Abstract
To explore the role of the kallikrein–kinin system in relation to ischemia/reperfusion injury in the kidney, we generated mice lacking both the bradykinin B1 and B2 receptor genes (B1RB2R-null, Bdkrb1−/−/Bdkrb2−/−) by deleting the genomic region encoding the two receptors. In 4-month-old mice, blood pressures were not significantly different among B1RB2R-null, B2R-null (Bdkrb2−/−), and WT mice. After 30 min of bilateral renal artery occlusion and 24 h of reperfusion, mortality rates, renal histological and functional changes, 8-hydroxy-2′-deoxyguanosine levels in total DNA, mtDNA deletions, and the number of TUNEL-positive cells in the kidneys increased progressively in the following order (from lowest to highest): WT, B2R-null, and B1RB2R-null mice. Increases in mRNA levels of TGF-β1, connective tissue growth factor, and endothelin-1 after ischemia/reperfusion injury were also exaggerated in the same order (from lowest to highest): WT, B2R-null, and B1RB2R-null. Thus, both the B1 and B2 bradykinin receptors play an important role in reducing DNA damage, apoptosis, morphological and functional kidney changes, and mortality during renal ischemia/reperfusion injury.
Keywords: hypoxia, mitochondria, oxidative stress
Ischemic tissue injuries, including stroke, myocardial infarction, and ischemic acute renal failure, are devastating complications in patients with hypertension, diabetes, atherosclerosis, and senescence. In organ transplantation, damage to allografts during any ischemic period before the transplant markedly influences short-term and long-term graft function and outcome (1). Furthermore, although reoxygenation after ischemia is essential for cell survival, damage also develops during the reperfusion period. Previous studies have shown that acute ATP depletion, intracellular Ca2+ accumulation, and increased generation of reactive oxygen species by mitochondria all play important roles in the pathogenesis of ischemia/reperfusion (I/R) injury (2).
Angiotensin-I-converting enzyme (ACE) inhibitors (ACEIs) reduce not only chronic cardiovascular remodeling after ischemia but also acute tissue injury caused by I/R in the heart (3), lung (4), liver (5), and kidneys (6, 7). Several observations indicate that the beneficial effects of ACEIs in the acute phase are mainly due to activation of the bradykinin nitric oxide (NO) cascade as a result of inhibition of the inactivation of bradykinin, rather than due to suppression of angiotensin II formation. Thus, ACEIs are much more effective than angiotensin type I receptor antagonists in protecting against I/R (8–10), and bradykinin B2 receptor (B2R) antagonists and NO synthase blockers markedly attenuate the protective effects of ACEIs (8, 9, 11).
Bradykinin and lysyl–bradykinin (kallidin) are generated from kininogens by the kallikreins and some other serine proteases. ACE, which is a carboxydipeptidase, inactivates both peptides by removing two amino acids from their carboxyl termini. In mammals, at least two receptors have been identified: the bradykinin B1 receptor (B1R) and the B2R. Expression of the B2R is constitutive and high in most tissues, except liver and spleen (12). The B1R is expressed at low levels in normal tissues but is induced after tissue injury or after animals are treated with endotoxins or cytokines (13). It has also been shown that expression of the B1R is markedly induced when B2Rs are absent (14, 15). Both the B1R and B2R are coupled with the Gq protein (the heterotrimeric G protein with αqβγ subunit composition) (13), and their stimulation activates endothelial NO synthase in the vascular endothelium (16–19).
In contrast to the consensus that ACEIs have beneficial effects on I/R injury, previous studies have produced conflicting results concerning the effect on I/R injury in different organs of stimulating the two bradykinin receptors, B1R and B2R (20–22). In addition, several studies have suggested that agonism of the B1R may have opposite effects from agonism of the B2R on the severity of I/R injury (23). Although expression of the B1R is much less than that of B2R in the kidney and heart of WT mice, it is markedly induced in B2R-null mice (14, 15), and expression of both receptors increases in I/R injury (24). However, whether they act synergistically or antagonistically and whether or not the kallikrein–kinin system has net protective effects in I/R injuries remain unanswered questions. To answer these questions, we have generated mice lacking both the B1R and B2R for comparison with WT mice and with mice lacking the B2R only.
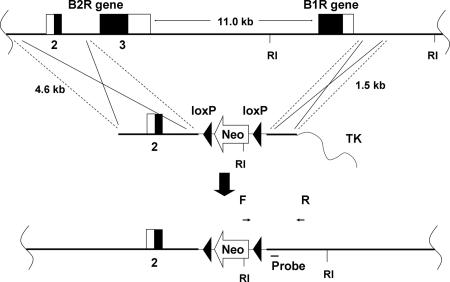
Because the genomic loci for the B1R and the B2R are close (11.0 kb), mice lacking both the B1R and B2R cannot be obtained practically by simply crossbreeding mice lacking B1Rs and mice lacking B2Rs. To generate mice lacking both receptor genes (B1RB2R-null, Bdkrb1−/−/Bdkrb2−/−) we therefore deleted the genomic region coding the two receptors, because no coding genes are predicted to be between the B1R and B2R (Fig. 1). Here we show that mice deficient in both receptors are extremely vulnerable to renal I/R injury. Furthermore, because the order of the severity of the injurious phenotypes increases progressively from WT to B2R-null to B1RB2R-null, we conclude that the both the receptors are important in reducing oxidative stress and protecting kidney tissue from I/R injury.
