Abstract
A previously isolated fission yeast γ-tubulin mutant containing apparently stabilized microtubules proliferated at an approximately identical rate as wild type, yet the mutant mitosis spindle dynamics were aberrant, particularly the kinetochore microtubule dynamics. Progression through mitosis in the mutant, however, resulted in mostly accurate chromosome segregation. In the absence of the spindle assembly checkpoint gene, mad2+, the spindle dynamics in the γ-tubulin mutant were greatly compromised, leading to a high incidence of chromosome missegregation. Unlike in wild-type cells, green fluorescent protein (GFP)-tagged Mad2 protein often accumulated near one of the poles of an elongating spindle in the γ-tubulin mutant. We isolated novel mad2 mutants that were defective in arresting mitotic progression upon gross perturbation of the spindle formation but remained functional for the viability of the γ-tubulin mutant. Further, the mad2 mutations did not appreciably destabilize minichromosomes in unperturbed mitoses. When overexpressed ectopically, these mutant Mad2 proteins sequestered wild-type Mad2, preventing its function in mitotic checkpoint arrest, but not in minichromosome stability. These results indicated that the Mad2 functions required for checkpoint arrest and chromosome stability in unperturbed mitosis are genetically discernible. Immunoprecipitation studies demonstrated that GFP-fused mutant Mad2 proteins formed a Mad1-containing complex with altered stability compared to that formed with wild-type Mad2, providing clues to the novel mad2 mutant phenotype.
ACCURATE segregation of replicated chromosomes in mitosis is essential for cell proliferation. In all eukaryotes, this process is dependent on the mitotic apparatus, the spindle, which is transiently formed in the mitotic phase of the cell-division cycle. The spindle is a microtubule (MT)-based bipolar structure primarily devoted to chromosome segregation. A crucial event in chromosome segregation is sister-chromatid separation, which occurs at the onset of the mitotic anaphase, after all of the sister kinetochores are properly linked to their respective spindle poles in a bi-oriented manner. The anaphase promoting complex (APC)-mediated ubiquitination and degradation of securin, which trigger the activation of separase protease and the subsequent disruption of sister-chromatid cohesion, constitute key events in sister-chromatid separation. Sister chromosome sets are then separated by the combined mechanisms of anaphase A and B, that is, the shrinkage of the pole-to-kinetochore MTs (kMTs) and the elongation of the pole-to-pole distance, respectively. Sister-chromatid separation occurs on individual chromosomes, but must be temporally and spatially regulated so that all chromosomes are simultaneously split into sister chromosomes and faithfully distributed into each of the two daughter nuclei. The spindle assembly checkpoint (SAC) is thought to be essential for this coordinated chromosome behavior in mitosis and to prevent aneuploidy (Cleveland et al. 2003; Lew and Burke 2003; Kwon and Scholey 2004; Pinsky and Biggins 2005).
SAC genes, such as BUB, MAD, and MPS1, were originally identified in budding-yeast mutants that were defective in arresting in M-phase when the spindle assembly was impaired with MT poisons such as benomyl (Hoyt et al. 1991; Li and Murray 1991; Weiss and Winey 1996). It was subsequently determined that the SAC genes are conserved among a wide range of species (Cleveland et al. 2003; Lew and Burke 2003; Pinsky and Biggins 2005), including Schizosaccharomyces pombe (He et al. 1997, 1998; Bernard et al. 1998; Millband and Hardwick 2002; Kim et al. 2003). SAC functions include monitoring the lack of kMT attachment to the kinetochore or the absence of tension at the kinetochore and blocking mitotic progression, primarily through producing diffusible APC inhibitors. Even a single affected kinetochore can produce the inhibitors (Rieder et al. 1995; Kerscher et al. 2003), thereby allowing time for the affected kinetochores to correct their linkage to kMTs and ensuring coordinated chromosome segregation. Mad2 is a key molecule in SAC that binds to Cdc20 (Slp1 in fission yeast) to block APC activation. According to a currently accepted theory (Musacchio and Hardwick 2002; De Antoni et al. 2005; Hardwick 2005; Yu 2006), Mad2 forms a complex with Mad1 and is recruited to kinetochores that are not occupied by kMTs; the bound complex then functions to activate free Mad2 to form the Mad2–Cdc20 complex. This activation process is thought to include a specific interaction with Mad2 that requires a segment in Mad2 distinct from the C-terminal segment involved in binding to both Mad1 and Cdc20. Thus, at the physiological level of Mad2 expression, its activity is dependent on Mad1, and the major role of Mad1 in SAC is thought to be the activation of Mad2.
The fission yeast is useful for investigating the molecular mechanisms and regulation of mitosis. As in other organisms (Kwon and Scholey 2004), proper formation of the spindle in fission yeast requires factors that interact with MTs. Among them are a kinesin family protein, Cut7 [a homolog of BimC, kinesin-5, according to the standard nomenclature (Lawrence et al. 2004)], which is required for the formation of the bipolar spindle and spindle elongation (Hagan and Yanagida 1990, 1992; Troxell et al. 2001); Pkl1 and Klp2 (homologous to Kar3, kinesin-14), which are required for proper spindle length (Klp2 probably has a role in antagonizing Cut7) (Pidoux et al. 1996; Troxell et al. 2001); and Klp5 and Klp6 (belonging to the Kip3-KinI-family, kinesin-8), which are required for proper metaphase spindle formation and chromosome segregation, probably through the regulation of kMT stability at the kinetochore (Garcia et al. 2002; West et al. 2002). Nonkinesin-like proteins, including Dis1 and its homolog Alp14/Mtc1, the TOG/XMAP215-family proteins that affect the MT stability and the kinetochore–kMT interaction (Nakaseko et al. 2001; Garcia et al. 2002), Ase1 for MT bundling (Loiodice et al. 2005; Yamashita et al. 2005), and kinetochore and its interacting proteins (Cleveland et al. 2003; Lew and Burke 2003; Sanchez-Perez et al. 2005) are also implicated in spindle dynamics.
We previously isolated a cold-sensitive γ-tubulin mutant, gtb1-93, in fission yeast and demonstrated that it has strong genetic interactions with Pkl1, Klp5/6, and Dis1 (Tange et al. 2004). At a restrictive temperature, the mutant produced an abnormally elongated spindle that failed to properly segregate chromosomes (Paluh et al. 2000). It contained apparently stabilized cytoplasmic MT bundles at both restrictive and permissive temperatures. Although the mutant proliferated at permissive temperatures at a rate nearly identical to that of wild type (Tange et al. 2004), as we demonstrate in this study, close examination of the mutant mitoses revealed peculiar abnormalities. More specifically, the kMT dynamics in metaphase through anaphase A appeared strikingly different from that of wild-type mitosis. Despite the abnormal mitosis, nuclear division ended with near-normal chromosome segregation. Near-normal mitosis in the γ-tubulin mutant required the SAC genes in varying degrees. By isolating new novel mutations in the mad2 gene, we demonstrated that the function of Mad2 required for the γ-tubulin mutant mitosis were genetically discernible from the canonical SAC function. Furthermore, the new mad2 mutants, unlike a complete loss-of-function mutation of the mad2 gene, did not appreciably destabilize chromosome stability in unperturbed mitosis.
MATERIALS AND METHODS
Strains and general genetic methods:
Yeast strains used are listed in supplemental Table 1 at http://www.genetics.org/supplemental/. Strains carrying disrupted mad2 (mad2Δ) and mad1Δ, both by ura4+ insertion, as well as a strain with the mad2+-GFP, were obtained from T. Matsumoto (Ikui et al. 2002). Strains with sid4+-GFP were described in Tange et al. (2004). The green fluorescent protein (GFP)-tagged atb2+ gene expressed under the control of the nda2 promoter at the lys1 locus was kindly provided by H. Masuda (for construction, see Masuda et al. 2006). GFP-tagged cdc13+ was obtained from M. Yanagida via the Yeast Genetic Resource Center. Gene disruption with the G-418-resistant gene was performed according to Bahler et al. (1998). YE containing G-418 (Sigma Chemical, St. Louis) at a concentration equivalent to 100 μg/ml was used for this selection. Strains carrying the lacO sequence at cen2 or ade6 together with the lacI-GFP expression system were obtained from A. Yamamoto. Rich media, YE, supplemented YE (YES), and YPD, and a synthetic medium, EMM2, were used (Alfa et al. 1993). For culture of the mad2Δ gtb1 double mutant or for a thiabendazole (TBZ)-sensitivity assay, 5 or 20 μg/ml TBZ, respectively, was added to the medium. For the minichromosome stability assay, YE or EMM2 (supplemented with 5 μg/ml adenine sulfate) were used. EMM2 was used when selective pressure for the plasmid was needed.
