Abstract
Valproic acid (VPA) is widely used to treat epilepsy and manic-depressive illness. Although VPA has been reported to exert a variety of biochemical effects, the exact mechanisms underlying its therapeutic effects remain elusive. To gain further insights into the molecular mechanisms of VPA action, a genetic screen for fission yeast mutants that show hypersensitivity to VPA was performed. One of the genes that we identified was vps45+, which encodes a member of the Sec1/Munc18 family that is implicated in membrane trafficking. Notably, several mutations affecting membrane trafficking also resulted in hypersensitivity to VPA. These include ypt3+ and ryh1+, both encoding a Rab family protein, and apm1+, encoding the μ1 subunit of the adaptor protein complex AP-1. More importantly, VPA caused vacuolar fragmentation and inhibited the glycosylation and the secretion of acid phosphatase in wild-type cells, suggesting that VPA affects membrane trafficking. Interestingly, the cell-wall-damaging agents such as micafungin or the inhibition of calcineurin dramatically enhanced the sensitivity of wild-type cells to VPA. Consistently, VPA treatment of wild-type cells enhanced their sensitivity to the cell-wall-digesting enzymes. Altogether, our results suggest that VPA affects membrane trafficking, which leads to the enhanced sensitivity to cell-wall damage in fission yeast.
VALPROIC acid (VPA) is a short-chain fatty acid widely used in humans as an anticonvulsant drug to control certain types of seizures in the treatment of epilepsy (Johannessen and Johannessen 2003). It is also used to treat various psychiatric illnesses such as bipolar disorder (Johannessen and Johannessen 2003). There are several hypotheses to explain the anticonvulsant activity of VPA (Loscher 1999). VPA may act through more than one target, such as the induction of histone acetylation, DNA demethylation, and chromatin decondensation (Gottlicher et al. 2001; Phiel et al. 2001; Johannessen and Johannessen 2003; Kramer et al. 2003; Marchion et al. 2005). There have been reports showing that VPA increases the expression of genes regulated by the transcription factor, activator protein-1 (Chen et al. 1997). VPA also has an antiproliferative effect on cells and is known to induce the differentiation of cell lines derived from myeloid leukemia, teratocarcinoma, glioma, and neuroblastoma (Gurvich et al. 2004; Achachi et al. 2005). Despite the progress that has been made in elucidating the biological and biochemical action of VPA, the exact mechanisms underlying their therapeutic effects have not been fully established.
The fission yeast Schizosaccharomyces pombe and the budding yeast Saccharomyces cerevisiae have become valuable tools for the study of basic cellular functions of eukaryotic cells, including mechanisms of membrane trafficking and cell cycle control (Gould et al. 1990; Babst et al. 2000). Both are excellent organisms for the identification of molecular targets and for the elucidation of molecular/cellular mechanisms of sensitivity to various drugs, since major signaling pathways and processes involved in the cellular response to cytotoxic agents are conserved between yeasts and mammalian cells (Perego et al. 1998, 2000; Sugiura et al. 2002).
To better understand the molecular basis for the mechanisms of action of VPA, we performed in fission yeast a genetic screen for mutants that show hypersensitivity to VPA. Here, we identified vps45+ that encodes a member of the Sec1/Munc18 protein (Toonen and Verhage 2003). The budding-yeast homolog of Vps45 plays an essential role in membrane trafficking at the step of fusion of Golgi-derived vesicles with the prevacuolar compartment (Cowles et al. 1994; Bryant et al. 1998). Consistently, the vps45 mutants displayed defects in membrane trafficking.
Notably, in addition to the vps45 mutation, we showed that a mutation in apm1+ encoding the μ1 subunit of the adaptor protein complex AP-1 (Kita et al. 2004), as well as a mutation in ypt3+ encoding Rab GTPase involved in membrane trafficking from and to the Golgi (Cheng et al. 2002), affected the sensitivity of cells to VPA, thus suggesting that a disturbance in the membrane trafficking events resulted in increased sensitivity to VPA. More importantly, VPA treatment caused vacuolar fragmentation and inhibited the secretion and the glycosylation of acid phosphatase, suggesting that VPA affects membrane trafficking. We also present evidence that cell-wall damage, including the treatment with micafungin or glucanases and the inhibition of calcineurin that encodes the Ca2+/calmodulin-dependent protein phosphatase (Rusnak and Mertz 2000; Sugiura et al. 2002), dramatically enhanced the sensitivity of wild-type cells to VPA. To our knowledge, this is the first demonstration that VPA affects membrane trafficking, thereby causing cell-wall damage.
