGF receptors. EGFR degradation was assessed by immunoblotting after EGF stimulation of serum-starved HeLa cells transfected with control or spartin siRNA. We observed a decreased rate of EGFR degradation at all time points after ligand stimulation in cells treated with spartin siRNA compared with cells treated with control siRNA (Figure 8A). Quantitative analyses of three independent experiments revealed that at 45 min after ligand stimulation, depletion of spartin resulted in ∼45% decrease of EGFR degradation compared with cells expressing physiological levels of spartin. At 90 and 150 min, cells treated with spartin siRNA showed ∼30% decrease of EGFR degradation compared with cells treated with control siRNA (Figure 8B).
Figure 8.
Spartin depletion decreases the degradation of EGFR. (A) HeLa cells treated with control or spartin siRNA were serum starved and then stimulated with EGF for the indicated times. Cell lysates were analyzed by immunoblotting with anti-EGFR antibodies, and blots were subsequently reprobed with anti-spartin and anti-β-actin antibodies. (B) Graphical representation of quantified optical densities of EGFR protein bands from three independent experiments. p < 0.05 at all time points using one-tailed Student's t test.
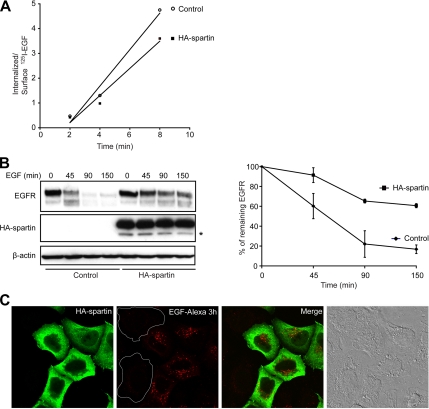
We also tested the effect of overexpressed spartin on the rate of EGFR internalization and degradation. HeLa cells transfected with HA-spartin showed ∼27% decrease in the rate of EGFR internalization as compared with cells transfected with empty vector (Figure 9A). HA-spartin also decreased the rate of EGFR degradation; quantitative analysis revealed that HA-spartin decreased the EGFR degradation by ∼50% at 90 and 150 min after ligand stimulation compared with control cells (Figure 9B). The decreased rate of EGFR degradation in cells transfected with HA-spartin and treated with EGF-Alexa was also clearly visualized using immunofluorescence (Figure 9C).
Figure 9.
Overexpression of HA-spartin inhibits the trafficking of EGFR. (A) HeLa cells transfected with either an empty vector (○) or HA-spartin (■) were incubated with 1.5 ng/ml 125I-EGF for 2–8 min, and the ratio of internalized to surface 125I-EGF was determined at the indicated times. (B) HeLa cells transfected with empty vector or HA-spartin were serum-starved and then stimulated with 1.5 ng/ml EGF for the indicated times. Left, cell lysates were analyzed by immunoblotting with anti-EGFR antibodies, and blots were subsequently reprobed with anti-HA and anti-β-actin antibodies. An asterisk (*) identifies a spartin degradation product. Right, graphical representation of quantified optical densities of EGFR protein bands from three independent experiments. p < 0.05 at all time points tested using one-tailed Student's t test. (C) HeLa cells transfected with HA-spartin were serum-starved, incubated with EGF-Alexa 555 (red) for 30 min at 4°C, and then warmed to 37°C for 3 h. After fixing and immunostaining for the HA-epitope (green), cells were analyzed by confocal fluorescence microscopy. Untransfected cell boundaries are outlined in white. An image acquired using differential interference contrast is shown in the far right panel. Scale bar, 15 μm.
Thus, both depletion of spartin and its overexpression have the same effect on EGFR trafficking. This bimodal action of lack of spartin and its overexpression on EGFR trafficking has been documented for other proteins implicated in sorting, such as Hrs (Bache et al., 2003; Morino et al., 2004), and may reflect interactions of the overexpressed proteins outside of the proper intracellular context, resulting in a dominant-negative impairment of EGR internalization and degradation.