Fig. 1.
Generation of B1RB2R-null mice lacking both B1R and B2R (Bdkrb1−/−/Bdkrb2−/−). The top line indicates the target locus in ES cells from C57BL/6J mice. RI, EcoRI site. The middle line indicates the targeting vector, including the neomycin phosphotransferase gene (Neo) and thymidine kinase (TK) genes. Bold lines indicate mouse genomic DNA sequences. The bottom line indicates the resulting locus after homologous recombination. Both of the receptor genes are now disrupted. Coding and noncoding exons of the receptor genes are shown as black and white bars, respectively, and are numbered. The location of the primers and probe used to identify recombinants are shown as arrows (F and R) and a bar (probe).
Results
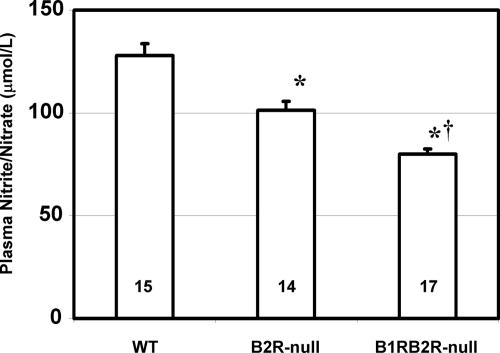
Three groups of mice were studied: homozygotes lacking both the B1R and B2R (B1RB2R-null, Bdkrb1−/−/Bdkrb2−/−), homozygotes lacking B2Rs only (B2R-null, Bdkrb2−/−), and WT. Systolic blood pressures, measured at 4 months age with a tail-cuff method before carrying out the I/R, were not significantly different among the three groups [WT, 108.7 ± 1.5 mmHg (1 mmHg = 133 Pa) (n = 15); B2R-null, 109.6 ± 2.0 mmHg (n = 14); B1RB2R-null, 110.7 ± 1.6 mmHg (n = 17)]. Kidney function was also not significantly different among the three groups of mice at 4 months of age, as determined by plasma urea nitrogen and creatinine (data not shown). However, fasting plasma levels of nitrite/nitrate, the terminal metabolites of NO, decreased significantly in the three groups of mice in the following order (from highest to lowest decrease): WT, B2R-null, B1RB2R-null mice; this suggested the importance of both the B1R and B2R in total NO production (Fig. 2).
Fig. 2.
Plasma levels of nitrite/nitrate in WT, B2R-null (Bdkrb2−/−), and B1RB2R-null mice. ∗, P < 0.01 vs. WT; †, P < 0.01 vs. B2R-null. The data are means ± standard errors, with the numbers of animals shown in black digits.
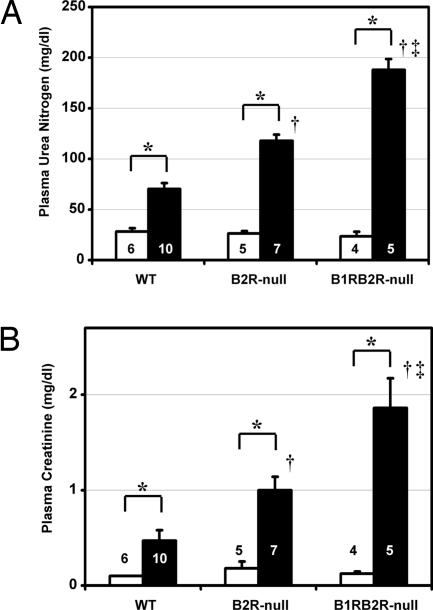
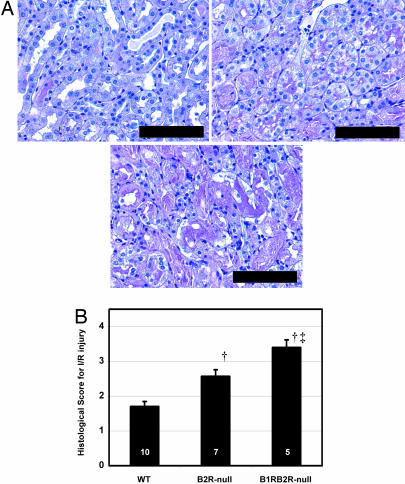
Twenty-four hours after the renal I/R procedure, the mortality rate was notably greater in B1RB2R-null (8 of 13) than in B2R-null (1 of 8) and WT (0 of 10) mice (P < 0.01 vs. WT, P < 0.05 vs. B2R-null; Fisher's exact test), although no mortality was observed in B1RB2R-null (0 of 10), B2R-null (0 of 7), or WT (0 of 5) mice in sham-operated groups. In the surviving animals subjected to I/R, plasma levels of urea nitrogen and creatinine in the B1RB2R-null mice were higher than in B2R-null mice, which were higher than in WT mice (Fig. 3), although there was no significant difference in the urea nitrogen and creatinine levels among the sham-operated groups of the three genotypes. The severity of histological changes in renal proximal tubules were in the same order (from highest to lowest): B1RB2R-null, B2R-null, WT mice (Fig. 4).