MATERIALS AND METHODS
Strains, media, and genetic and molecular biology methods:
The complete medium YPD (yeast extract–peptone–dextrose) and Edinburgh minimal medium (EMM) have been described previously (Toda et al. 1996). Standard genetic and recombinant-DNA methods (Moreno et al. 1991) were used except where noted. FK506 was provided by Fujisawa Pharmaceutical (Osaka, Japan).
Isolation of the vas1-1/vps45-v1 mutant and cloning of the vps45+ gene:
The vas1-1/vps45-v1 mutant was isolated in a screen of cells that had been mutagenized with nitrosoguanidine as described previously (Zhang et al. 2000). To clone the vps45+ gene, the vas1-1/vps45-v1 mutant (KP1370) was grown at 27° and transformed with an S. pombe genomic DNA library constructed in the vector pDB248 (Beach et al. 1982) (Table 1). Leu+ transformants were replica plated onto YPD plates containing VPA at 27° and the plasmid DNA was recovered from transformants that showed a plasmid-dependent rescue. These plasmids complemented the VPA sensitivity of the vas1-1/vps45-v1 mutant. By DNA sequencing, the suppressing plasmids were identified as containing the vps45+ gene (SPAC2G11.03c).
TABLE 1.
S. pombe strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| HM123 | h− leu1-32 | Our stock |
| KP162 | h− leu1-32 ypt3-i5 | Our stock |
| HM528 | h+ his2 | Our stock |
| KP630 | h− leu1-32 ura4-D18 apm1∷ ura4+ | Our stock |
| KP928 | h+ his2 leu1-32 ura4-D18 | Our stock |
| KP1248 | h− leu1-32 ura4-294 | Our stock |
| KP1370 | h− leu1-32 vps45-v1 | This study |
| KP1447 | h− leu1-32 ryh1-i6 | Our stock |
| KP1747 | h− leu1-32 ura4-D18 vps45∷ ura4+ | This study |
| KP2035 | h− leu1-32 ura4-294 nmt1GFP-syb1∷ura4+ | Our stock |
| KP2321 | h− leu1-32 ura4-294 nmt81 vps45-GFP∷ura4+ | This study |
| KP2322 | h− leu1-32 vps45∷ kanMX | This study |
| KP2589 | h− leu1-32 ura4-294 vps45∷ kanMX nmt1GFP-syb1∷ura4+ | This study |
To investigate the relationship between the cloned vps45+ gene and the vas1-1/vps45-v1 mutant, linkage analysis was performed as follows. The entire vps45+ gene was subcloned into the pUC-derived plasmid containing the S. cerevisiae LEU2 gene and was integrated by homologous recombination into the genome of the wild-type strain HM123. The integrant was mated with the vas1-1/vps45-v1 mutant. The resulting diploid was sporulated, and tetrads were dissected. A total of 30 tetrads were dissected. In all cases, only parental ditype tetrads were found, indicating allelism between the vps45+ gene and the vas1-1/vps45-v1 mutation (data not shown).
Tagging of the vps45+ gene:
The vps45+ gene was amplified by PCR with the genomic DNA of wild-type cells as a template. The sense primer was 5′-GAA GAT CTC ATG GAT TTA GTA TCA GCT TCC CAA TC-3′, and the antisense primer was 5′-GAA GAT CTG CGG CCG CCT TTT ATT CTG GTT GAC ATA TAC-3′. The amplified product containing the vps45+ gene was digested with BglII, and the resulting fragment was subcloned into BlueScriptSK (+) (Stratagene, La Jolla, CA).