DISCUSSION
In this study, we have identified a functional role for the Troyer syndrome protein spartin in endocytosis. Although spartin is predominantly cytosolic, it is recruited to enlarged endosomes induced by Vps4DN. It is also redistributed from the cytoplasm to the plasma membrane immediately after EGFR activation and is then present in EGF-positive endosomes. After cells were depleted of spartin using specific siRNAs, they exhibited a decreased rate of EGFR degradation compared with control cells. In addition, depletion of spartin results in decreased uptake and/or recycling of EGF. Taken together, our data indicate that spartin is an important component in the intracellular sorting of EGFR and very likely other ubiquitinated receptors.
Low levels of EGF trigger the internalization of EGFR via clathrin-mediated endocytosis (Sigismund et al., 2005). This complex, highly coordinated process begins with the recruitment of AP-2, an adaptor protein that drives the assembly of the clathrin lattice and binds to many accessory proteins, including amphiphysin, intersectin, epsins, auxilin, and Eps15 (Kirchhausen, 2000; Slepnev and De Camilli, 2000). Several lines of evidence support the role of Eps15 in endocytosis (Tebar et al., 1996; Carbone et al., 1997; Benmerah et al., 1998; Benmerah et al., 1999). Indeed, Eps15 is redistributed after EGFR activation; it is initially recruited to the plasma membrane and then follows the endocytosed EGFR (Torrisi et al., 1999). In general, the behavior of spartin in response to EGFR activation is similar to that of Eps15. Presently, the molecular mechanisms for the redistribution of spartin are unknown. However, one possibility might be that, upon EGFR stimulation, phosphorylated Eps15 recruits spartin and translocates the spartin-Eps15 complex to the plasma membrane, most likely to coated pits, where the presence of Eps15 is well documented (Tebar et al., 1996; Torrisi et al., 1999). Alternatively, EGFR activation might induce phosphorylation of spartin, and this modification could be responsible for the translocation of spartin to the plasma membrane, where spartin might associate with its binding partner, Eps15. Although we have not detected phosphorylation of spartin on Tyr residues (data not shown), we are currently examining Ser/Thr phosphorylation of spartin.
An important finding of our study is that acute depletion of spartin by siRNA in HeLa cells decreases the rate of EGFR degradation. After internalization of EGFR, the occupied receptor is sorted to the late endosomes for subsequent degradation by the lysosomes, and depletion of many proteins known to be involved in sorting of EGFR results in the diminished degradation of this receptor. For example, down-regulation of Hrs or STAM1 and STAM2, which are mono-ubiquitinated and contain ubiquitin-interacting motifs, decreases EGFR degradation by 40 and 35%, respectively, compared with control cells (Bache et al., 2003; Kanazawa et al., 2003). The colocalization of spartin with EGFR for 30 min after its internalization, together with the data showing that depletion of spartin decreases the rate of EGFR degradation, indicates that spartin regulates the sorting of ubiquitinated cargo receptors.
Another major finding of this study is the identification of the covalent modification of spartin by ubiquitination. Using immunoblotting, we observed protein bands representing endogenous spartin at ∼85 and ∼95 kDa in different human cell lines, which concurs with another report using a different antibody (Robay et al., 2006), and we demonstrated that the 95-kDa band represents a mono-ubiquitinated form of spartin. We estimate that ∼30–35% of endogenous spartin is mono-ubiquitinated and that this modification does not depend on EGF stimulation, indicating that spartin constitutively undergoes this post-translational modification. Other investigators showed that mono-ubiquitination of endogenous or overexpressed Eps15 in Her14 cells (NIH3T3 cells expressing EGFR) occurs upon EGF stimulation (van Delft et al., 1997; Klapisz et al., 2002; Fallon et al., 2006), whereas in other cell lines endogenous Eps15 is constitutively mono-ubiquitinated (Klapisz et al., 2002). Mono-ubiquitination of other proteins involved in trafficking of internalized cargo is EGF dependent. For example, overexpressed Hrs, epsin1, and epsin2 in COS-7 cells have been shown to be ubiquitinated only in response to EGF stimulation (Polo et al., 2002). It is reasonable to infer that although mono-ubiquitination of some sorting proteins is EGF dependent, other proteins are constitutively ubiquitinated. By this means they might present different molecular modules in the ubiquitin-receptor network and regulate specific aspects of clathrin-dependent or clathrin-independent endocytic pathways.