Cytologic methods:
For the experiment shown in Figure 1 or other experiments where indicated, 10 mm hydroxyurea (HU) was added to a logarithmic phase of culture at 33° for 3 hr, and cells were collected and washed on a glass filter, resuspended in fresh media (EMM2 or YE), and incubated for 75 to 90 min. Methanol fixation was performed as follows: Yeast cells were harvested by a glass filter, soaked in chilled methanol, and kept at −80° for 2 hr. Fixed cells were resuspended in PEMS buffer (100 mm PIPES, 1 mm EGTA, 1 mm MgCl2, 1.2 m sorbitol, pH 6.9) and digested with zymolyase before staining with 4′,6-diamidino-2-phenylindole (DAPI). Condensed chromosomes in the cut7 mutant and in carbendazim (CBZ)-treated cells were observed with DAPI staining after glutaraldehyde fixation. CBZ (50 μg/ml) was added to log-phase cultures in YES medium at 30° for the indicated time. Fluorescence in situ hybridization (FISH) was performed according to Goto et al. (2001), using the probes cos1228 (chromosome 2) and cos1322 (chromosome 3) (Mizukami et al. 1993), which were mapped, respectively, at ∼50 and 150 kb from the respective centromere (The Wellcome Trust Sanger Institute, S. pombe Genome Project). Microscopic observation of living cells was performed using the DeltaVision system (Applied Precision, Issaquah, WA) placed in an air-conditioned room set at 30° ± 0.5°. Relevant parameters for image acquisition are described in the legends to Figures 2–4 and 6. Images presented in this work were obtained by assembling the pixels with the highest signal intensities through all images from different focal planes acquired at the same time point without the computational removal of out-of-focus information. Spindle length, presented in Figure 2, A and B, was measured by taking the difference in focal plane into account.
Figure 1.—
Separation of chromosomes on the mitotic spindle. Synchronized cells with GFP-tagged α-tubulin at 30° in EMM2 medium were fixed with methanol and stained with DAPI. In A–D, microtubules (green) and chromosomes (red) (color panels) and chromosomes in black-and-white images are shown. Cells containing a spindle >∼5 μm were chosen, and then the spindle length and the segregation figures of chromosomes were recorded. The figures were classified as equal (representative image as in A and in blue boxes in E–G), incomplete separation (B, red), lagging chromosome near the spindle center (C, pink) or close to the spindle pole (green), and unequal (D, yellow). Each box in E–G represents one cell. Strains used were YT417 (wild type), YT413 (gtb1-93), and YT415 (gtb1-93 mad2Δ). See supplemental Table 1 (at http://www.genetics.org/supplemental/) for strains.
Figure 2.—
Live observation of spindle dynamics. Spindle length was determined as the distance between two separating Sid4-GFP signals. Living cells were observed every 20 sec, and at each time point 12 images were taken at serial focal planes at 0.2-μm steps (A and B). These parameters were changed to 30 sec, 6 images, and 0.4-μm steps in C and D. The time of the onset of anaphase B (phase 3) was set as time 0 and all figures obtained for each strain are assembled in a single figure. (A) Wild type (strain P18, number of cells (n) = 21). (B) gtb1-93 (P168, n = 24). (C) mad2Δ (YT522, n = 10). (D) gtb1-93 mad2Δ (YT519, n = 14).
Isolation of new mad2 alleles:
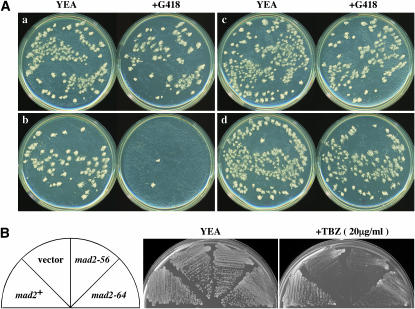
A polymerase chain reaction (PCR)-based mutagenesis of the mad2+ gene was performed using the Diversify PCR random mutagenesis kit (CLONTECH, Palo Alto, CA), and the fragments produced were cloned in a multicopy vector (pKD10; Shimanuki et al. 1997) and transformed into W986 (mad2Δ). Each transformant was first tested for TBZ sensitivity on YE solidified with agar (YEA) containing 20 μg/ml TBZ at 33°. The transformants were crossed with a strain in which the G-418-resistant marker (Bahler et al. 1998) was inserted 600 bp upstream of the start codon of the gtb1-93 mutant gene. Spores from this cross were spread on EMM2 plates (to select for Ura+ and Leu+ colonies) and incubated at 33° for 3 days followed by replica plating onto G418 plates to select for the gtb1-93 mutation linked to the drug-resistant marker. After incubating for 2 days, the number of large colonies growing on the drug-containing plates was compared with control plates (see Figure 7). Two strains displayed TBZ hypersensitivity and many large colonies on the G-418 plates were selected (see text). To create strains containing singly integrated copies of the mad2 alleles, we first used PCR to generate a 6-kb-long genomic DNA fragment containing the mad2 gene near the center of the fragment, in which the G-418-resistant gene (Bahler et al. 1998) was inserted 136 bp downstream from the termination codon of the mad2 gene. This fragment was transformed into HM713 (h− leu1 ura4 mad2∷ura4+), and G418-resistant and Ura− transformants were selected. Correct integration was confirmed by sequencing the relevant genomic DNA segment. For GFP tagging of these mad2 alleles, a GFP-tagging cassette with a nourseothricin-resistant (natr) marker was used (a kind gift from T. Toda) (Sato et al. 2005). Nourseothricin (clonNAT) was purchased from Werner BioAgents (Jena, Germany) and used at 100 μg/ml.
Figure 7.—
Genetic properties of new mad2 mutants. (A) The G-418 plate assay. Colonies grown on EMM2 plates from spores produced from crosses between P5 (kanr linked to the gtb1 mutation) and W986 (mad2) carrying a plasmid with mad2+ (a), empty vector alone (b), mad2-56 (c), or mad2-64 (d) were replica plated onto YE with or without G418 (see text for details). (B) The TBZ-sensitivity assay. HM713 (mad2Δ) carrying a plasmid with the indicated mad2 alleles grown on EMM2 medium was streaked on YE plates with or without TBZ and incubated at 33° for 3 days.
Immunoprecipitation analysis:
Strains containing GFP-tagged Mad2 were cultivated for logarithmic growth at 30° in YPD medium and harvested by centrifugation. When required, 10 or 12 mm HU was added to the culture, followed by a 3-hr incubation for S-phase arrest. To release the cells from the S-phase arrest, the cells were incubated for 75 min in the absence of HU. For strains carrying the cut7ts or nda3cs mutations, cells were incubated at 36° for 3 hr or at 18° for 6 hr, respectively, to obtain mitotically arrested cells. Cells were washed once with TE (10 mm Tris–HCl, 1 mm EDTA, pH 7.5), frozen in liquid nitrogen, and stored at −80° until use. Immunoprecipitation was performed according to Saitoh et al. (2005) with modifications. To the frozen cell pellet, an equal amount of extraction buffer [50 mm HEPES–Na, pH 7.5, 10% glycerol, 0.5 mm dithiothreitol, 125 mm NaCl, 0.2% NP-40, 1% proteinase inhibitor cocktail (P8215, Sigma), 1 mm Pefabloc SC (Roche, Nutley, NJ)] was added and cells were disrupted with glass beads using Multi-Beads Shocker (Yasui Kikai, Osaka, Japan). The soluble fraction of the extracts was obtained by centrifuging at 1500 × g for 5 min and then at 14,000 × g for 10 min. An aliquot of the soluble extract containing 2 mg of protein was brought to 100 μl with extraction buffer and incubated at 4° for 1 hr with or without 0.15% of sodium dodecyl sulfate (SDS). After incubation, the SDS concentration was adjusted to 0.05% by dilution, and the soluble extract was further incubated at 4° for 1 hr with 25 μl Dynabeads M-280 (Dynal Biotech ASA, Oslo, Norway) conjugated with sheep anti-mouse IgG, which was preincubated with 2 μg of mouse anti-GFP antibody (Roche Diagnostics). After washing three times with the extraction buffer, bound proteins were recovered in a loading buffer for SDS–polyacrylamide gel electrophoresis by heating at 100° for 5 min. Western blot analysis was performed with anti-Mad1 rabbit polyclonal antibody (a generous gift from T. Matsumoto) and with the anti-GFP antibody. For the reciprocal immunoprecipitation experiments, we attempted to create appropriately tagged Mad1 protein, but failed to obtain functionally active constructs.