Fig. 3.
Plasma levels of urea nitrogen (A) and creatinine (B) as indices of renal excretory function in WT, B2R-null, and B1RB2R-null mice. White and black columns indicate sham-operated and ischemia groups, respectively. ∗, P < 0.05 vs. the sham-operated group of the same genotype; †, P < 0.05 vs. WT; ‡, P < 0.05 vs. B2R-null. The data are means ± standard errors; the numbers of animals are shown in white or black digits.
Fig. 4.
Histological changes in the postischemic kidneys of WT, B2R-null, and B1RB2R-null mice. (A) Periodic acid Schiff's staining. Shown are stainings for WT (Upper Left), B2R-null (Upper Right), and B1RB2R-null (Lower) mice. (Scale bar, 100 μm.) (B) Semiquantitative scoring for histological changes in the proximal tubule according to the criteria of Jablonski et al. (56). The sham-operated mice all a score of zero. The black columns show the scores of the ischemia groups. †, P < 0.05 vs. WT; ‡, P < 0.05 vs. B2R-null. The data are means ± standard errors, with the numbers of animals shown in white digits.
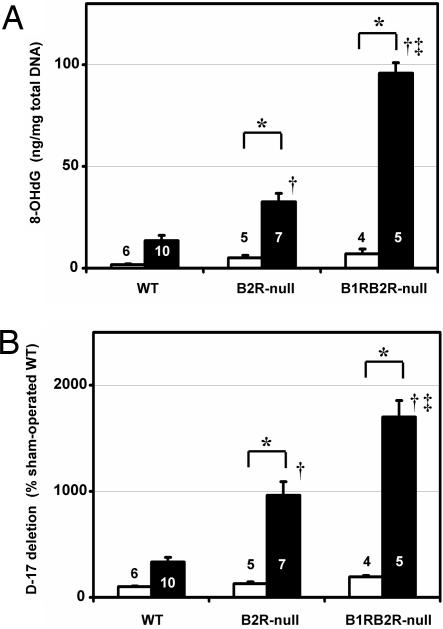
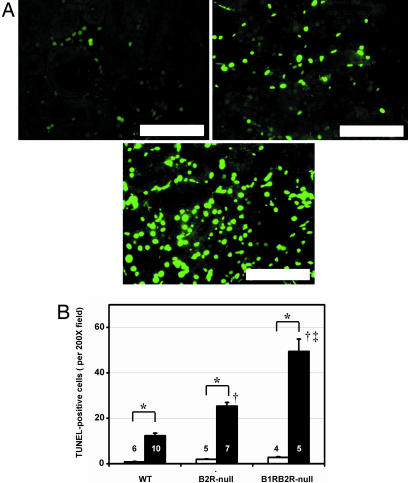
Oxidative stress, DNA damage, and apoptosis have all been suggested to play a causative role in the functional and morphological changes accompanying I/R injury (2, 25). In agreement with this suggestion, we found the levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG), which is considered to be an index of oxidative DNA modification (26), in total DNA of the kidney to be in the following order (from highest to lowest): B1RB2R-null, B2R-null, WT mice (Fig. 5A). The proportion of the D-17 deletion mutant in mtDNA, which increases with age (27) and diabetes (28) and has been shown to be an indicator of mtDNA damage, was also higher in the kidney subjected to the I/R injury in the same orders (from highest to lowest): B1RB2R-null, B2R-null, and WT (Fig. 5B). The number of apoptotic cells detected by TUNEL assay was likewise higher in B1RB2R-null mice than in B2R-null, which was higher than in WT mice (Fig. 6).
Fig. 5.
DNA alterations in the postischemic kidneys of WT, B2R-null, and B1RB2R-null mice. (A) The levels of 8-OHdG in total DNA of the kidney. (B) Relative proportion of D-17 deletions (as a percentage of WT) in the mtDNA. White and black columns indicate sham-operated and ischemia groups, respectively. The data are means ± standard errors, with the numbers of animals shown in white or black digits. ∗, P < 0.05 vs. the sham-operated group of the same genotype; †, P < 0.05 vs. WT; ‡, P < 0.05 vs. B2R-null.
Fig. 6.
TUNEL-positive cells in postischemic kidneys of WT, B2R-null, and B1RB2R-null mice. (A) Representative photomicrographs of the renal cortex. Shown are stainings for WT (Upper Left), B2R-null (Upper Right), and B1RB2R-null (Lower) mice. (Scale bar, 100 μm.) (B) Quantification of the number of apoptotic cells in the renal cortex. The white and black columns indicate sham-operated and ischemia groups, respectively. ∗, P < 0.05 vs. the sham-operated group of the same genotype; †, P < 0.05 vs. WT. ‡, P < 0.05 vs. B2R-null. The data are means ± standard errors, with the numbers of animals shown in white or black digits.