For ectopic expression of proteins, we used the thiamine-repressible nmt1 promoter (Maundrell 1993). Expression was repressed by the addition of 4 μm thiamine to EMM. To express Vps45-green fluorescent protein (GFP), the complete open reading frame of vps45+ was amplified by PCR and ligated to the C terminus of the GFP carrying the S65T mutation. The pREP81 vector contained the attenuated version of the nmt1 promoter (Basi et al. 1993). To obtain the chromosome-borne Vps45-GFP, the fused gene was subcloned into the vector containing the ura4+ marker under the control of the nmt81 promoter and integrated into the chromosome at the ura4+ gene locus of KP1248 (h− leu1-32 ura4-294) as described (Cheng et al. 2002; Kita et al. 2004). The resultant strain (h− leu1-32 ura4-294 nmt81 vps45-GFP∷ura4+) did not show VPA sensitivity and FK506 sensitivity, and the expression of pREP81-Vps45-GFP complemented both the VPA sensitivity and the FK506 sensitivity of the Δvps45 cells (data not shown).
Deletion of the vps45+ gene:
A one-step gene disruption by homologous recombination was performed (Rothstein 1983). The vps45∷ura4+ disruption was constructed as follows. The BglII fragment containing the vps45+ gene was subcloned into the BamHI site of BlueScriptSK (+). Then, a BamHI fragment containing the ura4+ gene was inserted into the BamHI site of the previous construct. The fragment containing the disrupted vps45+ gene was transformed into diploid cells. Stable integrants were selected on medium lacking uracil. The disruption of the gene was checked by genomic Southern hybridization (data not shown).
Microscopy and miscellaneous methods:
Methods in light microscopy, such as fluorescence microscopy and differential interference contrast microscopy, were performed as described (Kita et al. 2004). FM4-64 labeling and conventional electron microscopy was performed as described (Kita et al. 2004). Acid phosphatase staining was performed as described previously (Maeda et al. 2004). Measurement of the acid phosphatase secretion was performed as described previously (Kita et al. 2004).
Valproic acid studies:
VPA (2-propyl-pentanoic acid) was purchased from Sigma (St. Louis), dissolved in distilled water at 1 m, and used at the final concentrations. VPA was added to plates subsequent to autoclaving and cooling of the medium to 55° or added to the liquid medium subsequently after autoclaving and cooling of the medium to the temperature as indicated in the legends of Figures 1, 4, and 6. For the growth of cells in the presence of VPA, a 20-ml culture of cells in logarithmic growth phase was treated with VPA at the concentrations.
Pulse-chase analysis and immunoblot analysis of the S.pombe Cpy1 protein:
Pulse-chase analysis and immunoprecipitation of the vacuolar carboxypeptidase Y (CPY) were carried out as previously described (Tabuchi et al. 1997).
RESULTS
Isolation of vas1-1 as a VPA-sensitive mutant:
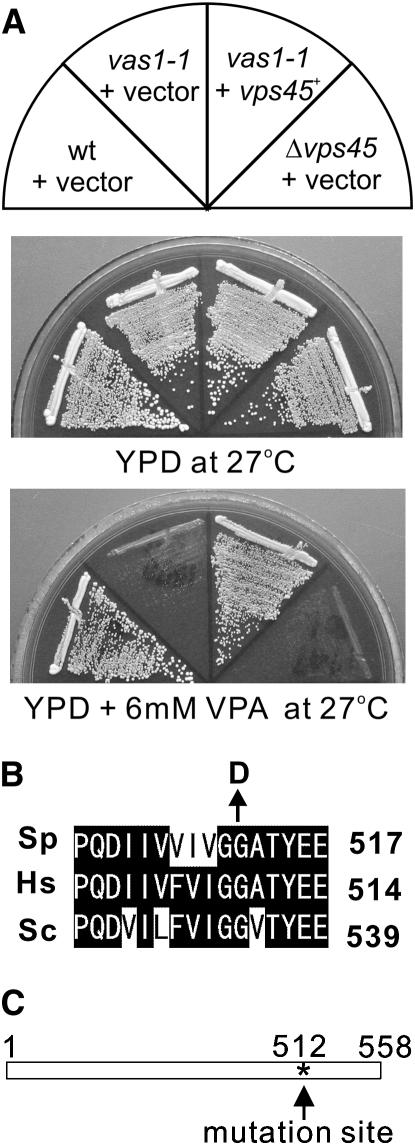
To identify genes that determine the sensitivity to VPA, we have developed a genetic screen for mutants that are hypersensitive to VPA and have identified six complementation groups (vas1–6 for valproic acid-sensitive). Here, we will describe vas1-1. As shown in Figure 1A, vas1-1 mutants grew equally well as compared with the wild-type cells at 27°. However, vas1-1 mutant cells did not grow on a YPD plate containing 6 mm VPA at 27°, whereas wild-type cells grew normally (Figure 1A).