Cdc2 kinase assay:
Cdc2 kinase activity in cell extracts was measured essentially as described in Moreno et al. (1989), but activity was assayed in the crude extracts. Histone H1 was purchased from Roche Applied Science (Tokyo). After the reaction, H1 histone was separated on a 15% SDS–polyacrylamide gel, and incorporated 32P was quantified using the Bio-Imaging analyzer system (Fuji Photo Film, Tokyo).
RESULTS
Aberrant mitosis in the γ-tubulin mutant:
As previously reported, the cold-sensitive γ-tubulin mutant (gtb1-93) doubles at a rate almost identical to that of wild type at permissive temperatures (Tange et al. 2004). There were several aberrant features, however, associated with its mitosis.
Strains with or without the gtb1-93 mutation containing the GFP-tagged α2-tubulin (Masuda et al. 2006) for visualizing spindles were treated with HU to synchronize cell cultures. After release from the S-phase block, mitotic cells appeared at approximately the same time in both strains. Methanol-fixed cells were stained with DAPI and examined for spindle length and chromosome separation. The spindle length in cells synchronized by HU-block release was longer than that in untreated cells. Nevertheless, in gtb1+ cells, when spindle length was ≥6 μm, two equally sized chromosomal masses became well separated [Figure 1, A and E (blue)]. In the gtb1 mutant, however, chromosomes were frequently not well separated in a spindle, even when spindle length exceeded 7 μm (Figure 1B), and there were lagging chromosomes in 11 of 85 mitoses (Figure 1, C and F). Despite this aberrancy, mutant cells in late anaphase or telophase (when the chromosomes nearly reached the ends of the cell) contained two equally sized chromosomal masses in 166 of 171 cells [the remaining cells contained large and small chromatin regions (n = 2) and lagging chromosomes (n = 3)]. Wild-type cells contained normally segregated chromosomes in all 191 cases examined. Thus, replicated chromosomes seemed to be distributed into daughter cells in near-normal fidelity at the permissive temperature in the mutant.
Live analysis of spindle dynamics in the γ-tubulin mutant:
To examine the mutant mitosis in more detail, we performed live analyses of spindle dynamics and chromosome behavior. Spindle dynamics in wild-type fission yeast are divided into three phases (Nabeshima et al. 1998; Mallavarapu et al. 1999). In phase 1, a bipolar spindle is formed; in phase 2, spindle length remains unchanged or only slightly elongates; and in phase 3, the spindle elongates to fully separate the chromosomes. Phases 2 and 3 correspond to prometaphase/metaphase and anaphase B, respectively. Anaphase A occurs at the end of phase 2, but occupies only a small part of it.
The spindle dynamics were determined using GFP-tagged Sid4, whose localization is strictly confined to the spindle pole body (SPB) throughout the cell cycle (Chang and Gould 2000). The distance between the two separating Sid4 signals was measured. Although the onset of phase 2 was not clear, the timing of the onset of phase 1 as well as that of phase 3 could be unambiguously determined. We ascertained in a separate experiment that, consistent with previous reports (Yanagida et al. 1999; Decottignies et al. 2001), Cdc13 (B cyclin)-GFP disappeared from the spindle and accumulated along the nuclear periphery at approximately the onset of phase 3 in both wild-type and mutant cells (data not shown). Spindle length at the onset of anaphase B was in the range of 2.2–3.6 μm (2.82 ± 0.39 μm; n = 21) in wild type, while that in the gtb1 mutant was between 3.0 and 5.0 μm (3.93 ± 0.45 μm; n = 24). Thus the metaphase spindle length in the mutant was 39% longer than that in the wild type. We then determined the duration of phases 1 and 2: 8.5 ± 1.8 min in the wild type and 8.9 ± 3.0 min in the mutant. This indicated that there was no significant difference in the average length of the duration from the start of phase 1 to the end of phase 2, although there was more variability in the mutant. There was little or no linear correlation between the duration of phases 1 and 2 and the spindle length at the onset of phase 3 in either the wild-type or the gtb1 mutant (supplemental Figure 3 at http://www.genetics.org/supplemental/). In Figure 2, A and B, all of the spindle elongation profiles were assembled in a single figure for each genotype by setting the anaphase B onset as time 0. The speed of SPB separation during phase 1 was faster in the mutant (∼1.7-fold) than in the wild type, but it was almost identical in phase 3 (Figure 2, A and B).
Anaphase A kMT dynamics were impaired in the gtb1 mutant:
We observed chromosome behavior by using the kinetochore protein Mis6 tagged with GFP (Saitoh et al. 1997). Unlike in wild-type cells, chromosome segregation of all chromosomes (anaphase A) did not occur simultaneously near the center of the spindle, but occurred at apparently more scattered positions along the spindle (data not shown). Because of the weak signal intensity of the Mis6-GFP and the swift movement of the signals, we were unable to trace individual kinetochores in this analysis. Therefore, we used the lacO/lacI-GFP system, which allowed for fluorescent marking of unique chromosomal loci. The loci that we used in this study were the cen2 (5 kb from the centromere) and the ade6 locus on chromosome 3 (Nabeshima et al. 1998; Yamamoto and Hiraoka 2003). Sid4-GFP was also used simultaneously to determine spindle length. We measured the distances between Sid4 and Sid4, Sid4 and cen2, and cen2 and cen2 at each time point. Representative results in the wild type as well as in the mutant are shown in Figure 3.
Figure 3.—
Live analysis of sister-chromatid separation. Fission yeast cells carrying both Sid4-GFP and cen2-GFP were observed every 15 sec with 10 steps (0.2 μm) along the z-axis at each time point. Cells in late phase 1 or in phase 2 were chosen for observation. Distances (d1–d4), as defined in the figure (Sid4-GFP: green dots; cen2-GFP: orange stars), were measured and plotted. (A and B) Wild type (P172). (C–F) gtb1-93 (P171).
Consistent with previous reports (Nabeshima et al. 1998; West et al. 2002), the pair of cen2 signals remained around the center of the spindle in wild-type phase 2 before the separation, although in some cases the pair of signals occasionally moved off the center and separated there. The duration of anaphase A determined by this method (from the separation of the sister-chromatid pair to the complete shortening of kMTs) was generally <45 sec. The onset of anaphase B (phase 3) occurred around the end of anaphase A (Figure 3, A and B). In contrast, there were several aberrant features in the mutant mitoses (Figure 3, C–F). First, sister centromere pairs tended not to be positioned near the spindle center, but to oscillate within the range of the spindle, and the separation of the pairs occasionally occurred away from the spindle center. In some cases, the separation occurred at or near the spindle pole (Figure 3F). Second, the kMT shrinkage rate in anaphase A was often different within pairs (for example, see Figure 3, D and E). Remarkably, one of each pair of kMTs sometimes even seemed to elongate while the other was shortening, even after the onset of anaphase A (Figure 3E). Probably related to this phenomenon, we encountered cases where sister-chromatid separation paused for a period [45 sec in Figure 3E, and 1 min in Figure 4 (right panels, green triangles)]. Third, the anaphase A movement of individual centromeres sometimes lasted far into anaphase B (see, for example, Figure 3, C and F). Almost identical results were obtained when using the ade6 marker (data not shown).
Figure 4.—
Timing of sister-chromatid separation of different chromosomes. Cells containing cen2-GFP and ade6-GFP as well as Sid4-GFP were observed in living cells every 15 sec. At each time point, 10 images were taken with a single z-axis step of 0.2 μm. P175 (WT) and P174 (gtb1) were used. In wild type, one of the sister-chromatid pairs was already separated in the fourth frame, but the other remained unseparated until the sixth frame. In the mutant, both pairs appeared to separate simultaneously (third frame from the top), but one of them (marked by green triangle) stopped separating for >1 min while the other (red triangle) continued to separate.
We then observed the cen2 and ade6 loci simultaneously in living cells. In wild-type cells, both of the loci began to separate almost simultaneously (within 15 sec) in 12 of 17 cases, but in other cases the separation timing was different by 30–50 sec (for example, see Figure 4, left panels). The differential separation appeared in approximately the same frequency in the mutant; in 11 of 14 mitoses, sister chromatids of chromosomes 2 and 3 separated simultaneously (Figure 4, right panels), while in others it occurred at a 30- to 45-sec interval. Thus, temporal coordination of sister-chromatid separation among chromosomes was not altered in the mutant, although spatial coordination was impaired. Thus, the mutant mitosis was characterized by a longer metaphase spindle, off-centered positioning of sister-chromatid separation, uncoordinated shrinkage of each kMT, and lagging chromosomes due to incomplete anaphase A in the middle of anaphase B. These features might explain the unusual distribution of chromosomes described in Aberrant mitosis in the γ-tubulin mutant.