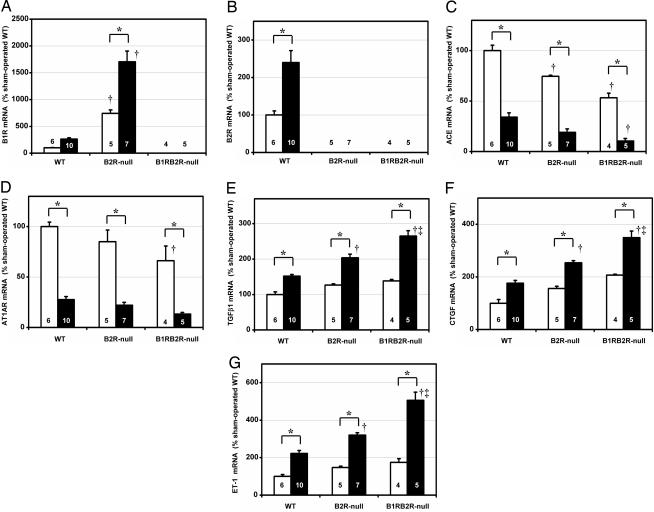
We next studied the mRNA levels of several I/R injury-related genes with quantitative RT-PCR. The levels of B1R mRNA were markedly increased in B2R-null mice, as previously shown, and this enhanced expression in B2R-null mice was increased further by I/R injury (Fig. 7A). Likewise, the levels of B2R mRNA were increased by I/R injury (Fig. 7B). These findings indicate that I/R injury increases the expression of both the B1R and the B2R.
Fig. 7.
The expression of mRNA relative to β-actin of B1R (A), B2R (B), ACE (C), AT1AR (D), TGF-β1 (E), CTGF (F), and ET-1 (G), as determined by quantitative RT-PCR and presented as percentages of sham-operated WT. The white and black columns indicate sham-operated and ischemia groups, respectively. The data are means ± standard errors, with the numbers of animals shown in white or black digits. ∗, P < 0.05 vs. the sham-operated group of the same genotype; †, P < 0.05 vs. WT. ‡, P < 0.05 vs. B2R-null.
We measured the mRNA levels of two components of the renin–angiotensin system. As previously reported, the mRNA levels of ACE and angiotensin II type 1A receptor (AT1AR) in the kidney were markedly decreased in I/R injury. The expression of ACE and AT1AR was less in B1RB2R-null mice than in B2R-null mice and less in B2R-null than in WT (Fig. 7 C and D).
We also studied gene expressions that are well known to be increased in I/R injury. The mRNA levels of TGF-β1 (29), connective tissue growth factor (CTGF) (30), and endothelin (ET)-1 (31) were increased in I/R injury, and these increases were greater in B1RB2R-null than in B2R-null mice and more in B2R-null than in WT mice (Fig. 7 E–G). Expression of the relaxin family peptide receptor 4, which has been suggested to serve as a bradykinin receptor (32), was not significantly affected by the I/R procedure or by the genotypes (not shown).
Discussion
The protective effect of ACEIs in cardiac I/R damage results largely from inhibition of the enzymatic breakdown of endogenous bradykinin and from the consequent enhancement of NO formation (8, 9, 11, 33). Our present finding that the basal levels of fasting plasma nitrite/nitrate are lower in B1RB2R-null than in B2R-null and WT mice and a previous report showing that urinary nitrite/nitrate excretion in B2R-null mice is lower than in WT mice (34) are additional indicators that the kallikrein–kinin system is important in basal NO production.
Recent studies have provided a likely explanation for this protection: I/R injuries are associated with mitochondrial Ca2+ overload resulting from a burst of reactive oxygen species, and the combination of mitochondrial Ca2+ overload and reactive oxygen species triggers the opening of mitochondrial permeability-transition pores and leads to cell apoptosis (35). This opening is effectively suppressed by bradykinin (36). NO, a second messenger of bradykinin receptors, reversibly suppresses mitochondrial oxidative metabolism (37–39) largely via inhibiting cytochrome c oxidase in a cGMP-independent manner (40, 41).
In contrast to the consensus that ACEIs have beneficial effects on I/R injury, previous reports are conflicting with respect to the effects of pharmacological agents interacting with the bradykinin receptors (20–22). In addition, several studies have suggested that agonism of the B1R and B2R may have different effects on the severity of I/R injury (21–23). In contrast, lack of the B1R (42) or B2R (43) aggravates the I/R injury in the heart and brain of mice, whereas adenovirus-mediated gene transfer of tissue kallikrein protects against ischemic stroke in rats (44). Transgenic expression of tissue kallikrein in mice attenuates ischemic cardiac damage (45), whereas tissue kallikrein knockout in mice aggravates the damage (24). In our current study, we show that I/R damage increases progressively in the order WT, B2R-null, and B1RB2R-null, as determined by morphological and functional changes to the kidney, demonstrating clearly that both the B1R and B2R are important in protecting the kidney from I/R injury.
Oxidative stress, DNA damage, and apoptotic changes have all been implicated in I/R injury (25). We have previously reported markedly increased levels of 8-OHdG in kidney DNA resulting from the absence of B2Rs in animals with diabetes (28). In our present study, we have clearly demonstrated that I/R increases 8-OHdG in kidney DNA in the following order (from lowest to highest): WT, B2R-null, and B1RB2R-null. The most common deletion mutant of mouse mtDNA (D-17), which increases with age (27) and diabetes (28), was also markedly increased in the kidney 24 h after the I/R procedure. The increase in this mtDNA alteration was enhanced progressively by absence of the B2R or of both the B1R and B2R. We also found that tubular morphological changes and the number of apoptotic cells after the I/R injury in the B1RB2R-null mice was greater than in B2R-null mice, which was greater than in WT.
