Abstract
Duchenne muscular dystrophy (DMD), the most common lethal genetic disorder in children, is an X-linked recessive muscle disease characterized by the absence of dystrophin at the sarcolemma of muscle fibers. We examined a putative endometrial progenitor obtained from endometrial tissue samples to determine whether these cells repair muscular degeneration in a murine mdx model of DMD. Implanted cells conferred human dystrophin in degenerated muscle of immunodeficient mdx mice. We then examined menstrual blood–derived cells to determine whether primarily cultured nontransformed cells also repair dystrophied muscle. In vivo transfer of menstrual blood–derived cells into dystrophic muscles of immunodeficient mdx mice restored sarcolemmal expression of dystrophin. Labeling of implanted cells with enhanced green fluorescent protein and differential staining of human and murine nuclei suggest that human dystrophin expression is due to cell fusion between host myocytes and implanted cells. In vitro analysis revealed that endometrial progenitor cells and menstrual blood–derived cells can efficiently transdifferentiate into myoblasts/myocytes, fuse to C2C12 murine myoblasts by in vitro coculturing, and start to express dystrophin after fusion. These results demonstrate that the endometrial progenitor cells and menstrual blood–derived cells can transfer dystrophin into dystrophied myocytes through cell fusion and transdifferentiation in vitro and in vivo.
INTRODUCTION
Skeletal muscle consists predominantly of syncytial fibers with peripheral, postmitotic myonuclei, and its intrinsic repair potential in adulthood relies on the persistence of a resident reserve population of undifferentiated mononuclear cells, termed “satellite cells.” In mature skeletal muscle, most satellite cells are quiescent and are activated in response to environmental cues, such as injury, to mediate postnatal muscle regeneration. After division, satellite cell progeny, termed myoblasts, undergo terminal differentiation and become incorporated into muscle fibers (Bischoff, 1994). Myogenesis is regulated by a family of myogenic transcription factors including MyoD, Myf5, myogenin, and MRF4 (Sabourin and Rudnicki, 2000). During embryonic development, MyoD and Myf5 are involved in the establishment of the skeletal muscle lineage (Rudnicki et al., 1993), whereas myogenin is required for terminal differentiation (Hasty et al., 1993; Nabeshima et al., 1993). During muscle repair, satellite cells recapitulate the expression program of the myogenic genes manifested during embryonic development.
Dystrophin is associated with a large oligomeric complex of glycoproteins that provide linkage to the extracellular membrane (Ervasti and Campbell, 1991). In Duchenne muscular dystrophy (DMD), the absence of dystrophin results in destabilization of the extracellular membrane-sarcolemma-cytoskeleton architecture, making muscle fibers susceptible to contraction-associated mechanical stress and degeneration. In the first phase of the disease, new muscle fibers are formed by satellite cells. After depletion of the satellite cell pool in childhood, skeletal muscles degenerate progressively and irreversibly and are replaced by fibrotic tissue (Cossu and Mavilio, 2000). Like DMD patients, the mdx mouse lacks dystrophin in skeletal muscle fibers (Hoffman et al., 1987; Sicinski et al., 1989). However, the mdx mouse develops only a mild dystrophic phenotype, probably because muscle regeneration by satellite cells is efficient for most of the animal's life span (Cossu and Mavilio, 2000).
Myoblasts represent the natural first choice in cellular therapeutics for skeletal muscle because of their intrinsic myogenic commitment (Grounds et al., 2002). However, myoblasts recovered from muscular biopsies are poorly expandable in vitro and rapidly undergo senescence (Cossu and Mavilio, 2000). An alternative source of muscle progenitor cells is therefore desirable. Cells with a myogenic potential are present in many tissues, and these cells readily form skeletal muscle in culture (Gerhart et al., 2001). We report here that human dystrophin expression in the mdx model of DMD is attributed to cell fusion of mdx myocytes with human menstrual blood–derived stromal cells.
MATERIALS AND METHODS
Isolation of Human Endometrial Cells from Menstrual Blood
Menstrual blood samples (n = 21) were collected in DMEM with antibiotics (final concentrations: 100 U/ml penicillin/streptomycin) and 2% fetal bovine serum (FBS), and processed within 24 h. Ethical approval for tissue collection was granted by the Institutional Review Board of the National Research Institute for Child Health and Development, Japan. The centrifuged pellets containing endometrium-derived cells were resuspended in high-glucose DMEM medium (10% FBS, penicillin/streptomycin), maintained at 37°C in a humidified atmosphere containing 5% CO2, and allowed to attach for 48 h. Nonadherent cells were removed by changing the medium. When the culture reached subconfluence, the cells were harvested with 0.25% trypsin and 1 mM EDTA and plated to new dishes. After 2–3 passages, the attached endometrial stromal cells were devoid of blood cells. Human EM-E6/E7/hTERT-2 cells, endometrium-derived progenitors, were obtained from surgical endometrial tissue samples and were immortalized by E6, E7, and hTERT (Kyo et al., 2003). C2C12 myoblast cells were supplied by RIKEN Cell Bank (The Institute of Physical and Chemical Research, Japan).
Flow Cytometric Analysis
Flow cytometric analysis was performed as previously described (Terai et al., 2005). Cells were incubated with primary antibodies or isotype-matched control antibodies, followed by additional treatment with the immunofluorescent secondary antibodies. Cells were analyzed on an EPICS ALTRA analyzer (Beckman Coulter, Fullerton, CA). Antibodies against human CD13, CD14, CD29, CD31, CD34, CD44, CD45, CD50, CD54, CD55, CD59, CD73, CD90, CD105, CD117 (c-kit), CD133, HLA-ABC, and HLA-DR were purchased from Beckman Coulter, Immunotech (Marseille, France), Cytotech (Hellebaek, Denmark), and BD Biosciences PharMingen (San Diego, CA).