Similar to other mono-ubiquitinated proteins involved in endocytic trafficking of cargo receptors (Klapisz et al., 2002; Oldham et al., 2002; Polo et al., 2002), spartin binds to the ubiquitin moiety. Moreover, like most proteins, spartin interacts with ubiquitin via its Ile44 hydrophobic pocket (Hicke et al., 2005). Currently, at least 15 different ubiquitin-binding domains have been characterized, including ubiquitin-interacting motif, ubiquitin E2 variant domain, and ubiquitin-associated domain (Hicke et al., 2005). However, spartin does not harbor any of these and thus likely contains a novel ubiquitin-binding region.
Knockdown of endogenous spartin or Eps15 in HeLa cells by siRNA, individually or in combination, decreases the rate of EGF uptake by ∼30% compared with cells treated with control siRNA. Because we applied physiological, low levels of 125I-EGF (1.5 ng/ml) which preferentially uses the clathrin-dependent pathway for EGFR internalization (Sigismund et al., 2005), our data indicate that spartin, in conjunction with Eps15, may function in clathrin-mediated endocytosis of EGFR. These results also suggest that spartin might be involved in the internalization of EGFR. Alternatively, spartin depletion might instead influence the rate of EGFR recycling, which is known to have a half-time of ∼5 min (Sorkin et al., 1991). Future detailed biochemical assays as well as experiments examining the presence of spartin on coated-pits will clarify the role of spartin in the internalization and/or recycling of EGFR.
The SPG20 mutation in the spartin gene results in a premature stop codon and possibly degradation of the truncated protein that results in loss of function and subsequently a length-dependent axonopathy of the corticospinal motor neurons, the cardinal feature of HSPs. This impairment might be caused by dysfunctions at pre- and/or postsynaptic structures, but at present we can only speculate about the function of spartin in neurons. A recent study found spartin in synaptic nerve terminals (Robay et al., 2006), and the depletion of EHS-1, an ortholog of Eps15 in Caenorhabditis elegans, resulted in a temperature-dependent defect in movement as well as a decreased number of synaptic vesicles (Salcini et al., 2001). Thus, spartin, similar to Eps15, might be an accessory protein involved in clathrin-mediated endocytosis of synaptic vesicles in presynaptic nerve terminals. Another possible role for spartin in neurons may be in the internalization, sorting, and/or degradation of specific receptors for neurotrophic factors such as BDNF or NGF, which are important for cell survival and neurite outgrowth. Interestingly, a number of proteins mutated in neurological disorders, including alsin, huntingtin, and parkin, have roles in endocytosis (Devon et al., 2006; Fallon et al., 2006; Rong et al., 2006). It remains to be determined whether lack of spartin affects endocytosis of growth factor receptors, which might lead to deficiency in survival signals and impaired neurite outgrowth.
In summary, we demonstrate the functional role of spartin in the regulation of clathrin-mediated endocytosis of EGFR. Future studies in neurons from spartin knockout mice, which we are currently generating, will assess the functional role of spartin in pre- and postsynaptic terminals and clarify mechanisms by which the lack of spartin results in a length-dependent axonopathy.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Nagle and D. Kauffman (National Institute of Neurological Disorders and Stroke [NINDS] DNA Sequencing Facility) for DNA sequencing and Drs. C. Smith and M. Karbowski for insightful discussions on FRAP experiments. We are grateful to Drs. S. Sigismund and P. P. Di Fiore for their advice regarding critical experiments. This work was supported by the Intramural Research Program of the NINDS, National Institutes of Health (NIH), NINDS K22 training grant NS 50137-01 to J.C.B., and the Howard Hughes Medical Institute-NIH Research Scholars Program (P.F.).