Some, but not all, spindle checkpoint genes are required for ensuring near-normal proliferation of the γ-tubulin mutant:
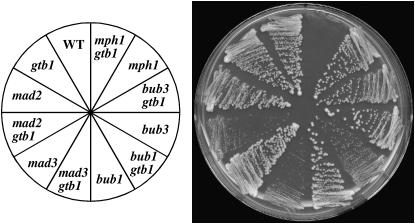
Given the aberrancy in spindle and chromosome dynamics in the mutant, we examined whether SAC function was involved in ensuring the near-normal chromosome segregation. We combined individual checkpoint mutants with the gtb1 mutation and examined the colony size of the double mutants grown at permissive temperatures for the gtb1 mutant (Figure 5). The combination of mad2Δ, mad3Δ, or bub1Δ mutations with the gtb1 mutation led to the formation of small colonies, containing many dead cells. The mph1Δ mutation seemed to exert a more deleterious effect. In contrast, on the basis of colony size, the bub3Δ mutation had little synergetic effect with the gtb1 mutation. Intriguingly, the mad1Δ gtb1-93 double mutant did not form colonies from spores, indicating that this checkpoint gene has some functions different from those of Mad2 in the gtb1 mutant. The mad1Δ gtb1 double-mutant cells made extremely small colonies when a low concentration of TBZ (5 μg/ml) was added to the medium, which relieves the gtb1 defect (Tange et al. 2004), but failed to form colonies when the cells were transferred to a TBZ-free medium. This indicated that the double mutant was not solely defective in germination from spores but in fact was lethal in the absence of the drug. The double mutant had a deregulated septation defect, which was not observed in the mad2Δ gtb1 double mutant (Y. Tange and O. Niwa, unpublished results).
Figure 5.—
Differential requirements of SAC genes for growth of the γ-tubulin mutant. Approximately the same amount of cells freshly grown on a YE plate at 33° was streaked out on the same medium and incubated at 30° for 3 days. Strains used are HM123 (WT), W604 (gtb1), HM713 (mad2), YT578 (mad2 gtb1), YT406 (mad3), YT410 (mad3 gtb1), YT405 (bub1), YT409 (bub1 gtb1), YT407 (bub3), YT411 (bub3 gtb1), YT408 (mph1), and YT412 (mph1 gtb1).
Mad2 is required for near-normal chromosome segregation in the γ-tubulin mutant mitosis:
As described above, the gtb1-93 mad2Δ double mutant was viable but produced very small colonies at permissive temperatures for the gtb1 mutation (see Figure 5). Cells composing the small colonies (excluding apparently dead cells) had ∼20% plating efficiency. To determine the reason for this low viability, we examined chromosome segregation as shown in Figure 1G. Two notable features were apparent in the double mutant compared to the gtb1 single mutant. One was the reduction of “incomplete separation” (shown in red in Figure 1) in spindles >7 μm, and the other was the high incidence of unequal nuclear division (Figure 1D and yellow in Figure 1G) and lagging chromosomes (Figure 1C and green and pink in Figure 1G), both indicating errors in chromosome segregation. FISH analysis using probes for chromosomes 2 and 3 revealed a high frequency of nondisjunction in the double mutant (supplemental Figure 1 at http://www.genetics.org/supplemental/). Thus, the low viability of the double mutant was accounted for by the high incidence of errors in chromosome segregation.
We then performed live analysis of the spindle dynamics in mad2Δ gtb1-93 double mutants and also in mad2Δ single mutants (Figure 2, C and D). As anticipated from its nearly normal growth, the mad2Δ single mutant had spindle dynamics comparable to the wild type (Figure 2C), although the duration of phase 1 plus phase 2 appeared to be less than in wild-type cells (7.5 ± 1.7 min; n = 8), which was ∼1 min shorter than in the wild type. Spindle length at the onset of anaphase B (2.4 ± 0.27 μm) might also be shorter than that of wild-type spindles. In sharp contrast, there was a drastic change in the double mutant (Figure 2D). It was difficult to determine the timing of anaphase B onset in the double mutant, even with the aid of Cdc13 localization analysis. Thus, the results shown in Figure 2D are only approximate. Nevertheless, it was clear that in the double mutant the length of spindles at the onset of anaphase B were highly variable. Also, the duration of phase 2 was very short, and in some cells it was almost missing. Live observation of sister-chromatid separation with the cen2 and ade6 loci revealed unequal chromosome segregation in 7 of 30 mitoses in the double mutant (four nondisjunction and three lagging chromosomes). These live analyses demonstrated that in the absence of functional Mad2 in the gtb1 mutant, the duration of the progression through phases 1 and 2 was greatly shortened and the formation of the prometaphase/metaphase spindle was impaired. This in turn suggested that anaphase B tended to start without prior proper formation of the bipolar metaphase spindle that would be required for accurate chromosome segregation.
As reported previously (Tange et al. 2004), the cold-sensitive growth phenotype of the γ-tubulin mutant was suppressed by an α2-tubulin mutant, which itself was hypersensitive to the MT-destabilizing drug TBZ, or partially suppressed by the addition of a low concentration of TBZ into the medium. These MT-destabilizing conditions alleviated the harmful effects of the mad2 mutation on the gtb1 mutant growth (data not shown), suggesting that the occurrence of improperly stabilized MTs affects the spindle formation so that it becomes heavily dependent on Mad2 (and probably also on some other checkpoint genes) to proceed with near-normal dynamics.
Mad2 localization is altered in the mutant:
Because the near-normal mitosis in the gtb1 mutant was very dependent on Mad2, we examined whether the localization of the protein was altered. We used GFP-tagged Mad2 (Ikui et al. 2002) and observed it in living cells. Consistent with previous reports (Ikui et al. 2002; Saitoh et al. 2005) during interphase of wild-type cells, Mad2-GFP was localized to the nuclear periphery as well as to the chromatin region, and during the prophase to prometaphase it was highly accumulated at kinetochores, which were often clustered (Figure 6, A and B). After the proper attachment of kinetochores to spindle MTs was established in metaphase, the intense kinetochore signals diminished. Thus, on the phase 2 spindles, Mad2-GFP localization was observed only near the poles and very faintly along the spindle (Figure 6C). The localization of Mad2 was altered in the mutant in two aspects. One was that phase 2 spindles had brighter Mad2 signals at the ends of the spindle, often at only one of the poles (Figure 6E). Moreover, phase 2 spindles in the mutant sometimes contained a bright dot on the spindle between the poles, which was moving along the spindle (Figure 6H). From its behavior, we assumed that the bright dot between the spindle poles was a pair of kinetochores, but this was not verified. Less intense dot signals sometimes coexisted with the bright dot on the same spindle. Mad2 localization was also altered in phase 3. In wild type there were only faint signals at the poles of the spindles (Figure 6D), but in the mutant, brighter signals were often observed, generally near one of the poles (Figure 6, F and G). The signals were not dot-like in shape but usually elongated toward the spindle center, and in some cases we noted multiple dots moving inward from the pole. The phase 3 spindles in the mutant occasionally contained multiple dot signals between the poles, which were moving to the spindle end (see supplemental Figures 4–7 at http://www.genetics.org/supplemental/ for movies). The mutant-specific Mad2 localization on the metaphase–anaphase spindle might reflect the aberrant kMT dynamics during these phases. As has been suggested in other organisms (Buffin et al. 2005), Mad2 protein might flow from the kinetochores to the spindle poles during anaphase in fission yeast. In the mutant, there might be an increased chance of detachment of kMTs from kinetochores that would lead to Mad2 recruitment to the kinetochores and subsequent transfer toward the pole. Also, because of the prolonged existence of kMTs in the mutant, more Mad2 proteins might be transferred to the pole and accumulate there. The possibility that the localization of Mad2 protein along the spindle and at the poles was independent from the kinetochore, however, could not be ruled out. According to Saitoh et al. (2005), in a subset of kinetochore mutants in which Mad2 localization to unattached kinetochores was reduced, the accumulation of Mad2 at the spindle pole was rather enhanced.
Figure 6.—
Dynamic localization of Mad2-GFP in mitotic cells. (A–D) Wild type (W949). (E–H) gtb1 (W948). (A) Prophase. (B) Prometaphase. (C) Metaphase (phase 2). (D) Anaphase (phase 3). (E–G) Early anaphase to late anaphase. (H) Metaphase (phase 2): sequential images showing dynamic movement of intense Mad2-GFP dot along the spindle. Original images are available as movies in supplemental Figures 4 and 5 (wild type) and supplemental Figures 6 and 7 (gtb1) at http://www.genetics.org/supplemental/. Images were taken every 15 sec, nine images with 0.3-μm steps in the z-axis at each time point.