In Vitro Lentivirus-mediated Gene (EGFP) Transfer into EM-E6/E7/hTERT-2 Cells
Infection of EM-E6/E7/hTERT-2 cells with lentivirus having a CMV promoter and enhanced green fluorescent protein (EGFP) reporter resulted in high levels of EGFP expression in all cells. Cells were analyzed for EGFP expression by flow cytometry (Miyoshi et al., 1997, 1998).
In Vitro Myogenesis
Menstrual blood–derived cells or EM-E6/E7/hTERT-2 cells were seeded onto collagen I–coated cell culture dishes (Biocoat, BD Biosciences, Bedford, MA) at a density of 1 × 104/ml in growth medium (DMEM, supplemented with 20% FBS). Forty-eight hours after seeding onto collagen I–coated dishes, cells were treated with 5-azacytidine for 24 h. Cell cultures were then washed twice with PBS and maintained in differentiation medium (DMEM, supplemented with either 2% horse serum (HS) or 1% insulin-transferrin-selenium supplement [ITS]). The differentiation medium was changed twice a week until the experiment was terminated.
RT-PCR Analysis of EM-E6/E7/hTERT-2 Cells and Menstrual Blood–derived Cells
Total RNA was prepared using Isogen (Nippon Gene, Tokyo, Japan). Human skeletal muscle RNA was purchased from TOYOBO (Osaka, Japan). RT-PCR of Myf5, MyoD, desmin, myogenin, myosin heavy chain-IIx/d (MyHC-IIx/d), and dystrophin was performed with 2 μg of total RNA. RNA for RT-PCR was converted to cDNA with a first-stand cDNA synthesis kit (Amersham Pharmacia Biotechnology, Piscataway, NJ) according to the manufacturer's recommendations. The sequences of PCR primers that amplify human but not mouse genes are listed in Supplementary Table 1. PCR was performed with TaKaRa recombinant Taq (Takara Shuzo, Kyoto, Japan) for 30 cycles, with each cycle consisting of 94°C for 30 s, 62°C or 65°C for 30 s, and 72°C for 20 s, with an additional 10-min incubation at 72°C after completion of the last cycle.
Immunohistochemical and Immunocytochemical Analysis
Immunohistochemical analysis was performed as previously described (Mori et al., 2005). Briefly, the sections were incubated for 1 h at room temperature with mouse mAb against vimentin (Cone V9, DakoCytomation, Fort Collins, CO). After washing in PBS, sections were incubated with horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin, diluted, and washed in cold PBS. Staining was developed by using a solution containing diaminobenzidine and 0.01% H2O2 in 0.05 M Tris-HCl buffer, pH 6.7. Slides were counterstained with hematoxylin. In the cases of fluorescence, frozen sections fixed with 4% PFA were used. The antibodies against human dystrophin (NCL-DYS3; Novocastra, Newcastle upon Tyne, United Kingdom) or anti-human nuclei mouse mAb (clone 235-1, Chemicon, Temecula, CA) was used as a first antibody, and goat anti-mouse IgG conjugated with Alexa Fluor 488 or goat anti-mouse IgG antibody conjugated with Alexa Fluor 546 (Molecular Probes, Eugene, OR) was used as a second antibody.
Immunocytochemical analysis was performed as previously described (Mori et al., 2005), with antibodies to skeletal myosin (Sigma, St. Louis, MO; product no. M 4276), MF20 (which reacts with all sarcomere myosin in striated muscles, Developmental Studies Hybridoma Bank, University of Iowa, IA), α-sarcomeric actin (Sigma, product no. A 7811), and desmin (Bio-Science Products, Emmenbruecke, Switzerland; no. 010031, clone: D9) in PBS containing 1% bovine serum albumin. As a methodological control, the primary antibody was omitted. In the cases of fluorescence, slides were incubated with Alexa Fluor 546–conjugated goat anti-mouse IgG antibody.
Western Blotting
Western blot analysis was performed as previously described (Mori et al., 2005). Blots were incubated with primary antibodies (desmin, myogenin [Clone F5D, Santa Cruz Biotechnology], and dystrophin [NCL-DYSA, Novocastra]) for 1–2 h at room temperature. After washing three times in the blocking buffer, blots were incubated for 30 min with a horseradish peroxidase–conjugated secondary antibody (0.04 μg/ml) directed against the primary antibody. The blots were developed with enhanced chemiluminescence substrate according to the manufacturer's instructions.
Fusion Assay
EM-E6/E7/hTERT-2 cells (2500/cm2) or EGFP-labeled EM-E6/E7/hTERT-2 cells (2500/cm2) were cocultured with C2C12 myoblasts (2500/cm2) for 2 d in DMEM supplemented with 10% FBS and then cultured for 7 additional days in DMEM with 2% HS to promote myotube formation. The cultures were fixed in 4% paraformaldehyde and stained with a mouse anti-human nuclei IgG1 mAb and the mouse anti-human dystrophin IgG2a mAb (or anti-myosin heavy chain IgG2b mAb MF-20). The cells were visualized with appropriate Alexa-fluor–conjugated goat anti-mouse IgG1 and IgG2a (or IgG2b) secondary antibodies (Molecular Probes). Total cell nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole).
In Vivo Cell Implantation
Six- to 8-wk-old NOD/Shi-scid/IL-2 receptor −/− (NOG, CREA, Shizuoka, Japan) mice and 6- to 8-wk-old mdx-scid mice were implanted with EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells in seven independent experiments. The cells (2 × 107) were suspended in PBS in a total volume of 100 μl and were directly injected into the right thigh muscle of NOG mice or mdx-scid mice. The mice were examined 3 wk after cell implantation, and the right thigh muscle was analyzed for human vimentin and dystrophin by immunohistochemistry. The antibodies to vimentin and dystrophin (NCL-DYS3) react with human vimentin and dystrophin-equivalent protein, but not murine protein.