Traditionally, the kallikrein–kinin system is viewed as a major counteracting force to the renin–angiotensin system. In the current study, we demonstrated that the expression of both ACE and AT1AR is suppressed in I/R injury, as previously reported (46), and this suppression is exaggerated in absence of bradykinin receptors. The decrease in mRNA for ACE and AT1AR in I/R injury cannot be explained merely by the loss of the proximal tubular cells, which is the major site for ACE and AT1AR gene expression in the kidney (47, 48), because, even in the WT mice subjected to I/R, where there is little morphological change in proximal tubules, the expression of ACE and AT1AR was substantially decreased. Given the well documented ability of angiotensin II to stimulate reactive oxygen species and to cause TGF-β1 production, the down-regulation of the renin–angiotensin system in I/R injury may be protective.
Previous studies have shown that TGF-β1, CTGF, and ET-1 increase in hypoxic tissue damages (29–31), and that their expression increases with aging (49, 50) and diabetes (51–53). In the present study, we found that these three genes were more expressed in B1RB2R-null mice than B2R-null mice, and more expressed in B2R-null mice than WT, suggesting that both B1R and B2R are protective against renal cell damage in I/R injury. However, it is still unclear whether increased expression of these genes in I/R injury plays a primary/causative or secondary/protective role.
Our results show that the absence of the B1R and B2R, and to a lesser extent, the absence of B2R alone, enhances a series of changes including oxidative DNA damages, mtDNA deletions, apoptosis, and the expression of TGF-β1, CTGF, and ET-1, all of which are common to other oxidative stress-related disorders. Reduced plasma levels of end products of NO are associated with the absence of B1R and B2R. Together, these data suggest that both the B1R and B2R, presumably acting in part via NO, mitigate renal I/R injury by blunting the marked increase in oxidative stress that accompanies the I/R. Because ACEIs increase the level of bradykinin, our findings emphasize the importance of using ACEIs in situations in which I/R injury is likely to occur. Pretransplant treatment of the donor with ACEIs, as well as posttransplant treatment of the recipient, merits consideration (5).
In summary, we have shown that the kallikrein–kinin system, including both bradykinin B1R and B2R, is important in protecting the kidney from damage caused by I/R. The B1RB2R-null mice that we have generated should be useful for studying the role of kallikrein–kinin system in other physiological and pathophysiological conditions.
Methods
Animals.
Mice having the null allele for the B2R, generated by Borkowski et al. (54), were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in our mouse facility. The mice were backcrossed to WT C57BL/6J at least six times. Mice lacking both the B1R and B2R were generated in our laboratory and the University of North Carolina Animal Models Core Facility. To do this, we targeted C57BL/6J ES cells by using the scheme illustrated in Fig. 1. The sequences of the primers for PCR screening of the recombinants were as follows: forward, 5′-ACGCGTCACCTTAATATGCG-3′ (in the Neo gene of the OS dupdel gene-targeting vector; Open Biosystems, Huntsville, AL) (denoted as F in Fig. 1), and reverse, 5′-GTCCATTATCACCAAGGTGG-3′ (in the Bdkrb1 locus) (denoted as R in Fig. 1). Targeting was confirmed by a Southern blot of genomic DNA digested with EcoRI by using a 434-bp KpnI–NcoI fragment (probe in Fig. 1) within the short homology arm as the probe. The targeted ES cells were injected into C57BL/6J blastocysts. Chimerism in the resulting mice was assessed in their toe DNA by the amount of Neo gene relative to β-actin gene by real-time PCR with an Applied Biosystems 7700 Sequence Detection System (PerkinElmer, Boston, MA). Genotyping of subsequent progeny mice was performed by real-time PCR with primers and probes for detection of the presence of the Neo–Bdkrb1 junction [forward, 5′-CTCGACATTGGGTGGAAACA-3′ (in the Neo gene); reverse, 5′-GGTGCTTCTGTCTTGTGACA-3′ (in the Bdkrb1 locus); probe, 5′-FAM-AGCCTCTCCACCCAGGCCTGG-TAMRA-3′ (FAM and TAMRA indicate 6-carboxyfluorescein and 5′-6-carboxytetramethylrhodamine, respectively)] and the absence of the third exon of Bdkrb2 (forward, 5′-ATCTTGCAGGTGCTGAGGAA-3′; reverse, 5′-AGCACAAAGAGCCCCAGGA-3′; probe, 5′-FAM-CAGCACTAGCACGGTGGCCTTCCT-TAMRA-3′). Systolic blood pressure and pulse rate were measured with the tail-cuff method described previously (55). All experiments performed were approved by the University of North Carolina Institutional Animal Care and Use Committee.
Renal I/R.
Mice were anesthetized with isoflurane (1.5%) inhalation, and core body temperature was maintained at 37°C by using a homeothermic blanket. After a midline laparotomy, heparin (10 units/kg i.m.) was injected. In I/R groups, renal arteries and veins were bilaterally occluded by using microaneurysm clamps for 30 min. In sham-operated groups, renal arteries and veins were not occluded, but the abdomen was kept open for 30 min. In I/R groups, 30 min after the occlusion, the incision was double-sutured after the clamps were removed. Twenty-four hours after the removal of the clamps, mice were killed and blood and renal tissues were collected.