Figure 1.—
Mutation in the vas1+/vps45+ gene causes VPA-sensitive phenotype. (A) The VPA sensitivities of the vas1-v1/vps45-v1 mutant cells. Cells transformed with the multicopy vector pDB248 or the vector containing the vps45+ gene were streaked onto each plate containing YPD or YPD plus 6 mm VPA and then incubated for 4 days at 27°. (B) Alignment of partial protein sequences of S. pombe (Sp) Vps45 with related proteins from human (Hs) and S. cerevisiae (Sc). Sequence alignment was performed using the Clustal W program. Solid background indicates identical amino acids. Arrow indicates the highly conserved glycine 512, which, when mutated to aspartic acid, resulted in VPA-sensitive function in Vps45. (C) Linear representation of the structure of Vps45 and where the vas1-v1 mutation resides. Asterisk indicates the mutation site.
The vas1-1 is an allele of the vps45+ gene that encodes a member of the Sec1/Munc18 family:
The vas1+ gene was cloned by complementation of the VPA-sensitive growth defect of the vas1-1 mutant (Figure 1A, YPD at 27°+VPA, +vps45+). Nucleotide sequencing of the cloned DNA fragment revealed that the vas1+ gene is identical to the vps45+ gene (SPAC2G11.03c), which encodes a protein of 558 amino acids that is highly similar to the human hVps45 (42.42% identity) (Pevsner et al. 1996) and S. cerevisiae Vps45p (39.08% identity) (Cowles et al. 1994; Pevsner et al. 1996) (Figure 1B). Linkage analysis was performed (see materials and methods) and results indicated the allelism between the vps45+ gene and the vas1-1 mutation. We therefore renamed vas1-1 as vps45-v1. The vps45 deletion cells were viable, but the Δvps45 cells also showed VPA sensitivity, similar to that of vps45-v1 cells (Figure 1A, Δvps45+ vector).
To identify the mutation site in the vps45-v1 allele, the genomic DNA from the vps45-v1 mutant was isolated, and the full-length coding region of the vps45-v1 gene was sequenced. The G-to-A nucleotide substitution caused a highly conserved glycine to be altered to an aspartic acid residue at the amino acid position 512 that lies in the domain of unknown function (Figure 1, B and C, solid arrow).
Vps45 is involved in Golgi-to-vacuole protein transport in fission yeast:
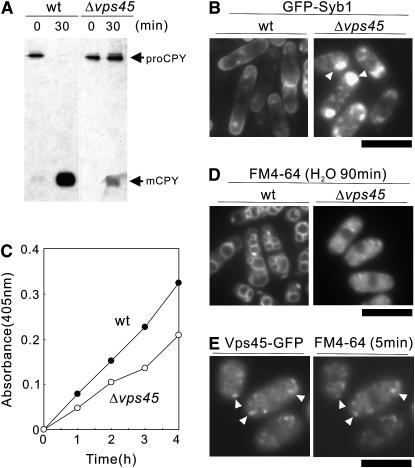
In budding yeast, the vacuolar protein-sorting (VPS) pathway mediates the localization of proteins from the trans-Golgi to the vacuole via a prevacuolar endosome compartment. Mutations in the vps genes affected the vesicle-mediated Golgi-to-vacuole protein transport and resulted in the secretion of vacuolar proteins (Bankaitis et al. 1986; Rothman and Stevens 1986; Robinson et al. 1988). To investigate whether Vps45 is required for VPS, pulse-chase analysis of CPY was performed. During a 15-min pulse incubation of wild-type cells with Express 35S-label, an immunoreactive band with an apparent molecular mass of 110 kDa (proCPY) was detected. After 30 min of chase, the molecular mass of this 110-kDa form was converted to the 32-kDa form (mature form of CPY) in wild-type cells (Figure 2A, wt). In Δvps45 cells, the maturation of CPY was severely impaired, indicating that Δvps45 cells missort CPY (Figure 2A, Δvps45). These results are consistent with the previous reports in budding yeast that the budding yeast Vps45 is required for trafficking to the vacuole (Piper et al. 1994) and suggest that S. pombe Vps45 is the functional ortholog of S. cerevisiae Vps45p.