RESULTS
Surface Marker Expression of Endometrium-derived Cells
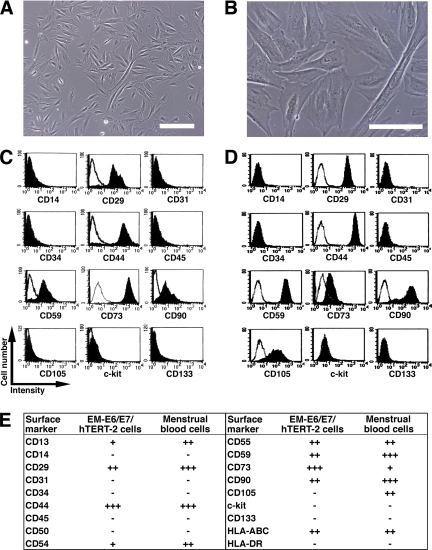
We investigated myogenic differentiation of primary cells without gene introduction from menstrual blood, because menstrual blood on the first day of the period is considered to include endometrial tissue. We successfully cultured a large number of primary cells from menstrual blood. Menstrual blood–derived cells showed at least two morphologically different cell groups: small spindle-like cells and large stick-like cells, regarded as being passage day (PD) 1 or 2 (Figure 1, A and B, respectively). Surface markers of the menstrual blood–derived cells were evaluated by flow cytometric analysis. Surface markers of EM-E6/E7/hTERT-2 cells (Figure 1C) and menstrual blood–derived cells (Figure 1D) were evaluated by flow cytometric analysis (Figure 1E). In these experiments, the cells were cultured in the absence of any inductive stimuli. EM-E6/E7/hTERT-2 cells were positive for CD13, CD29 (integrin β1), CD44 (Pgp-1/ly24), CD54, CD55, CD59, CD73, and CD90 (Thy-1), implying that EM-E6/E7/hTERT-2 cells expressed mesenchymal cell–related antigens in our experimental setting. Menstrual blood–derived cells were positive for CD13, CD29, CD44, CD54, CD55, CD59, CD73, CD90, and CD105, implying that proliferated and propagated cells express mesenchymal cell-related cell surface markers. Unlike EM-E6/E7/hTERT-2 cells, the menstrual blood–derived adherent cells were positive for CD105. EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells expressed neither hematopoietic lineage markers, such as CD34, nor monocyte-macrophage antigens such as CD14 (a marker for macrophage and dendritic cells), or CD45 (leukocyte common antigen). The lack of expression of CD14, CD34, or CD45 suggests that EM-E6/E7/hTERT-2 cells and the menstrual blood–derived cell culture in the present study is depleted of hematopoietic cells. The cells were also negative for expression of CD31 (PECAM-1), CD50, c-kit, and CD133. The cell population was positive for HLA-ABC, but not for HLA-DR. These results demonstrate that almost all cells derived from endometrium are of mesenchymal origin or stromal origin.
Figure 1.
Surface marker expression of endometrium-derived cells. (A and B) Morphology of menstrual blood–derived cells, regarded as being PD 1 or 2. Scale bars, 200 μm (A), 100 μm (B). (C and D) Flow cytometric analysis of cell surface markers of EM-E6/E7/hTERT-2 cells (C) and menstrual blood–derived cells (D). (E) Further phenotypic analysis in EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells are summarized. Peak intensity was estimated in comparison with isotype controls. +++, strongly positive (>100 times the isotype control); ++, moderately positive (<100 times but more than 10 times the isotype control); +, weakly positive (<10 times but more than twice the isotype control); −, negative (less than twice the isotype control).
Implanted Endometrium-derived Cells Induce De Novo Myogenesis in Immunodeficient NOG Mice
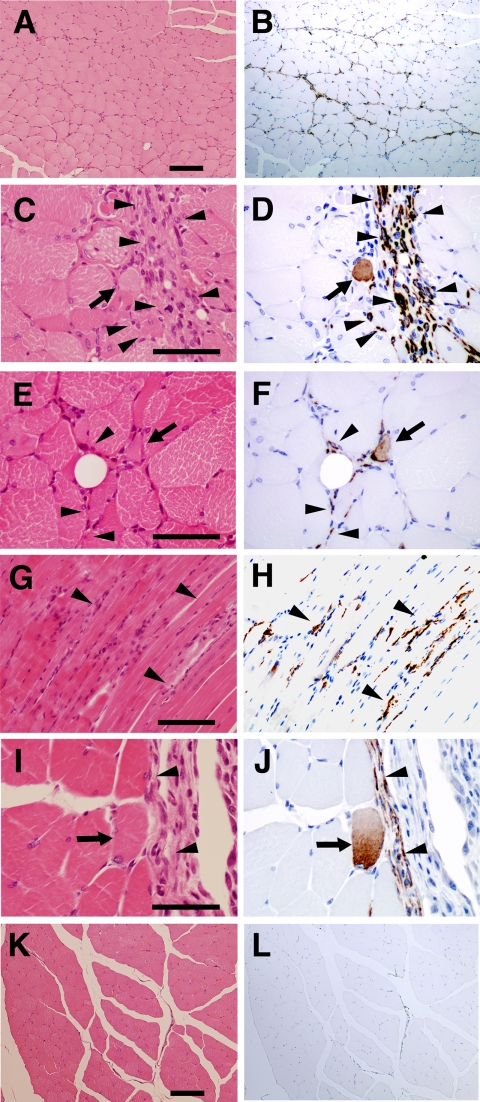
EM-E6/E7/hTERT-2 cells originate from the endometrial gland and are considered as endometrial progenitor cells or bipotential cells capable of differentiating into both glandular epithelial cells and endometrial stromal cells (Kyo et al., 2003). To determine whether EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells generate complete endometrial structure in vivo, like endometriosis, the cells without any treatment or induction were injected into the right thigh muscle of immunodeficient NOG mice. PBS without cells was injected into the left thigh muscles as a control. We failed to detect any endometrial structure in the cell-injected site. Immunohistochemical analysis using an antibody specific to human vimentin, an intermediate filament associated with a mesenchymal cell, revealed that the injected EM-E6/E7/hTERT-2 cells (Figure 2, A–F) or menstrual blood–derived cells (Figure 2, G–J) extensively migrated or infiltrated between muscular fibers (Figure 2, arrowheads). To investigate if the donor cells between muscular fibers occur as a result of cell migration, we performed a time-course analysis of implanted cells, as probed by human-specific antibody to vimentin (Supplementary Figure 1). Donor cells at 3 h after implantation are observed at the injection site, which is considered to be due to just injection of cells. Cells at 1–3 wk after implantation are detected between myocytes in the muscle bundle or muscular fascicle as well as in the interstitial tissue, implying that the donor cells between myotubes result from cell migration. Interestingly, some of the vimentin-positive implanted cells exhibited round-shaped structure (Figure 2, D, F, and J, arrows), suggesting that endometrium-derived cells are capable of differentiating into myoblasts/myotubes, and can contribute to skeletal muscle repair in patients suffering from genetic disorders such as DMD, similar to previous reports for marrow stromal cells (Dezawa et al., 2005) and synovial membrane cells (De Bari et al., 2003).