Measurement of Biochemical Parameters.
One month before the ischemia experiment (after fasting for 4 h), and at the end of the 24-h reperfusion period, mice were anesthetized by using avertin (2.5%; 0.1 mg/3 g i.p.), and blood was collected from the retroorbital sinus with heparinized glass pipettes. Samples were centrifuged (7,000 × g for 5 min) to separate plasma. Plasma samples were frozen and stored at −80°C before analyses for biochemical parameters. Nitrite/nitrate in the plasma, partly derived from endogenously produced NO, was measured with a nitric oxide colorimetric assay kit, using the Griess method (Calbiochem, San Diego, CA). Plasma urea nitrogen and creatinine concentrations were measured by using the Vitros 250 chemistry system (Ortho-Clinical Diagnostics, Raritan, NJ). 8-OHdG levels in DNase I-digested kidney DNA (3 μg) were determined with ELISA (Japan Institute for Control of Aging/Nikken SEIL, Shizuoka, Japan).
Histological Evaluation.
The left kidney was fixed with 4% (wt/vol) paraformaldehyde. Renal tissue sections were stained with periodic acid Schiff's reagent with hematoxylin and examined by optical microscopy. The histological changes in the proximal tubule were semiquantified by using the criteria described by Jablonski et al. (56).
Quantitative RT-PCR.
Total RNA was extracted from whole kidney, and the mRNAs for B1R, B2R, TGF-β1, CTGF, ET-1, and relaxin family peptide receptor 4 were assayed by quantitative RT-PCR, as described previously (15, 28, 57). Expression of ET-1 and relaxin family peptide receptor 4 was assayed with primers and probes for the following: ET-1 (forward, 5′-TGCCACCTGGACATCATCTG-3′; reverse, 5′-ACGCTTGGACCTGGAAGAAC-3′; probe, 5′-FAM-TCCCGAGCGCGTCGTACCGTATG-TAM-RA3′); relaxin family peptide receptor 4 (forward, 5′-AATGGCA-GGACAGCCGAGT-3′; reverse, 5′-GAGAGTGACTACATGGTTGG-3′; probe, 5′-FAM-AGCCCGCTCTGTCCGTGT-CCTG-TAMRA-3′).
Quantification of mtDNA Deletion Mutants.
Occurrence of the most common deletion mutant of mtDNA, D-17 (27), was assayed in kidney DNA samples by using quantitative PCR with primers flanking the deletion normalized by the amount of cytochrome b gene, as previously described (28).
Fluorescence Microscopy.
TUNEL assay was performed in the paraformaldehyde-fixed kidney by using ApopTag Fluorescein In Situ Apoptosis Detection Kit (Chemicon, Temecula, CA), as previously described (28). The numbers of TUNEL-positive cells were computationally counted (ImageJ version 1.33u; National Institutes of Health).
Statistical Analysis.
The data are expressed as means ± standard errors. To compare groups, we used two-factor ANOVA or the χ2 test. Post hoc pairwise comparisons were by the Tukey–Kramer honestly significant difference test or Fisher's exact test (JMP version 5.1.2; SAS Institute Inc., Cary, NC).
Acknowledgments
We thank John Hagaman, Kuikwon Kim, Nobuyo Maeda, Nobuyuki Takahashi, Randy Thresher, and Xianwen Yi for assistance. This work was supported by The Howard H. Holderness Distinguished Medical Scholars Program (to R.W.M.), Career Development Award 2-2006-108 from the Juvenile Diabetes Research Foundation International (to M.K.), and National Institutes of Health Grants HL49277, HL70523, and HL71266 (to O.S.).
Abbreviations
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- ACE
angiotensin-I-converting enzyme
- ACEI
ACE inhibitor
- AT1AR
angiotensin II type 1A receptor
- B1R
bradykinin B1 receptor
- B2R
bradykinin B2 receptor
- CTGF
connective tissue growth factor
- ET
endothelin
- I/R
ischemia/reperfusion.
Footnotes
The authors declare no conflict of interest.