Isolation of mad2 mutants that are defective in general SAC but retain the function for γ-tubulin mutant viability:
The differential requirements of SAC genes, particularly of Mad1 and Mad2, for the γ-tubulin mutant led us to examine whether the function of Mad2 for ensuring near-normal mitosis in the mutant differs from the function to arrest mitotic progression responding to gross perturbation of spindle formation [for the sake of simplicity, we hereafter use gtb1-CS (for chromosome stability) and general SAC to distinguish Mad2 functions required in these different conditions]. To address this directly, we attempted to isolate mad2 mutants that were defective in general SAC function but retained gtb1-CS activity. We isolated two mad2 mutant genes (mad2-56 and mad2-64) on a multicopy plasmid. These mad2 alleles supported the growth of the gtb1 mutant as well as the wild-type mad2 (Figure 7A).
Both mad2-56 and mad2-64 were isolated on the basis of their hypersensitivity to TBZ, indicative of the deficiency in general SAC (Figure 7B). We verified this finding by two different criteria. The first criterion was the ability to block mitotic progression in the cut7ts mutant; the existence of hypercondensed chromosomes was considered to be an indication of general SAC activity (Kim et al. 1998). With the mad2+ allele, 44.0% cells contained hypercondensed chromosomes, while with mad2-56 and mad2-64 this value was reduced to 4.6 and 6.7%, respectively, values only slightly higher than the control value with the vector (Table 1). Importantly, when the mutant alleles were introduced into mad2+ cells with the multicopy plasmid, they had a clear dominant-negative effect over the mad2+ gene, suggesting that they sequestered the active Mad2 product, preventing its checkpoint function. The second criterion used was the accumulation of Cdc2 kinase activity after mitotic arrest (He et al. 1997). In both nda3cs and cut7ts mutants, the mad2+-dependent increase in Cdc2 kinase activity was significantly reduced in the newly isolated mad2 mutants (supplemental Figure 2 at http://www.genetics.org/supplemental/). Taken together, we concluded that these mad2 alleles were not functional in blocking mitotic progression when spindle assembly was grossly compromised, but remained functional for gtb1-CS.
TABLE 1.
Mad2-dependent mitotic arrest assessed in the cut7 mutant
| mad2 allele in host cell | mad2 allele on plasmid | % of cells with condensed chromosomes (total no. of cells) |
|---|---|---|
| mad2Δ | mad2+ | 44.0 (184) |
| None | 2.2 (180) | |
| mad2-56 | 4.6 (151) | |
| mad2-64 | 6.7 (180) | |
| mad2-64M52I | 42.9 (163) | |
| mad2-64D177V | 31.6 (190) | |
| mad2+ | mad2+ | 67.2 (119) |
| None | 35.6 (174) | |
| mad2-56 | 7.8 (255) | |
| mad2-64 | 7.8 (218) | |
| mad2+ | No plasmid | 52.7 (163) |
| mad2Δ | 1.7 (241) | |
| mad2+∷kanr | 53.2 (171) | |
| mad2-56∷kanr | 5.2 (271) | |
| mad2-64∷kanr | 6.5 (245) |
Strains containing plasmid were incubated at 36° for 4 hr in minimal medium, but those without plasmid were incubated in YPD medium at 36° for 3 hr. Cells were fixed with glutaraldehyde and stained with DAPI. Cells that had hypercondensed chromosomes were scored. Yeast strains used were P43 (mad2Δ) and HM783 (mad2+) for the tests with plasmids, and P43, HM783, YT541 (mad2+∷kanr), YT542 (mad2-56∷kanr), and YT543 (mad2-64∷kanr) for chromosomal mad2 alleles.
Mutant Mad2 proteins formed a Mad1-containing complex with altered stability:
Mutation sites in the new mad2 alleles were determined. In mad2-56, Ser at 185 was changed to Arg and in mad2-64, Met52 and Asp177 were changed to Ile and Val, respectively. Either single mutation in the mad2-64 allele alone did not confer an extensive SAC defect, although the D177V mutation displayed slightly reduced SAC activity (Table 1).
Because both of the mutant proteins had an amino acid exchange in the carboxyl terminal segment of Mad2, which is implicated in the stable complex formation with Mad1 (Luo et al. 2002; Musacchio and Hardwick 2002; Sironi et al. 2002; De Antoni et al. 2005), we tested whether the amount and/or stability of the Mad1-containing complex was altered. GFP-tagged Mad2 proteins were expressed under the control of a native mad2 promoter. Cell extracts were immunoprecipitated with anti-GFP antibody, and the amounts of co-immunoprecipitated Mad1 were compared by Western blot analysis (see materials and methods). In a control experiment, we ascertained that the immunoprecipitation of Mad2-GFP and Mad1 was dependent on added anti-GFP-antibody and on the GFP-tagging of Mad2 (data not shown). Comparable amounts of Mad2-GFP were recovered in both wild type and mutants (Figure 8). The mobility of GFP-fused mad2-64 mutant proteins was slower in the electrophoresis, although it is not clear why. The amounts of co-immunoprecipitated Mad1 were not appreciably different between wild type and mutants. They were not affected by increasing the salt concentration to 0.5 m NaCl (data not shown). However, when the extracts were incubated in the presence of a low concentration of the detergent SDS, prior to the addition of the antibody, the amount of Mad1 was greatly reduced in the mutant extracts, but not in the wild-type extract (Figure 8). The reduction of Mad1 by SDS treatment in the mutant extracts was not due to degradation of the protein possibly induced by the SDS treatment, because there was no reduction of the protein in SDS-treated whole extracts (Figure 8). These results strongly suggested that the mutant Mad2 proteins form a Mad1/Mad2 complex that has a different stability from that formed with wild-type Mad2. We then addressed whether the amount of the SDS-resistant form of the complex changes in a cell-cycle-related manner or in response to SAC-inducing conditions (see materials and methods), but failed to see any notable changes at least in the soluble cell extracts (data not shown).
Figure 8.—
Immunoprecipitation analysis of the mutant Mad2 proteins. Strains containing mad2+-GFP (YT579), mad2-56-GFP (YT580), and mad2-64-GFP (YT581) were used. Immunoprecipitated proteins were detected by Western blot analysis using the indicated antibodies. IP, immunoprecipitated. WCE, whole-cell extracts. Protein size markers are on the right: open triangle (80 kDa) and closed triangle (50 kDa). See materials and methods for experimental details.
We also examined whether the localization properties of mutant Mad2 were altered from wild-type protein, using the GFP-tagged genes. There was no notable difference in the localization profile under any experimental conditions that we tested when using singly integrated genes (i.e., in mitotically arrested cells induced by the nda3cs mutation and by CBZ treatment, and in wild-type cells that were synchronized by HU arrest and release). It remains to be examined, however, whether there are any differences in the amount and/or in the dynamic nature of the localization. In cells that expressed the GFP-tagged Mad2 proteins from the multicopy plasmid, wild-type protein accumulated at a site in the interphase nucleus, presumed to be at the SPB or at the clustered centromeres, whereas there was very little accumulation of the mutant proteins (data not shown).
Minichromosome stability in unperturbed mitosis was only marginally affected by the new mad2 mutations:
As another way to access Mad2 activity, we measured minichromosome stability by the half-sector method (Allshire et al. 1995). In the presence of the wild-type mad2 allele, mitotic loss occurred approximately once/3 × 103 cell divisions (Table 2). In the absence of Mad2, minichromosome instability increased 6-fold. When cells carried the mad2-56 or mad2-64 alleles, the minichromosome remained stable, and the stability was only 1.3- or 1.1-fold different from that of wild type. When the same cells were challenged with 7.5 μg/ml TBZ to perturb the spindle assembly, minichromosome stability was greatly reduced in cells carrying either the mad2-56 or mad2-64 alleles (11- and 6-fold, respectively), but it was only slightly affected in mad2+-carrying cells (1.8-fold). This is consistent with the fact that the new mad2 alleles were defective in general SAC.
TABLE 2.