Figure 2.
Implantation of endometrium-derived cells-derived cells into the muscle of NOG mice. EM-E6/E7/hTERT-2 cells (A–F) or menstrual blood–derived cells (G–J) cultured in absence of any stimuli were directly injected into the right thigh muscle of NOG mice. Immunohistochemical analysis was performed using antibody that reacts to human vimentin but not to murine vimentin. (A, C, E, G, I, and K) hematoxylin and eosin stain. (B, D, F, H, J, and L) immunohistochemistry. Note that vimentin-positive EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells with a spindle morphology (C–J, arrowheads) extensively migrated into muscular bundles at 3 wk after injection, and some of the injected cells exhibited round structure (D, F, and J, arrows). Isotype mouse IgG1 served as a negative control (L). Scale bars, 100 μm (A, B, K, and L), 50 μm (C–F, I, and J), 90 μm (G and H).
Induction of Myogenic Differentiation in Endometrial Progenitor Cells In Vitro
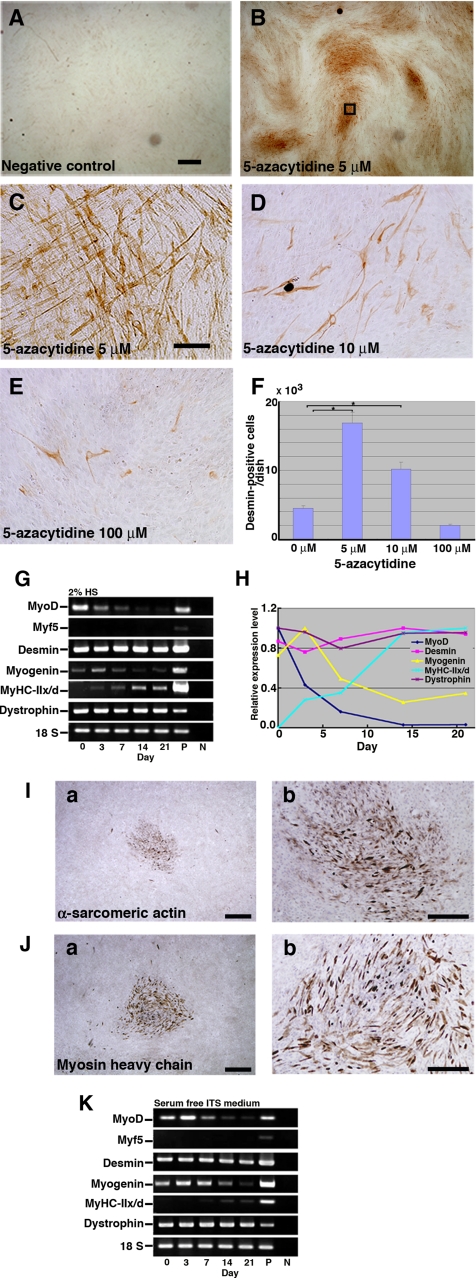
EM-E6/E7/hTERT-2 cells at 2 wk (cultured in the DMEM supplemented with 20% FBS) after exposure to different concentrations (5, 10, and 100 μM) of 5-azacytidine were analyzed by immunostaining using anti-desmin antibody (Figure 3, A–E). The number of desmin-positive cells was significantly higher in experimental groups with 5 or 10 μM 5-azacytidine than in untreated control groups (p < 0.05) (Figure 3F). To investigate whether EM-E6/E7/hTERT-2 cells are capable of differentiating into skeletal muscle cells in vitro, the cells were exposed to 5 μM 5-azacytidine for 24 h and then subsequently cultured in the DMEM supplemented with 2% HS or serum-free ITS for up to 21 d. Skeletal myoblastic differentiation of the cells was analyzed by evaluating expression of MyoD, Myf5, desmin, myogenin, MyHC-IIx/d, and dystrophin by RT-PCR. The MyoD, desmin, myogenin, and dystrophin genes were constitutively expressed, but MyHC-IIx/d and Myf5 genes were not. The decline of MyoD was observed in both the 2% HS (Figure 3, G and H) and the serum-free ITS (Figure 3K). The expression of MyHC-IIx/d, as determined by RT-PCR and immunocytochemistry, significantly increased with 2% HS (Figure 3G) and serum-free ITS (Figure 3K). Immunocytochemical analysis indicated that α-sarcomeric actin (Figure 3I) and MyHC (Figure 3J) were detected in the cells incubated with 2% HS for 21 d.
Figure 3.