Abbreviations used:
- EGFR
epidermal growth factor receptor
- ESCRT
endosomal sorting complex required for transport
- FRAP
fluorescence recovery after photobleaching
- GST
glutathione S-transferase
- HA
hemagglutinin
- HSP
hereditary spastic paraplegia
- MIT
contained within microtubule-interacting and trafficking molecules
- Vps4DN
Vps4-AE228Q
- YFP
yellow fluorescence protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0833) on March 1, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Babst M., Katzmann D. J., Snyder W. B., Wendland B., Emr S. D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 2002;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Babst M., Wendland B., Estepa E. J., Emr S. D. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17:2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache K. G., Raiborg C., Mehlum A., Stenmark H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 2003;278:12513–12521. doi: 10.1074/jbc.M210843200. [DOI] [PubMed] [Google Scholar]
- Bakowska J. C., Jenkins R., Pendleton J., Blackstone C. The Troyer syndrome (SPG20) protein spartin interacts with Eps15. Biochem. Biophys. Res. Commun. 2005;334:1042–1048. doi: 10.1016/j.bbrc.2005.06.201. [DOI] [PubMed] [Google Scholar]
- Benmerah A., Bayrou M., Cerf-Bensussan N., Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1999;112:1303–1311. doi: 10.1242/jcs.112.9.1303. [DOI] [PubMed] [Google Scholar]
- Benmerah A., Lamaze C., Bègue B., Schmid S. L., Dautry-Varsat A., Cerf-Bensussan N. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N., Horman A., Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J. Cell Biol. 2002;157:91–101. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N., Woodman P. ATPase-defective mammalian VPS4 localizes to aberrant endosomes and impairs cholesterol trafficking. Mol. Biol. Cell. 2000;11:227–239. doi: 10.1091/mbc.11.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone R., Fré S., Iannolo G., Belleudi F., Mancini P., Pelicci P. G., Torrisi M. R., Di Fiore P. P. eps15 and eps15R are essential components of the endocytic pathway. Cancer Res. 1997;57:5498–5504. [PubMed] [Google Scholar]
- Carter R. E., Sorkin A. Endocytosis of functional epidermal growth factor receptor-green fluorescent protein chimera. J. Biol. Chem. 1998;273:35000–35007. doi: 10.1074/jbc.273.52.35000. [DOI] [PubMed] [Google Scholar]
- Chen H., Slepnev V. I., Di Fiore P. P., De Camilli P. The interaction of epsin and Eps15 with the clathrin adaptor AP-2 is inhibited by mitotic phosphorylation and enhanced by stimulation-dependent dephosphorylation in nerve terminals. J. Biol. Chem. 1999;274:3257–3260. doi: 10.1074/jbc.274.6.3257. [DOI] [PubMed] [Google Scholar]
- Ciccarelli F. D., Proukakis C., Patel H., Cross H., Azam S., Patton M. A., Bork P., Crosby A. H. The identification of a conserved domain in both spartin and spastin, mutated in hereditary spastic paraplegia. Genomics. 2003;81:437–441. doi: 10.1016/s0888-7543(03)00011-9. [DOI] [PubMed] [Google Scholar]
- Crosby A. H., Proukakis C. Is the transportation highway the right road for hereditary spastic paraplegia? Am. J. Hum. Genet. 2002;71:1009–1016. doi: 10.1086/344206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross H. E., McKusick V. A. The Troyer syndrome. A recessive form of spastic paraplegia with distal muscle wasting. Arch. Neurol. 1967;16:473–485. doi: 10.1001/archneur.1967.00470230025003. [DOI] [PubMed] [Google Scholar]
- Devon R. S., et al. Als2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proc. Natl. Acad. Sci. USA. 2006;103:9595–9600. doi: 10.1073/pnas.0510197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico A., Ballabio A., Rugarli E. I. Spastin, the protein mutated in autosomal dominant hereditary spastic paraplegia, is involved in microtubule dynamics. Hum. Mol. Genet. 2002;11:153–163. doi: 10.1093/hmg/11.2.153. [DOI] [PubMed] [Google Scholar]
- Errico A., Claudiani P., D'Addio M., Rugarli E. I. Spastin interacts with the centrosomal protein NA14, and is enriched in the spindle pole, the midbody and the distal axon. Hum. Mol. Genet. 2004;13:2121–2132. doi: 10.1093/hmg/ddh223. [DOI] [PubMed] [Google Scholar]