Effect of mad2 mutations on minichromosome stability
| mad2 allele at chromosomal locus | mad2 allele on plasmid | TBZ | Total no. of colonies | % half-sectored colonies |
|---|---|---|---|---|
| mad2Δa | mad2+ | 0 μg/ml | 247,165 | 0.032 |
| None | 250,158 | 0.19 | ||
| mad2-56 | 260,861 | 0.047 | ||
| mad2-64 | 266,571 | 0.029 | ||
| mad2+ | 7.5 μg/ml | 41,076 | 0.054 | |
| None | 7,066 | 1.8 | ||
| mad2-56 | 40,922 | 0.43 | ||
| mad2-64 | 36,916 | 0.21 | ||
| mad2+b | mad2+ | 0 μg/ml | 38,422 | 0.005 |
| None | 36,545 | 0.012 | ||
| mad2-56 | 44,037 | 0.014 | ||
| mad2-64 | 36,188 | 0.006 | ||
| mad2+∷kanrc | NAd | 0 μg/ml | 72,048 | 0.011 |
| mad2Δ | 53,651 | 0.15 | ||
| mad2-56∷kanr | 33,853 | 0.035 | ||
| mad2-64∷kanr | 64,886 | 0.020 | ||
| mad2+∷kanr | 7.5 μg/ml | 66,316 | 0.030 | |
| mad2Δ | 6,170 | 0.91 | ||
| mad2-56∷kanr | 16,994 | 0.78 | ||
| mad2-64∷kanr | 31,399 | 0.26 |
h− leu1 ade6-210 mad2Δ Ch16 (P112; see supplemental Table 1 at http://www.genetics.org/supplemental/) was transformed with a plasmid carrying the indicated mad2 alleles. Minichromosome stability assay was performed on synthetic medium selective for the plasmid marker.
Same as in footnote a, but P113 was used (h− leu1 ade6-210 Ch16).
h− leu1 ade6-210 Ch16 with the indicated mad2 alleles at the chromosomal loci were used to determine minichromosome stability on YE plates. Strains used were P224, P112, P225, and P227.
NA, not applicable.
With regard to the dominant-negative effect of the new mad2 mutants over wild-type mad2 in cut7 mutants (described above), we tested minichromosome stability in wild-type cells that carried the plasmid with different mad2 alleles. Stability was not altered in these cells (Table 2). Further, the growth of the gtb1 mutant was not affected by the multicopy plasmid bearing the new mad2 alleles (data not shown). These results also demonstrated that the functions of Mad2 required for general SAC and for gtb1-CS and minichromosome stability are genetically discernible.
Activity of a single copy of the new mad2 alleles:
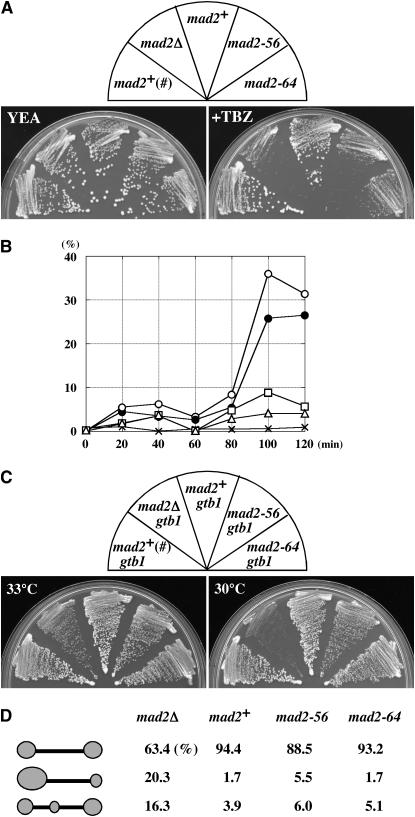
Because the new mad2 alleles were first isolated in plasmids and subsequent tests (described above) were performed with the plasmid-borne genes, we created strains containing a single integrated copy of the mad2 allele (see materials and methods). Using these mad2 alleles, we first tested whether these alleles are recessive to the wild type. Diploid cells heterozygous at the mad2 locus (mad2+/mad2-56, mad2+/mad2-64, and mad2+/mad2-null) had TBZ resistance similar to that of homozygous mad2+/mad2+ wild-type diploid cells (data not shown). This suggested that both of the mad2 mutants were recessive to wild type with regard to general SAC function. Thus, the observed dominant-negative effects of the plasmid-borne mad2 alleles (see Table 1) were likely due to overproduction of the mutant proteins. We then tested these single-copy mad2 alleles for TBZ sensitivity (Figure 9A), the activity to support the growth of the γ-tubulin mutant (Figure 9, C and D), the checkpoint function to arrest mitosis in the cut7 mutant and in CBZ-treated cells (Table 1, Figure 9B), and the effect on minichromosome stability (Table 2). The results indicated that the single copy of the new mad2 mutants had activities that were comparable to those observed with multicopy plasmids. The results demonstrated that the differential activities of the new mad2 mutants displayed in various aspects of Mad2-requiring cellular processes were not solely due to the high gene dosage.
Figure 9.—
Activity of a single copy of the new mad2 alleles. mad2+ (#) indicates the normal wild-type mad2 allele, while mad2+, mad2-56, and mad2-64 indicate that these alleles were replaced with the mad2Δ allele together with the kanr marker gene (see materials and methods). mad2Δ denotes the mad2 allele disrupted by insertion of the ura4+ gene, the kanr marker was not used here. (A) TBZ sensitivity test. YE containing 20 μg/ml TBZ was used together with a control plate. Incubation was at 33° for 3 days. Strains used were HM123, HM713, YT547, YT549, and YT553. (B) Activity of mitotic arrest. Strains used were the same as in A. CBZ (50 μg/ml) was added to a logarithmic-phase culture and incubated for the indicated time periods. Percentage of cells containing hypercondensed chromosomes was measured. mad2+(#) (solid circles); mad2+ (open circles); mad2Δ (crosses); mad2-56 (open triangles); mad2-64 (open squares). More than 200 cells were scored for each strain. (C) Capability of different mad2 alleles to support the growth of gtb1 mutant. Incubation was at 33° or 30° for 3 days. Strains used were W604, YT578, YT555, YT556, and YT559. (D) Patterns of chromosome segregation in late anaphase. Strains with indicated mad2 alleles in the gtb1-93 background (same as in C) were incubated at 30° in YE for 1.5 hr after the treatment with HU and stained with DAPI. More than 170 mitoses were scored for each strain.
DISCUSSION
The defective mitotic phenotype in the γ-tubulin mutant was strikingly similar to that described in the klp5/klp6 mutant in several aspects (Garcia et al. 2002; West et al. 2002). First, both had longer metaphase spindles and lagging chromosomes until the middle of anaphase B. Second, sister-chromatid separation occurred at scattered sites on the metaphase spindle. Third, the spindle elongation rates in anaphase B as well as the chromosome segregation in late anaphase were normal or nearly normal. These properties might be explained by deregulated kMT dynamics or by generally slower shrinkage rates of kMTs. The Klp5/6 kinesins localize near the kinetochores and are implicated in the disassembly of the kMTs at the plus ends to generate the force for the bipolar attachment of sister kinetochores. The lack of Klp5/6 might give rise to the increased stability of kMTs and/or defects in the kinetochore–kMT interaction (Garcia et al. 2002; West et al. 2002; Sanchez-Perez et al. 2005). The gtb1-93 mutant contained apparently stabilized cytoplasmic MT bundles, which were very similar in shape to those in the klp5/6 mutant (Paluh et al. 2000; Tange et al. 2004); the cold sensitivity of the gtb1-93 mutant was enhanced in the presence of a TBZ-resistant β-tubulin (Yamamoto 1981; Y. Tange and O. Niwa, unpublished data); and defects in the gtb1-93 mutant were relieved under various MT-destabilizing conditions (Tange et al. 2004; this study). These findings suggest that the peculiar spindle dynamics in the γ-tubulin mutant, particularly the defective anaphase A, are due to stabilized MTs. Kinetochore MTs formed in the mutant might somehow resist disassembly of the plus-end MTs, which is required for the movement in anaphase A. Analogous γ-tubulin mutants in Aspergillus also have an anaphase A defect (Prigozhina et al. 2004; Li et al. 2005). It is not well understood how the altered form of γ-tubulin affects MT stability, although it is most likely at the plus end (see Zimmerman and Chang 2005). Despite the apparent similarity in spindle dynamics, the requirement of the klp5/6 mutant for SAC genes is different from that of the gtb1 mutant. The klp5/6 mutant is heavily dependent on Bub1, but less dependent on Mad2 and Mph1 (West et al. 2002). This fact suggests that underlying mechanistic defects that require SAC gene functions are different between these mutants. Further studies are needed to examine this issue.