Expression of myogenic-specific genes during myogenic differentiation of EM-E6/E7/hTERT-2 cells. (A–E) Immunocytochemical analysis of EM-E6/E7/hTERT-2 cells using an antibody to desmin. (A) Omission of only the primary antibody to desmin serves as a negative control. (C) Higher magnification of inset in B. (F) Myogenic differentiation of EM-E6/E7/hTERT-2 cells with exposure to different concentrations (B, 5 μM; C, 5 μM; D, 10 μM; E, 100 μM) of 5-azacytidine. To estimate myogenic differentiation, the number of all the desmin-positive cells was counted for each dish (n = 3). Data were analyzed for statistical significance using ANOVA. EM-E6/E7/hTERT-2 cells were cultured in the DMEM supplemented with 2% HS, and serum-free ITS. (G and K) RT-PCR analysis with PCR primers allows amplification of the human MyoD, Myf5, desmin, myogenin, myosin heavy chain type IIx/d (MyHC-IIx/d), and dystrophin cDNA (from top to bottom). RNAs were isolated from EM-E6/E7/hTERT-2 cells at the indicated day after treatment with 5-azacytidine. RNAs from human muscle and H2O served as positive (P) and negative (N) controls, respectively. Only the 18S PCR primer reacted with the human and murine cDNA. (H) Time course of MyoD, desmin, myogenin, MyHC-IIx/d, and dystrophin expression in the cells incubated with 2% HS for up to 21 d after 5-azacytidine treatment. Relative mRNA levels were determined using Multi Gauge Ver 2.0 (Fuji Film). The signal intensities of MyoD, desmin, and dystrophin mRNA at day 0, myogenin mRNA at day 3, and MyHC-II/d mRNA at day 21 were regarded as equal to 100%. (I and J) The cells were exposed to 5 μM 5-azacytidine for 24 h and then subsequently cultured in DMEM supplemented with 2% HS for 21 d. α-Sarcomeric actin (I) and skeletal myosin heavy chain (J) was detected by immunocytochemical analysis. Scale bars, 2 mm (A and B), 300 μm (C–E), 900 μm (Ia and Ja), 425 μm (Ib and Jb).
In Vitro Myogenic Differentiation of Menstrual Blood-derived Cells
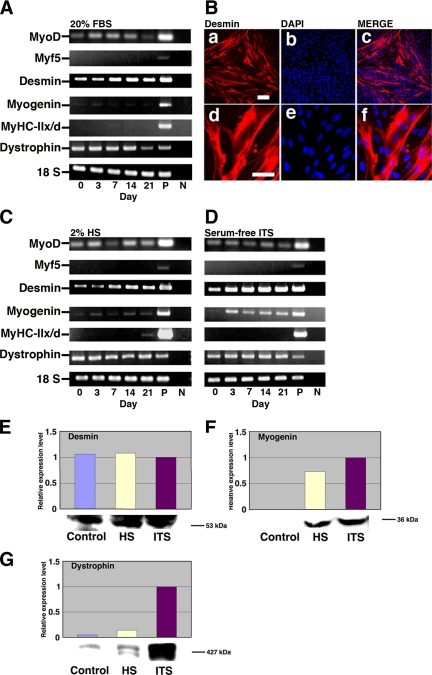
Menstrual blood–derived cells at 3 wk (cultured in DMEM supplemented with 20% FBS) after exposure to different concentrations (5, 10, and 100 μM) of 5-azacytidine were analyzed by immunostaining using anti-desmin antibody (data not shown). The number of desmin-positive cells was significantly higher in experimental groups with 5 or 10 μM 5-azacytidine than with 100 μM 5-azacytidine; for further in vitro experiments, the menstrual blood–derived cells were exposed to 5 μM 5-azacytidine for 24 h and then subsequently cultured in DMEM supplemented with low serum (2% HS) or serum-free ITS for up to 21 d (Figure 4). Myogenic potential of human menstrual blood–derived cells was analyzed by evaluating the expression of Myf5, MyoD, desmin, myogenin, MyHC-IIx/d, and dystrophin by RT-PCR. MyoD, desmin, and dystrophin genes were constitutively expressed in menstrual blood–derived cells, but MyHC-IIx/d and Myf5 were not (Figure 4A). For cells treated with 2% HS or serum-free ITS, the mRNA level of desmin and myogenin significantly increased after 3 d, and desmin steadily increased until day 21 (Figure 4, C and D). MyHC-IIx/d started to be expressed at a low level at day 21 of induction (Figure 4C). We then analyzed desmin expression by immunocytochemistry. Menstrual blood–derived cells were exposed to 5 μM 5-azacytidine for 24 h and then subsequently cultured in DMEM supplemented with 20% FBS for up to 2 wk. Desmin was readily detected in colonies of the menstrual blood–derived cells (Figure 4B). Western blot analysis indicated that desmin, myogenin, and dystrophin were highly expressed in the cells incubated for 3 wk (Figure 4, E–G). These results suggest that menstrual blood–derived cells are, like the EM-E6/E7/hTERT-2 cells, able to differentiate into skeletal muscle.
Figure 4.
Expression of myogenic-specific genes in differentiated menstrual blood–derived cells. Menstrual blood–derived cells were cultured in DMEM supplemented with 20% FBS, 2% HS, or serum-free ITS medium. (A) RT-PCR analysis with PCR primers that allows amplification of the human MyoD, Myf5, desmin, myogenin, MyHC-IIx/d, and dystrophin cDNA (from top to bottom). RNAs were isolated from menstrual blood–derived cells in DMEM supplemented with 20% FBS at the indicated day after treatment with 5 μM 5-azacytidine for 24 h. RNAs from human muscle and H2O served as positive (P) and negative (N) controls. Only the 18S PCR primer reacted with the human and murine cDNA. (B) Immunocytochemical analysis using an antibody to desmin (a–f) was performed on the menstrual blood–derived cells at 2 wk after exposure to 5 μM of 5-azacytidine for 24 h. The desmin-positive cells are shown at higher magnification (d–f). Merge of a and b is shown in c, and merge of d and e is shown in f. The images were obtained with a laser scanning confocal microscope. Scale bars, 200 μm (a–c) and 75 μm (d—f). (C and D) RT-PCR analysis of menstrual blood–derived cells on DMEM supplemented with 2% HS (C) or serum-free ITS medium (D) at the indicated day after exposure to 5 μM 5-azacytidine for 24 h. (E–G) Western blot analysis was performed on the cells cultured in myogenic medium indicated for 21 d. The blot was stained with desmin (E), myogenin (F), and dystrophin (G) antibodies followed by an HRP-conjugated secondary antibody.