- Evans K. J., Gomes E. R., Reisenweber S. M., Gundersen G. G., Lauring B. P. Linking axonal degeneration to microtubule remodeling by spastin-mediated microtubule severing. J. Cell Biol. 2005;168:599–606. doi: 10.1083/jcb.200409058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon L., et al. A. regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat. Cell Biol. 2006;8:834–842. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- Fink J. K. Hereditary spastic paraplegia. Curr. Neurol. Neurosci. Rep. 2006;6:65–76. doi: 10.1007/s11910-996-0011-1. [DOI] [PubMed] [Google Scholar]
- Garrus J. E., et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Haffner C., Takei K., Chen H., Ringstad N., Hudson A., Butler M. H., Salcini A. E., Di Fiore P. P., De Camilli P. Synaptojanin 1: localization on coated endocytic intermediates in nerve terminals and interaction of its 170 kDa isoform with Eps15. FEBS Lett. 1997;419:175–180. doi: 10.1016/s0014-5793(97)01451-8. [DOI] [PubMed] [Google Scholar]
- Haglund K., Sigismund S., Polo S., Szymkiewicz I., Di Fiore P. P., Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- Harding A. E. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1:1151–1155. doi: 10.1016/s0140-6736(83)92879-9. [DOI] [PubMed] [Google Scholar]
- Hicke L., Schubert H. L., Hill C. P. Ubiquitin-binding domains. Nat. Rev. Mol. Cell Biol. 2005;6:610–621. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- Hoeller D., et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat. Cell Biol. 2006;8:163–169. doi: 10.1038/ncb1354. [DOI] [PubMed] [Google Scholar]
- Huang F., Khvorova A., Marshall W., Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- Kanazawa C., Morita E., Yamada M., Ishii N., Miura S., Asao H., Yoshimori T., Sugamura K. Effects of deficiencies of STAMs and Hrs, mammalian class E Vps proteins, on receptor downregulation. Biochem. Biophys. Res. Commun. 2003;309:848–856. doi: 10.1016/j.bbrc.2003.08.078. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Clathrin. Annu. Rev. Biochem. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- Klapisz E., Sorokina I., Lemeer S., Pijnenburg M., Verkleij A. J., van Bergen en Henegouwen P.M.P. A ubiquitin-interacting motif (UIM) is essential for Eps15 and Eps15R ubiquitination. J. Biol. Chem. 2002;277:30746–30753. doi: 10.1074/jbc.M203004200. [DOI] [PubMed] [Google Scholar]
- Lu J., Rashid F., Byrne P. C. The hereditary spastic paraplegia protein spartin localises to mitochondria. J. Neurochem. 2006;98:1908–1919. doi: 10.1111/j.1471-4159.2006.04008.x. [DOI] [PubMed] [Google Scholar]
- Morino C., Kato M., Yamamoto A., Mizuno E., Hayakawa A., Komada M., Kitamura N. A role for Hrs in endosomal sorting of ligand-stimulated and unstimulated epidermal growth factor receptor. Exp. Cell Res. 2004;297:380–391. doi: 10.1016/j.yexcr.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Oldham C. E., Mohney R. P., Miller S.L.H., Hanes R. N., O'Bryan J. P. The ubiquitin-interacting motifs target the endocytic adaptor protein epsin for ubiquitination. Curr. Biol. 2002;12:1112–1116. doi: 10.1016/s0960-9822(02)00900-4. [DOI] [PubMed] [Google Scholar]
- Panavas T., Pikula A., Reid P. D., Rubinstein B., Walker E. L. Identification of senescence-associated genes from daylily petals. Plant Mol. Biol. 1999;40:237–248. doi: 10.1023/a:1006146230602. [DOI] [PubMed] [Google Scholar]
- Patel H., Cross H., Proukakis C., Hershberger R., Bork P., Ciccarelli F. D., Patton M. A., McKusick V. A., Crosby A. H. SPG20 is mutated in Troyer syndrome, an hereditary spastic paraplegia. Nat. Genet. 2002;31:347–348. doi: 10.1038/ng937. [DOI] [PubMed] [Google Scholar]
- Polo S., Sigismund S., Faretta M., Guidi M., Capua M. R., Bossi G., Chen H., De Camilli P., Di Fiore P. P. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- Proukakis C., Cross H., Patel H., Patton M.A., Valentine A., Crosby A. H. Troyer syndrome revisited. A clinical and radiological study of a complicated hereditary spastic paraplegia. J. Neurol. 2004;251:1105–1110. doi: 10.1007/s00415-004-0491-3. [DOI] [PubMed] [Google Scholar]