We demonstrated that the quasi-normal chromosome segregation in the γ-tubulin mutant is heavily dependent on Mad2. Some observations that we made in this study might provide a clue to Mad2 dependency. The absence of Mad2 had only a subtle effect on the spindle dynamics in otherwise wild-type mitosis, but it had a profound effect in the γ-tubulin mutant: mitotic progression was accelerated and the formation of the prometaphase/metaphase spindle was almost abolished. The γ-tubulin mutant contains MTs that are more stable than those in wild type, which might enhance the bundling of MTs to form bipolar spindles and spindle elongation, whereas the kinetochore–kMT attachment as well as the regulation of kMT depolymerization at the kinetochore might be impaired, which would result in the lack of tension at the kinetochore or in the loss of a proper attachment of the kMTs to the kinetochore during metaphase/anaphase A. The presence of such affected kinetochores might be monitored by a checkpoint system that includes Mad2 that would allow time for the proper attachment of kMTs to the kinetochores. Such a monitoring system, if present, should not be identical with general SAC, because it did not require Bub3 and was not dependent on the Mad2 activity that is required for general SAC, as demonstrated in this study. Another possibility is that Mad2 has a direct role in chromosome segregation, perhaps for the attachment of kinetochores to kMTs. In this case, the requirement for the hypothesized activity of Mad2 in chromosome segregation would be much greater in the γ-tubulin mutant than in the wild type. Both of these possibilities are compatible with the observation that Mad2-GFP often remained on the metaphase/anaphase spindles as bright dots. It should be noted that Vanoosthuyse et al. (2004) reported a separation-of-function mutation in fission yeast bub1, in which the SAC activity is lost but that for chromosome stability is retained, analogous to the mad2 mutants isolated in this study.
In this study, we isolated novel mad2 alleles that were defective in general SAC but retained activity for gtb1-CS and for minichromosome stability in unperturbed mitosis. These alleles were in fact leaky mutants; that is, neither of them had completely lost the activity for general SAC, and they had slightly reduced activity for gtb1-CS and for minichromosome stability. Thus, as the first approximation, it might be that general SAC function requires a high level of Mad2 activity, while for the gtb1-CS and minichromosome stability, low activity of Mad2 is sufficient. The new mad2 mutants were not merely low-activity mutants, however, as they did not have additive activity when ectopically overexpressed; they robustly sequestered active Mad2 from general SAC function. Importantly, the same high level of expression of the mutants did not have harmful effects either on the growth of the gtb1 mutant or on minichromosome stability. Thus, we suggest that the new mad2 mutants were rather specifically impaired in the activity that is required for general SAC but not for gtb1-CS and minichromosome stability. Because an attempt to isolate mad2 mutants that are specifically defective in gtb1-CS was unsuccessful, it remains to be determined whether the activity required for gtb1-CS is also required for general SAC.
Both alleles have a missense mutation in the C-terminal segment of Mad2, which is implicated in the formation of a stable complex with Mad1 and with Slp1/Cdc20 (Luo et al. 2002; Musacchio and Hardwick 2002; Sironi et al. 2002; De Antoni et al. 2005). In this regard, it is interesting that the mad2-64 mutant has an additional mutation in the N-terminal region; neither of the two mutations induced a significant defect in general SAC function. The mutated amino acids in the mad2-64 mutant might cause a conformational change in the Mad2 protein, so that they affect the activation of Mad2. The results obtained in this study suggest that the mutant Mad2 proteins form a complex with Mad1 with altered stability. A stable Mad1/Mad2 complex is required for the activation of Mad2 in the formation of the Mad2/Cdc20 complex, although the precise mechanism involved in the activation is still a matter of debate (Yu 2006). It is possible that the mutant Mad2 protein forms an unstable complex with Mad1, which would cause inefficient production of the Mad2/Cdc20 complex. Further, because the same structural motif of Mad2 is used for the formation of the Mad2/Cdc20 complex, it is likely that the stability of Mad2/Cdc20(Slp1) is also affected in the new mad2 mutants. It will be interesting to examine if the Mad2/Slp1(Cdc20) complex formation is affected in the new mad2 mutant.
We fortuitously found that Mad1 has an essential function in the survival of the γ-tubulin mutant. Although further detailed investigation is required, a defective phenotype of the mad1Δ gtb1-93 double mutant was clearly related to a septation defect. This observation is reminiscent of the fact that fission yeast Mad1 was identified as a suppressor of a septation mutant (Kim et al. 2003). In addition, in the absence of Mad1, minichromosome instability is greatly increased, independent of Mad2. Our study (Y. Tange and O. Niwa, unpublished data) also showed that minichromosome stability is highly variable between different SAC gene mutants. Together, the findings of this study clearly show the need to elucidate the function of individual SAC genes for the fidelity of chromosome transmission during unperturbed mitosis.
Acknowledgments
We are grateful to T. Matsumoto for the anti-Mad1 serum and yeast strains. We also thank M. Yanagida, A. Yamamoto, and T. Toda for the yeast strains and plasmids and A. Kurabayashi for FISH analysis. This work was supported by the Kazusa DNA Research Institute and partly by a grant (no. 16370083) from the Japan Society for the Promotion of Science to O.N.
References
- Alfa, C., P. Fantes, J. Hyams, M. McLeod and E. Warbrick, 1993. Experiments With Fission Yeast: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Allshire, R. C., E. R. Nimmo, K. Ekwall, J.-P. Javerzat and G. Cranston, 1995. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9: 218–233. [DOI] [PubMed] [Google Scholar]
- Bahler, J., J.-Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie et al., 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951. [DOI] [PubMed] [Google Scholar]
- Bernard, P., K. Hardwick and J.-P. Javerzat, 1998. Fission yeast bub1 is a mitotic centromere protein essential for the spindle checkpoint and the preservation of correct ploidy through mitosis. J. Cell Biol. 143: 1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffin, E., C. Lefebvre, J. Huang, M. E. Gagou and R. E. Karess, 2005. Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex. Curr. Biol. 15: 856–861. [DOI] [PubMed] [Google Scholar]
- Chang, L., and K. L. Gould, 2000. Sid4p is required to localize components of the septation initiation pathway to the spindle pole body in fission yeast. Proc. Natl. Acad. Sci. USA 97: 5249–5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland, D. W., Y. Mao and K. F. Sullivan, 2003. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112: 407–421. [DOI] [PubMed] [Google Scholar]
- De Antoni, A., C. G. Pearson, D. Cimini, J. C. Canman, V. Sala et al., 2005. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr. Biol. 15: 214–225. [DOI] [PubMed] [Google Scholar]
- Decottignies, A., P. Zarzov and P. Nurse, 2001. In vivo localisation of fission yeast cyclin-dependent kinase cdc2p and cyclin B cdc13p during mitosis and meiosis. J. Cell Sci. 114: 2627–2640. [DOI] [PubMed] [Google Scholar]
- Garcia, M. A., N. Koonrugsa and T. Toda, 2002. Two kinesin-like Kin I family proteins in fission yeast regulate the establishment of metaphase and the onset of anaphase A. Curr. Biol. 12: 610–621. [DOI] [PubMed] [Google Scholar]
- Goto, B., K. Okazaki and O. Niwa, 2001. Cytoplasmic microtubular system implicated in de novo formation of a Rabl-like orientation of chromosomes in fission yeast. J. Cell Sci. 114: 2427–2435. [DOI] [PubMed] [Google Scholar]
- Hagan, I., and M. Yanagida, 1990. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature 347: 563–566. [DOI] [PubMed] [Google Scholar]
- Hagan, I., and M. Yanagida, 1992. Kinesin-related cut7 protein associates with mitotic and meiotic spindles in fission yeast. Nature 356: 74–76. [DOI] [PubMed] [Google Scholar]
- Hardwick, K. G., 2005. Checkpoint signaling: Mad2 conformers and signal propagation. Curr. Biol. 15: R122–R124. [DOI] [PubMed] [Google Scholar]
- He, X., T. E. Patterson and S. Sazer, 1997. The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc. Natl. Acad. Sci. USA 94: 7965–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X., M. H. Jones, M. Winey and S. Sazer, 1998. mph1, a member of the Mps1-like family of dual specificity protein kinases, is required for the spindle checkpoint in S. pombe. J. Cell Sci. 111: 1635–1647. [DOI] [PubMed] [Google Scholar]
- Hoyt, M. A., L. Totis and B. T. Roberts, 1991. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66: 507–517. [DOI] [PubMed] [Google Scholar]