Regeneration of Dystrophin by Cell Implantation in the DMD Model mdx-scid Mouse
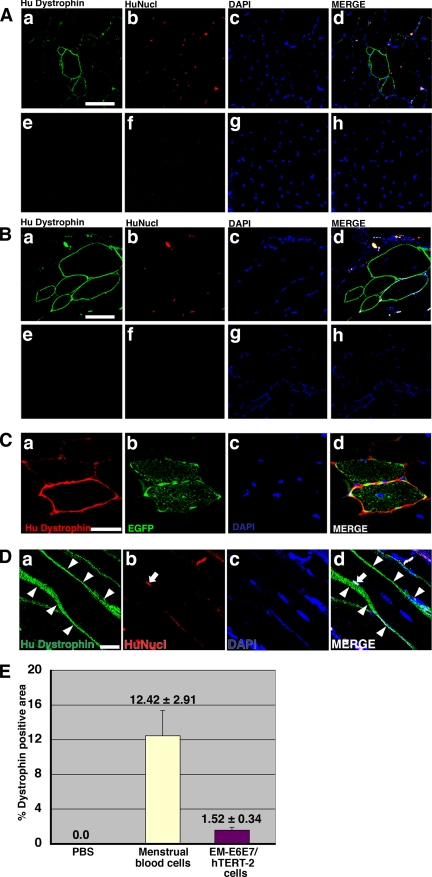
To investigate whether human EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells can generate muscle tissue in vivo, cells without any treatment or induction were implanted directly into the right thigh muscles of mdx-scid mice (Supplementary Figure 2). The left thigh muscles were injected with PBS as an internal control. After 3 wk, myotubes in the muscle tissues injected with human EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells expressed human dystrophin as a cluster (Figure 5, A, C, and D, EM- E6/E7/hTERT-2 cells, and 5B, menstrual blood–derived cells). Quantification analysis revealed that the percentage of dystrophin-positive myofibers after implantation of menstrual blood–derived cells was high, compared with that after implantation of EM-E6/E7/hTERT-2 cells (Figure 5E). Donor cells with EGFP fluorescence participated in myogenesis 3 wk after implantation (Supplementary Figure 3). EGFP-labeled EM-E6/E7/hTERT-2 cells became positive for human dystrophin (Figure 5C). Dystrophin was not detected in the muscle of mdx-scid mice and NOG mice without cell implantation because the antibody to dystrophin used in this study is human-specific, implying that dystrophin is transcribed from dystrophin genes of human donor cells but not from reversion of dystrophied myocytes in mdx-scid mice.
Figure 5.
Conferral of dystrophin to mdx myocytes by human endometrial cells. (A and B) Immunohistochemistry analysis using an antibody against human dystrophin molecule (green), human nuclei (HuNucl, red), and DAPI staining (blue) on thigh muscle sections of mdx-scid mice after direct injection of EM-E6/E7/hTERT-2 cells (A) or menstrual blood–derived cells (B) without any treatment or induction. (C) EGFP-labeled EM-E6/E7/hTERT-2 cells without any treatment or induction were directly injected into the thigh muscle of mdx-scid mice. Immunohistochemistry revealed the incorporation of implanted cells into newly formed EGFP-positive myofibers, which expressed human dystrophin 3 wk after implantation. (A and B) As a methodological control, the primary antibody to dystrophin was omitted (e and f). (D) Immunohistochemistry analysis using an antibody against human dystrophin molecule (green, arrowheads), human nuclei (HuNucl, red, arrow), and DAPI staining (blue) on thigh muscle sections of mdx-scid mice after direct injection of human EM-E6/E7/hTERT-2 cells without any treatment or induction. (A and B) Merge of a–c is shown in d, and merge of e–g is shown in h. (C and D) Merge of a–c is shown in d. Scale bars, 50 μm (A and B), 20 μm (C and D). (E) Quantitative analysis of human dystrophin-positive myotubes. Menstrual blood–derived cells or EM-E6/E7/hTERT-2 cells without any treatment or induction were directly injected into thigh muscle of mdx-scid mice. The percentage of human dystrophin–positive-myofiber areas was calculated 3 wk after implantation of the EM-E6/E7/hTERT-2 cells or menstrual blood–derived cells. Injection of PBS without cells into mdx-scid myofibers was used as a control.
To determine if dystrophin expression in the donor cells is due to transdifferentiation or fusion, immunohistochemistry with an antibody against human nuclei (HuNucl) and DAPI stain was performed. If dystrophin expression is explained by fusion, dystrophin-positive myocytes must be demonstrated to have both human and murine nuclei. We examined almost all the 7-μm-thick serial histological sections parallel to the muscular bundle (longitudinal section) of the muscular tissues by confocal microscopy and found that dystrophin-positive myocytes have nuclei derived from both human and murine cells in the longitudinal section of the myocytes (Figure 5D), implying that dystrophin expression is attributed to fusion between murine host myocytes and human donor cells, rather than myogenic differentiation of EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells per se.
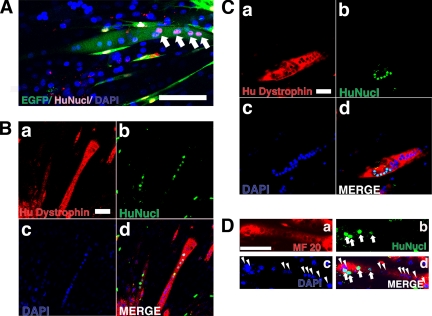
Detection of Human Endometrial Cell Contribution to Myotubes in an In Vitro Myogenesis Model
To simulate in vivo phenomena, human endometrial cells were cocultured in vitro with murine C2C12 myoblasts for 2 d under proliferative conditions and then switched to differentiation conditions for an additional 7 d. Figure 6A provides an example of how human and mouse nuclei in the EGFP-positive myotubes were detected. Multinucleated myotubes were revealed by the presence of specific human dystrophin (Figure 6, B and C) and myosin heavy chain (Figure 6D). Dystrophin was detected in cytoplasm in culture condition (Figure 6, B and C) despite evidence of cell surface localization in vivo. Human dystrophin and human nuclei were unequivocally identified by staining with antibodies to human dystrophin and human nuclei, whereas the numerous mouse nuclei present in this field, as shown by DAPI staining, are negative (Figure 6, B and C).