References
- 1.Deschenes M, Belle SH, Krom RA, Zetterman RK, Lake JR. Transplantation. 1998;66:302–310. doi: 10.1097/00007890-199808150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Padanilam BJ. Am J Physiol. 2003;284:F608–F627. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- 3.Li K, Chen X. J Mol Cell Cardiol. 1987;19:909–915. doi: 10.1016/s0022-2828(87)80619-3. [DOI] [PubMed] [Google Scholar]
- 4.Morrell NW, Atochina EN, Morris KG, Danilov SM, Stenmark KR. J Clin Invest. 1995;96:1823–1833. doi: 10.1172/JCI118228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthuber M, Farkas S, Rihl M, Menger MD, Schildberg FW, Jauch KW, Messmer K. Hepatology. 1997;25:648–651. doi: 10.1002/hep.510250326. [DOI] [PubMed] [Google Scholar]
- 6.Oosterlinck W, Roelandt R, De Sy WA, Praet M. Eur Urol. 1985;11:36–39. doi: 10.1159/000472446. [DOI] [PubMed] [Google Scholar]
- 7.Kakoki M, Hirata Y, Hayakawa H, Suzuki E, Nagata D, Nishimatsu H, Kimura K, Goto A, Omata M. Hypertens Res. 2000;23:527–533. doi: 10.1291/hypres.23.527. [DOI] [PubMed] [Google Scholar]
- 8.Kitakaze M, Minamino T, Node K, Komamura K, Shinozaki Y, Mori H, Kosaka H, Inoue M, Hori M, Kamada T. Circulation. 1995;92:950–961. doi: 10.1161/01.cir.92.4.950. [DOI] [PubMed] [Google Scholar]
- 9.Liu YH, Yang XP, Sharov VG, Sigmon DH, Sabbath HN, Carretero OA. Hypertension. 1996;27:7–13. doi: 10.1161/01.hyp.27.1.7. [DOI] [PubMed] [Google Scholar]
- 10.Guba M, Steinbauer M, Buchner M, Frolich D, Farkas S, Jauch KW, Anthuber M. Shock. 2000;13:190–196. doi: 10.1097/00024382-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Brunner F, Kukovetz WR. Circulation. 1996;94:1752–1761. doi: 10.1161/01.cir.94.7.1752. [DOI] [PubMed] [Google Scholar]
- 12.Ma JX, Wang DZ, Chao L, Chao J. Gene. 1994;149:283–288. [PubMed] [Google Scholar]
- 13.Couture R, Girolami JP. Eur J Pharmacol. 2004;500:467–485. doi: 10.1016/j.ejphar.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 14.Duka I, Kintsurashvili E, Gavras I, Johns C, Bresnahan M, Gavras H. Circ Res. 2001;88:275–281. doi: 10.1161/01.res.88.3.275. [DOI] [PubMed] [Google Scholar]
- 15.Kakoki M, Takahashi N, Jennette JC, Smithies O. Proc Natl Acad Sci USA. 2004;101:13302–13305. doi: 10.1073/pnas.0405449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sangsree S, Brovkovych V, Minshall RD, Skidgel RA. Am J Physiol. 2003;284:H1959–H1968. doi: 10.1152/ajpheart.00036.2003. [DOI] [PubMed] [Google Scholar]
- 17.Lamontagne D, Nakhostine N, Couture R, Nadeau R. J Cardiovasc Pharmacol. 1996;28:645–650. doi: 10.1097/00005344-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 18.D'Orleans-Juste P, de Nucci G, Vane JR. Br J Pharmacol. 1989;96:920–926. doi: 10.1111/j.1476-5381.1989.tb11903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond GR, Cocks TM. Br J Pharmacol. 1995;116:2473–2481. doi: 10.1111/j.1476-5381.1995.tb15098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang WC, Chien CT, Lin WW, Lin SL, Chen YM, Lai CF, Wu KD, Chao J, Tsai TJ. Free Radic Biol Med. 2006;41:1304–1314. doi: 10.1016/j.freeradbiomed.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Souza DG, Lomez ES, Pinho V, Pesquero JB, Bader M, Pesquero JL, Teixeira MM. J Immunol. 2004;172:2542–2548. doi: 10.4049/jimmunol.172.4.2542. [DOI] [PubMed] [Google Scholar]
- 22.Wang PH, Cenedeze MA, Pesquero JB, Pacheco-Silva A, Camara NO. Int Immunopharmacol. 2006;6:1960–1965. doi: 10.1016/j.intimp.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Lagneux C, Bader M, Pesquero JB, Demenge P, Ribuot C. Int Immunopharmacol. 2002;2:815–822. doi: 10.1016/s1567-5769(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 24.Griol-Charhbili V, Messadi-Laribi E, Bascands JL, Heudes D, Meneton P, Giudicelli JF, Alhenc-Gelas F, Richer C. FASEB J. 2005;19:1172–1174. doi: 10.1096/fj.04-3508fje. [DOI] [PubMed] [Google Scholar]
- 25.Sheu SS, Nauduri D, Anders MW. Biochim Biophys Acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN. Proc Natl Acad Sci USA. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanhauser SM, Laipis PJ. J Biol Chem. 1995;270:24769–24775. doi: 10.1074/jbc.270.42.24769. [DOI] [PubMed] [Google Scholar]
- 28.Kakoki M, Kizer CM, Yi X, Takahashi N, Kim HS, Bagnell CR, Edgell CJ, Maeda N, Jennette JC, Smithies O. J Clin Invest. 2006;116:1302–1309. doi: 10.1172/JCI26958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spurgeon KR, Donohoe DL, Basile DP. Am J Physiol. 2005;288:F568–F577. doi: 10.1152/ajprenal.00330.2004. [DOI] [PubMed] [Google Scholar]