- Ikui, A. E., K. Furuya, M. Yanagida and T. Matsumoto, 2002. Control of localization of a spindle checkpoint protein, Mad2, in fission yeast. J. Cell Sci. 115: 1603–1610. [DOI] [PubMed] [Google Scholar]
- Kerscher, O., L. B. Crotti and M. A. Basrai, 2003. Recognizing chromosomes in trouble: association of the spindle checkpoint protein Bub3p with altered kinetochores and a unique defective centromere. Mol. Cell. Biol. 23: 6406–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. H., D. P. Lin, S. Matsumoto, A. Kitazono and T. Matsumoto, 1998. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science 279: 1045–1047. [DOI] [PubMed] [Google Scholar]
- Kim, I. G., D. K. Rhee, J. W. Jeong, S. C. Kim, M. Won et al., 2003. Mad1p, a component of the spindle assembly checkpoint in fission yeast, suppresses a novel septation-defective mutant, sun1, in a cell-division cycle. FEMS Microbiol. Lett. 227: 183–188. [DOI] [PubMed] [Google Scholar]
- Kwon, M., and J. M. Scholey, 2004. Spindle mechanics and dynamics during mitosis in Drosophila. Trends Cell Biol. 14: 194–205. [DOI] [PubMed] [Google Scholar]
- Lawrence, C. J., R. K. Dawe, K. R. Christie, D. W. Cleveland, S. C. Dawson et al., 2004. A standardized kinesin nomenclature. J. Cell Biol. 167: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, D. J., and D. J. Burke, 2003. The spindle assembly and spindle position checkpoints. Annu. Rev. Genet. 37: 251–282. [DOI] [PubMed] [Google Scholar]
- Li, R., and A. W. Murray, 1991. Feedback control of mitosis in budding yeast. Cell 66: 519–531. [DOI] [PubMed] [Google Scholar]
- Li, S., C. E. Oakley, G. Chen, X. Han, B. R. Oakley et al., 2005. Cytoplasmic dynein's mitotic spindle pole localization requires a functional anaphase-promoting complex, γ-tubulin, and NUDF/LIS1 in Aspergillus nidulans. Mol. Biol. Cell 16: 3591–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiodice, I., J. Staub, T. G. Setty, N. P. Nguyen, A. Paoletti et al., 2005. Ase1p organizes antiparallel microtubule arrays during interphase and mitosis in fission yeast. Mol. Biol. Cell 16: 1756–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, X., Z. Tang, J. Rizo and H. Yu, 2002. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol. Cell 9: 59–71. [DOI] [PubMed] [Google Scholar]
- Mallavarapu, A., K. Sawin and T. Mitchison, 1999. A switch in microtubule dynamics at the onset of anaphase B in the mitotic spindle of Schizosaccharomyces pombe. Curr. Biol. 9: 1423–1426. [DOI] [PubMed] [Google Scholar]
- Masuda, H., R. Miyamoto, T. Haraguchi and Y. Hiraoka, 2006. The carboxy-terminus of Alp4 alters microtubule dynamics to induce oscillatory nuclear movement led by the spindle pole body in Schizosaccharomyces pombe. Genes Cells 11: 337–352. [DOI] [PubMed] [Google Scholar]
- Millband, D. N., and K.G. Hardwick, 2002. Fission yeast Mad3p is required for Mad2p to inhibit the anaphase-promoting complex and localizes to kinetochores in a Bub1p-, Bub3p-, and Mph1p-dependent manner. Mol. Cell. Biol. 22: 2728–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami, T., W. I. Chang, I. Garkavtsev, N. Kaplan, D. Lombardi et al., 1993. A 13 kb resolution cosmid map of the 14 Mb fission yeast genome by nonrandom sequence-tagged site mapping. Cell 73: 121–132. [DOI] [PubMed] [Google Scholar]
- Moreno, S., J. Hayles and P. Nurse, 1989. Regulation of p34cdc2 protein kinase during mitosis. Cell 58: 361–372. [DOI] [PubMed] [Google Scholar]
- Musacchio, A., and K. G. Hardwick, 2002. The spindle checkpoint: structural insights into dynamic signaling. Nat. Rev. Mol. Cell. Biol. 3: 731–741. [DOI] [PubMed] [Google Scholar]
- Nabeshima, K., T. Nakagawa, A. F. Straight, A. Murray, Y. Chikashige et al., 1998. Dynamics of centromeres during metaphase-anaphase transition in fission yeast: Dis1 is implicated in force balance in metaphase bipolar spindle. Mol. Biol. Cell 9: 3211–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaseko, Y., G. Goshima, J. Morishita and M. Yanagida, 2001. M phase-specific kinetochore proteins in fission yeast: microtubule-associating Dis1 and Mtc1 display rapid separation and segregation during anaphase. Curr. Biol. 11: 537–549. [DOI] [PubMed] [Google Scholar]
- Paluh, J. L., E. Nogales, B. R. Oakley, K. McDonald, A. L. Pidoux et al., 2000. A mutation in γ-tubulin alters microtubule dynamics and organization and is synthetically lethal with the kinesin-like protein pkl1p. Mol. Biol. Cell 11: 1225–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux, A. L., M. LeDizet and W. Z. Cande, 1996. Fission yeast pkl1 is a kinesin-related protein involved in mitotic spindle function. Mol. Biol. Cell 7: 1639–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky, B. A., and S. Biggins, 2005. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 15: 486–493. [DOI] [PubMed] [Google Scholar]
- Prigozhina, N. L., C. E. Oakley, A. M. Lewis, T. Nayak, S. A. Osmani et al., 2004. γ-Tubulin plays an essential role in the coordination of mitotic events. Mol. Biol. Cell 15: 1374–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C. L., R. W. Cole, A. Khodjakov and G. Sluder, 1995. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130: 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh, S., K. Takahashi and M. Yanagida, 1997. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell 90: 131–143. [DOI] [PubMed] [Google Scholar]
- Saitoh, S., K. Ishii, Y. Kobayashi and K. Takahashi, 2005. Spindle checkpoint signaling requires the mis6 kinetochore subcomplex, which interacts with mad2 and mitotic spindles. Mol. Biol. Cell 16: 3666–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Perez, I., S. J. Renwick, K. Crawley, I. Karig, V. Buck et al., 2005. The DASH complex and Klp5/Klp6 kinesin coordinate bipolar chromosome attachment in fission yeast. EMBO J. 24: 2931–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, M., S. Dhut and T. Toda, 2005. New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast 22: 583–591. [DOI] [PubMed] [Google Scholar]
- Shimanuki, M., F. Miki, D.-Q. Ding, Y. Chikashige, Y. Hiraoka et al., 1997. A novel fission yeast gene, kms1+, is required for the formation of meiotic prophase-specific nuclear architecture. Mol. Gen. Genet. 254: 238–249. [DOI] [PubMed] [Google Scholar]
- Sironi, L., M. Mapelli, S. Knapp, A. De Antoni, K. T. Jeang et al., 2002. Crystal structure of the tetrameric Mad1-Mad2 core complex: implications of a ‘safety belt’ binding mechanism for the spindle checkpoint. EMBO J. 21: 2496–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tange, Y., A. Fujita, T. Toda and O. Niwa, 2004. Functional dissection of the γ-tubulin complex by suppressor analysis of gtb1 and alp4 mutations in Schizosaccharomyces pombe. Genetics 167: 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxell, C. L., M. A. Sweezy, R. R. West, K. D. Reed, B. D. Carson et al., 2001. pkl1+ and klp2+: two kinesins of the Kar3 subfamily in fission yeast perform different functions in both mitosis and meiosis. Mol. Biol. Cell 12: 3476–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoosthuyse, V., R. Valsdottir, J.-P. Javerzat and K. G. Hardwick, 2004. Kinetochore targeting of fission yeast Mad and Bub proteins is essential for spindle checkpoint function but not for all chromosome segregation roles of Bub1p. Mol. Cell. Biol. 24: 9786–9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, E., and M. Winey, 1996. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 132: 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, R. R., T. Malmstrom and J. R. McIntosh, 2002. Kinesins klp5+ and klp6+ are required for normal chromosome movement in mitosis. J. Cell Sci. 115: 931–940. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M., 1981. Genetic analysis of resistant mutants to antimitotic benzimidazole compounds in Schizosaccharomyces pombe. Mol. Gen. Genet. 180: 231–234. [DOI] [PubMed] [Google Scholar]
- Yamamoto, A., and Y. Hiraoka, 2003. Monopolar spindle attachment of sister chromatids is ensured by two distinct mechanisms at the first meiotic division in fission yeast. EMBO J. 22: 2284–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, A., M. Sato, A. Fujita, M. Yamamoto and T. Toda, 2005. The roles of fission yeast ase1 in mitotic cell division, meiotic nuclear oscillation, and cytokinesis checkpoint signaling. Mol. Biol. Cell 16: 1378–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida, M., Y. M. Yamashita, H. Tatebe, K. Ishii, K. Kumada et al., 1999. Control of metaphase-anaphase progression by proteolysis: cyclosome function regulated by the protein kinase A pathway, ubiquitination and localization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354: 1559–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H., 2006. Structural activation of Mad2 in the mitotic spindle checkpoint: the two-state Mad2 model versus the Mad2 template model. J. Cell Biol. 173: 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, S., and F. Chang, 2005. Effects of γ-tubulin complex proteins on microtubule nucleation and catastrophe in fission yeast. Mol. Biol. Cell 16: 2719–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]