Figure 6.
Detection of human endometrial cell contribution to myotubes in an in vitro myogenesis model. EGFP-labeled EM-E6/E7/hTERT-2 cells (A) or EM-E6/E7/hTERT-2 cells (B) or menstrual blood–derived cells (C and D) were cocultured with C2C12 myoblasts for 2 d under conditions that favored proliferation. The cultures were then changed to differentiation media for 7 d to induce myogenic fusion. (A) Myotubes were revealed by EGFP (green); human nuclei were detected by antibody specific to human nuclei (HuNucl, red, arrows). (B–D) Myotubes were revealed by specific human dystrophin mAb NCL-DYS3 (B and C, red) or anti-myosin heavy chain mAb MF-20 (D, red). (D) Human nuclei were detected by antibody specific to human nuclei (HuNucl, green, arrows). Total cell nuclei in the culture were stained with DAPI (blue, arrowheads). (B–D) Merge of a–c are shown in d. The cultures were then changed to differentiation media for 7 d to induce myogenic fusion. Scale bars, 100 μm (A–D).
DISCUSSION
Skeletal muscle has a remarkable regenerative capacity in response to an extensive injury. Resident within adult skeletal muscle is a small population of myogenic precursor cells (or satellite cells) that are capable of multiple rounds of proliferation (estimated at 80–100 doublings), which are able to reestablish a quiescent pool of myogenic progenitor cells after each discrete regenerative episode (Mauro, 1961; Schultz and McCormick, 1994; Seale and Rudnicki, 2000; Hawke and Garry, 2001). Although muscle regeneration is a highly efficient and reproducible process, it ultimately is exhausted, as observed in senescent skeletal muscle or in patients with muscular dystrophy (Gussoni et al., 1997; Cossu and Mavilio, 2000). In the present study, we investigated the myogenic potential of human endometrial tissue-derived immortalized EM-E6/E7/TERT-2 cells and primary cells derived from human menstrual blood. Human menstrual blood–derived cells proliferated over at least 25 PDs (9 passages) for more than 60 d and stopped dividing before 30 PDs. This cessation of cell division is probably due to replicative senescence or shortening of telomere length. Cell life span of menstrual blood cells is relatively short when compared with human fetal cells (Imai et al., 1994; Terai et al., 2005), and this shorter cell life span may be attributed to shorter telomere length of adult cells (i.e., endometrial stromal cells) at the start of cell cultivation, as is the case with hematopoietic stem cells (Suda et al., 1984).
Menstrual blood–derived cells had a high replicative ability similar to progenitors or stem cells that display a long-term self-renewal capacity and had a much higher growth rate in our experimental conditions than marrow-derived stromal cells (Mori et al., 2005). In addition, the myogenic potential of menstrual blood–derived cells, i.e., a high frequency of desmin-positive cells after induction, is much greater than expected. The higher myogenic differentiation ratio can be explained just by alteration of cell characteristics from epithelial and mesenchymal bipotential cells or heterogeneous populations of cells to cells with the mesenchymal phenotype in our cultivation condition, as determined by cell surface markers (Figure 1, C–E). MyoD-positive cells are present in many fetal chick organs such as brain, lung, intestine, kidney, spleen, heart, and liver (Gerhart et al., 2001), and these cells can differentiate into skeletal muscle in culture. Constitutive expression of MyoD, desmin, and myogenin, all markers for skeletal myogenic differentiation in both immortalized EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells, implies either that most of these cells are myogenic progenitors or that these cells have myogenic potential. Expression of MyoD, one of the basic helix-loop-helix transcription factors that directly regulate myocyte cell specification and differentiation (Edmondson and Olson, 1993), occurs at the early stage of myogenic differentiation, whereas myogenin is expressed later, related to cell fusion and differentiation (Aurade et al., 1994).
Acquisition or recovery of dystrophin expression in dystrophic muscle is attributed to two different mechanisms: 1) myogenic differentiation of implanted or transplanted cells and 2) cell fusion of implanted or transplanted cells with host muscle cells. Recovery of dystrophin-positive cells is explained by muscular differentiation of implanted marrow stromal cells and adipocytes (Dezawa et al., 2005; Rodriguez et al., 2005). In contrast, implantation of normal myoblasts into dystrophin-deficient muscle can create a reservoir of normal myoblasts that are capable of fusing with dystrophic muscle fibers and restoring dystrophin (Mendell et al., 1995; Terada et al., 2002; Wang et al., 2003; Dezawa et al., 2005; Rodriguez et al., 2005). In this study using menstrual blood–derived cells, our findings—that the implantation of immortalized EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells improved the efficiency of muscle regeneration and dystrophin delivery to dystrophic muscle in mice—is explained by both possibilities or the latter possibility alone, because cells expressing human dystrophin had both murine and human nuclei, located in the center and periphery of dystrophic muscular fiber, respectively (Figures 5D, in vivo, and 6, A–D, in vitro).