- 30.Ohnishi H, Oka T, Kusachi S, Nakanishi T, Takeda K, Nakahama M, Doi M, Murakami T, Ninomiya Y, Takigawa M, et al. J Mol Cell Cardiol. 1998;30:2411–2422. doi: 10.1006/jmcc.1998.0799. [DOI] [PubMed] [Google Scholar]
- 31.Stewart DJ, Kubac G, Costello KB, Cernacek P. J Am Coll Cardiol. 1991;18:38–43. doi: 10.1016/s0735-1097(10)80214-1. [DOI] [PubMed] [Google Scholar]
- 32.Boels K, Schaller HC. Br J Pharmacol. 2003;140:932–938. doi: 10.1038/sj.bjp.0705521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massoudy P, Becker BF, Gerlach E. J Cardiovasc Pharmacol. 1995;25:440–447. doi: 10.1097/00005344-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Schanstra JP, Duchene J, Praddaude F, Bruneval P, Tack I, Chevalier J, Girolami JP, Bascands JL. Am J Physiol. 2003;284:H1904–H1908. doi: 10.1152/ajpheart.01150.2002. [DOI] [PubMed] [Google Scholar]
- 35.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Am J Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 36.Park SS, Zhao H, Mueller RA, Xu Z. J Mol Cell Cardiol. 2006;40:708–716. doi: 10.1016/j.yjmcc.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Loke KE, Curran CM, Messina EJ, Laycock SK, Shesely EG, Carretero OA, Hintze TH. Hypertension. 1999;34:563–567. doi: 10.1161/01.hyp.34.4.563. [DOI] [PubMed] [Google Scholar]
- 38.Adler S, Huang H, Loke KE, Xu X, Tada H, Laumas A, Hintze TH. Am J Physiol. 2001;280:F838–F843. doi: 10.1152/ajprenal.2001.280.5.F838. [DOI] [PubMed] [Google Scholar]
- 39.Brown GC, Bolanos JP, Heales SJ, Clark JB. Neurosci Lett. 1995;193:201–204. doi: 10.1016/0304-3940(95)11703-y. [DOI] [PubMed] [Google Scholar]
- 40.Brunori M, Giuffre A, Forte E, Mastronicola D, Barone MC, Sarti P. Biochim Biophys Acta. 2004;1655:365–371. doi: 10.1016/j.bbabio.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Sarti P, Lendaro E, Ippoliti R, Bellelli A, Benedetti PA, Brunori M. FASEB J. 1999;13:191–197. doi: 10.1096/fasebj.13.1.191. [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Carretero OA, Sun Y, Shesely EG, Rhaleb NE, Liu YH, Liao TD, Yang JJ, Bader M, Yang XP. Hypertension. 2005;45:747–753. doi: 10.1161/01.HYP.0000153322.04859.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia CF, Smith RS, Jr, Shen B, Yang ZR, Borlongan CV, Chao L, Chao J. Hypertension. 2006;47:752–761. doi: 10.1161/01.HYP.0000214867.35632.0e. [DOI] [PubMed] [Google Scholar]
- 44.Xia CF, Yin H, Borlongan CV, Chao L, Chao J. Hypertension. 2004;43:452–459. doi: 10.1161/01.HYP.0000110905.29389.e5. [DOI] [PubMed] [Google Scholar]
- 45.Koch M, Spillmann F, Dendorfer A, Westermann D, Altmann C, Sahabi M, Linthout SV, Bader M, Walther T, Schultheiss HP, et al. Eur J Pharmacol. 2006;550:143–148. doi: 10.1016/j.ejphar.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 46.Allred AJ, Chappell MC, Ferrario CM, Diz DI. Am J Physiol. 2000;279:F636–F645. doi: 10.1152/ajprenal.2000.279.4.F636. [DOI] [PubMed] [Google Scholar]
- 47.Bruneval P, Hinglais N, Alhenc-Gelas F, Tricottet V, Corvol P, Menard J, Camilleri JP, Bariety J. Histochemistry. 1986;85:73–80. doi: 10.1007/BF00508656. [DOI] [PubMed] [Google Scholar]
- 48.Mujais SK, Kauffman S, Katz AI. J Clin Invest. 1986;77:315–318. doi: 10.1172/JCI112293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim KH, Park GT, Lim YB, Rue SW, Jung JC, Sonn JK, Bae YS, Park JW, Lee YS. Biochem Biophys Res Commun. 2004;318:819–825. doi: 10.1016/j.bbrc.2004.04.108. [DOI] [PubMed] [Google Scholar]
- 50.Kumazaki T, Fujii T, Kobayashi M, Mitsui Y. Exp Cell Res. 1994;211:6–11. doi: 10.1006/excr.1994.1051. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy M, Godson C, Cannon S, Kato S, Mackenzie HS, Martin F, Brady HR. J Biol Chem. 1999;274:5830–5834. doi: 10.1074/jbc.274.9.5830. [DOI] [PubMed] [Google Scholar]
- 53.Minchenko AG, Stevens MJ, White L, Abatan OI, Komjati K, Pacher P, Szabo C, Obrosova IG. FASEB J. 2003;17:1514–1516. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- 54.Borkowski JA, Ransom RW, Seabrook GR, Trumbauer M, Chen H, Hill RG, Strader CD, Hess JF. J Biol Chem. 1995;270:13706–13710. doi: 10.1074/jbc.270.23.13706. [DOI] [PubMed] [Google Scholar]
- 55.Krege JH, Hodgin JB, Hagaman JR, Smithies O. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 56.Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Kim HS, Lee G, John SW, Maeda N, Smithies O. Proc Natl Acad Sci USA. 2002;99:4602–4607. doi: 10.1073/pnas.072083799. [DOI] [PMC free article] [PubMed] [Google Scholar]