- Puertollano R., Bonifacino J. S. Interactions of GGA3 with the ubiquitin sorting machinery. Nat. Cell Biol. 2004;6:244–251. doi: 10.1038/ncb1106. [DOI] [PubMed] [Google Scholar]
- Reid E. Science in motion: common molecular pathological themes emerge in the hereditary spastic paraplegias. J. Med. Genet. 2003;40:81–86. doi: 10.1136/jmg.40.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid E., Connell J., Edwards T. L., Duley S., Brown S. E., Sanderson C. M. The hereditary spastic paraplegia protein spastin interacts with the ESCRT-III complex-associated endosomal protein CHMP1B. Hum. Mol. Genet. 2005;14:19–38. doi: 10.1093/hmg/ddi003. [DOI] [PubMed] [Google Scholar]
- Robay D., Patel H., Simpson M. A., Brown N. A., Crosby A. H. Endogenous spartin, mutated in hereditary spastic paraplegia, has a complex subcellular localization suggesting diverse roles in neurons. Exp. Cell Res. 2006;312:2764–2777. doi: 10.1016/j.yexcr.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Rong J., McGuire J. R., Fang Z.-H., Sheng G., Shin J.-Y., Li S.-H., Li X.-J. Regulation of intracellular trafficking of huntingtin-associated protein-1 is critical for TrkA protein levels and neurite outgrowth. J. Neurosci. 2006;26:6019–6030. doi: 10.1523/JNEUROSCI.1251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcini A. E., et al. The Eps15 C. elegans homologue EHS-1 is implicated in synaptic vesicle recycling. Nat. Cell Biol. 2001;3:755–760. doi: 10.1038/35087075. [DOI] [PubMed] [Google Scholar]
- Scott A., Gaspar J., Stuchell-Brereton M. D., Alam S. L., Skalicky J. J., Sundquist W. I. Structure and ESCRT-III protein interactions of the MIT domain of human VPS4A. Proc. Natl. Acad. Sci. USA. 2005;102:13813–13818. doi: 10.1073/pnas.0502165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev V. I., De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat. Rev. Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- Soderblom C., Blackstone C. Traffic accidents: molecular genetic insights into the pathogenesis of the hereditary spastic paraplegias. Pharmacol. Ther. 2006;109:42–56. doi: 10.1016/j.pharmthera.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Sorkin A., Krolenko S., Kudrjavtceva N., Lazebnik J., Teslenko L., Soderquist A. M., Nikolsky N. Recycling of epidermal growth factor-receptor complexes in A431 cells: identification of dual pathways. J. Cell Biol. 1991;112:55–63. doi: 10.1083/jcb.112.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A., Mazzotti M., Sorkina T., Scotto L., Beguinot L. Epidermal growth factor receptor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J. Biol. Chem. 1996;271:13377–13384. doi: 10.1074/jbc.271.23.13377. [DOI] [PubMed] [Google Scholar]
- Sorkina T., Bild A., Tebar F., Sorkin A. Clathrin, adaptors and eps15 in endosomes containing activated epidermal growth factor receptors. J. Cell Sci. 1999;112:317–327. doi: 10.1242/jcs.112.3.317. [DOI] [PubMed] [Google Scholar]
- Tebar F., Sorkina T., Sorkin A., Ericsson M., Kirchhausen T. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 1996;271:28727–28730. doi: 10.1074/jbc.271.46.28727. [DOI] [PubMed] [Google Scholar]
- Torrisi M. R., Lotti L. V., Belleudi F., Gradini R., Salcini A. E., Confalonieri S., Pelicci P. G., Di Fiore P. P. Eps15 is recruited to the plasma membrane upon epidermal growth factor receptor activation and localizes to components of the endocytic pathway during receptor internalization. Mol. Biol. Cell. 1999;10:417–434. doi: 10.1091/mbc.10.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta N., Orso G., Rossetto M. G., Daga A., Broadie K. The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr. Biol. 2004;14:1135–1147. doi: 10.1016/j.cub.2004.06.058. [DOI] [PubMed] [Google Scholar]
- van Delft S., Schumacher C., Hage W., Verkleij A. J., van Bergen en Henegouwen P.M.P. Association and colocalization of Eps15 with adaptor protein-2 and clathrin. J. Cell Biol. 1997;136:811–821. doi: 10.1083/jcb.136.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.