DMD is a devastating X-linked muscle disease characterized by progressive muscle weakness attributable to a lack of dystrophin expression at the sarcolemma of muscle fibers (Mendell et al., 1995; Rodriguez et al., 2005), and there are no effective therapeutic approaches for muscular dystrophy at present. Human menstrual blood–derived cells are obtained by a simple, safe, and painless procedure and can be expanded efficiently in vitro. In contrast, isolation of mesenchymal stem cells/mesenchymal cells from other sources, such as bone marrow and adipose tissue, is accompanied by a painful and complicated operation. Efficient fusion systems of our immortalized human EM-E6/E7/hTERT-2 cells and menstrual blood–derived cells with host dystrophic myocytes may contribute substantially to a major advance toward eventual cell-based therapies for muscle injury or chronic muscular disease. Finally, we would like to reemphasize that human menstrual blood–derived cells possess high self-renewal capacity, whereas biopsied myoblasts capable of differentiating into muscular cells are poorly expandable in vitro and rapidly undergo senescence (Cossu and Mavilio, 2000).
Supplementary Material
ACKNOWLEDGMENTS
We express our sincere thanks to J. Hata for support throughout this work, to H. Abe for providing expert technical assistance, to K. Saito for secretarial work, and to A. Crump for reviewing the manuscript. This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan; the Ministry of Health, Labor, and Welfare Sciences Research Grants; by a research grant on Health Science focusing on Drug Innovation from the Japan Health Science Foundation; by the program for promotion of Fundamental Studies in Health Science of the Pharmaceuticals and Medical Devices Agency; by a research grant for Cardiovascular Disease from the ministry of Health, Labor, and Welfare; and by a grant for Child Health and Development from the Ministry of Health, Labor, and Welfare.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0872) on February 21, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Aurade F., Pinset C., Chafey P., Gros F., Montarras D. Myf5, MyoD, myogenin and MRF4 myogenic derivatives of the embryonic mesenchymal cell line C3H10T1/2 exhibit the same adult muscle phenotype. Differentiation. 1994;55:185–192. doi: 10.1046/j.1432-0436.1994.5530185.x. [DOI] [PubMed] [Google Scholar]
- Bischoff R. The satellite cell and muscle regeneration. In: Engel A., Franzini-Armstrong C., Fischman D. A., editors. Myology: Basic and Clinical. New York: McGraw-Hill, Health Professions Division; 1994. pp. 97–118. [Google Scholar]
- Cossu G., Mavilio F. Myogenic stem cells for the therapy of primary myopathies: wishful thinking or therapeutic perspective? J. Clin. Invest. 2000;105:1669–1674. doi: 10.1172/JCI10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bari C., Dell'Accio F., Vandenabeele F., Vermeesch J. R., Raymackers J. M., Luyten F. P. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J. Cell Biol. 2003;160:909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezawa M., Ishikawa H., Itokazu Y., Yoshihara T., Hoshino M., Takeda S., Ide C., Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. Helix-loop-helix proteins as regulators of muscle-specific transcription. J. Biol. Chem. 1993;268:755–758. [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Bast B., Neely C., Iem S., Amegbe P., Niewenhuis R., Miklasz S., Cheng P. F., George-Weinstein M. MyoD-positive myoblasts are present in mature fetal organs lacking skeletal muscle. J. Cell Biol. 2001;155:381–392. doi: 10.1083/jcb.200105139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds M. D., White J. D., Rosenthal N., Bogoyevitch M. A. The role of stem cells in skeletal and cardiac muscle repair. J. Histochem. Cytochem. 2002;50:589–610. doi: 10.1177/002215540205000501. [DOI] [PubMed] [Google Scholar]
- Gussoni E., Blau H. M., Kunkel L. M. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat. Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J. H., Edmondson D. G., Venuti J. M., Olson E. N., Klein W. H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Hawke T. J., Garry D. J. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Imai S., Fujino T., Nishibayashi S., Manabe T., Takano T. Immortalization-susceptible elements and their binding factors mediate rejuvenation of regulation of the type I collagenase gene in simian virus 40 large T antigen-transformed immortal human fibroblasts. Mol. Cell. Biol. 1994;14:7182–7194. doi: 10.1128/mcb.14.11.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo S., Nakamura M., Kiyono T., Maida Y., Kanaya T., Tanaka M., Yatabe N., Inoue M. Successful immortalization of endometrial glandular cells with normal structural and functional characteristics. Am. J. Pathol. 2003;163:2259–2269. doi: 10.1016/S0002-9440(10)63583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J. R., et al. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N. Engl. J. Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Miyoshi H., Blomer U., Takahashi M., Gage F. H., Verma I. M. Development of a self-inactivating lentivirus vector. J. Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Takahashi M., Gage F. H., Verma I. M. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc. Natl. Acad. Sci. USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., et al. Combination of hTERT and bmi-1, E6, or E7 induces prolongation of the life span of bone marrow stromal cells from an elderly donor without affecting their neurogenic potential. Mol. Cell. Biol. 2005;25:5183–5195. doi: 10.1128/MCB.25.12.5183-5195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y., Hanaoka K., Hayasaka M., Esumi E., Li S., Nonaka I., Nabeshima Y. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez A. M., et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J. Exp. Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M. A., Schnegelsberg P. N., Stead R. H., Braun T., Arnold H. H., Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Sabourin L. A., Rudnicki M. A. The molecular regulation of myogenesis. Clin. Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- Schultz E., McCormick K. M. Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- Seale P., Rudnicki M. A. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev. Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Suda J., Suda T., Ogawa M. Analysis of differentiation of mouse hemopoietic stem cells in culture by sequential replating of paired progenitors. Blood. 1984;64:393–399. [PubMed] [Google Scholar]
- Terada N., Hamazaki T., Oka M., Hoki M., Mastalerz D. M., Nakano Y., Meyer E. M., Morel L., Petersen B. E., Scott E. W. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Terai M., Uyama T., Sugiki T., Li X. K., Umezawa A., Kiyono T. Immortalization of human fetal cells: the life span of umbilical cord blood-derived cells can be prolonged without manipulating p16INK4a/RB braking pathway. Mol. Biol. Cell. 2005;16:1491–1499. doi: 10.1091/mbc.E04-07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Willenbring H., Akkari Y., Torimaru Y., Foster M., Al-Dhalimy M., Lagasse E., Finegold M., Olson S., Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.