Figure 2.—
Defects in membrane trafficking in the Δvps45 mutant cells. (A) Processing of CPY in vivo. Wild-type strain and Δvps45 mutant cells were pulse labeled with Express-35S-label for 10 min at 27° and chased. The immunoprecipitates were separated on an SDS–10% polyacrylamide gel. The autoradiograms of the fixed dried gels are shown. proCPY, precursor form of CPY; mCPY, mature form of CPY. (B) Intracellular localization of GFP-Syb1 in Δvps45 mutant cells. Wild-type strain and Δvps45 mutant cells expressing chromosome-borne GFP-Syb1 were cultured in YPD medium at 27°. The localization of GFP-Syb1 was examined by fluorescence microscopy. Bar, 10 μm. (C) Defective secretion of acid phosphatase in Δvps45 cells. Wild-type strain and Δvps45 cells were grown to an optical density at 660 nm of 0.3 and assayed for secreted acid phosphatase activity. The data shown are representative of multiple experiments. (D) Δvps45 mutant cells are defective in vacuole fusion. Wild-type (wt) strain and Δvps45 mutant cells were grown in YPD medium at 27°. Cells were collected, labeled with FM 4-64 fluorescent dye (see materials and methods), resuspended in water, and examined by fluorescence microscope. Photographs were taken after 90 min. Bar, 10 μm. (E) Vps45-GFP is concentrated at the Golgi/endosomal compartments. Wild-type cells, expressing chromosome-borne Vps45-GFP cultured in EMM in the absence of thiamine for 12 hr, were incubated with the FM4-64 dye for 5 min to visualize early endosomes. Arrowheads indicate the dot-like structures of Vps45-GFP and early endosomes stained with FM4-64, respectively. Bar, 10 μm.
We next monitored the localization of GFP-Syb1, the synaptobrevin in fission yeast, as a GFP-fusion protein (Edamatsu and Toyoshima 2003; Kita et al. 2004). Synaptobrevin is a vesicle-associated membrane protein and copurifies with secretory vesicles. As a secretory vesicle SNARE, Syb1 would be expected to cycle between the cell surface and the endocytic pathway. To assess the Golgi-to-endosome- or Golgi-to-plasma-membrane trafficking pathway, the localization of GFP-Syb1 was monitored. The fluorescence of GFP-Syb1 in wild-type cells was detected as punctate structures in the cytoplasm and was enriched in the medial region and cell ends (Figure 2B, wt). In clear contrast, in Δvps45 cells, GFP-Syb1 failed to localize to the cell surface and the medial regions. Instead, they were observed as large dots with bright fluorescence in the cytoplasm at 27° (Figure 2B, Δvps45, arrowheads). Thus, Δvps45 cells displayed a defective localization of GFP-Syb1, indicating that Vps45 is involved in Golgi-to-endosome membrane trafficking.
The Δvps45 cells also showed a defective secretion of acid phosphatase, a protein that follows the classical secretory pathway from the endoplasmic reticulum to the extracellular periplasmic space (see materials and methods). The Δvps45 cells secreted a lesser amount of acid phosphatase than the wild-type cells at 27° (Figure 2C), indicating that Δvps45 cells are defective in secretion.
We also examined the vacuolar fusion induced by osmotic stress in wild-type strain and Δvps45 cells labeled with FM4-64, a vacuole-specific vital dye (Vida and Emr 1995). Hypotonic stress causes a dramatic fusion of vacuoles in S. pombe (Bone et al. 1998). When cells were collected, washed, and resuspended in water for 90 min, the wild-type cells had evidently large vacuoles that resulted from vacuole fusion (Figure 2D, wt). In contrast, vacuoles remained small and numerous in Δvps45 cells suspended in water, indicating that Vps45 is required for vacuole fusion (Figure 2D, Δvps45).
Altogether, these phenotypes involved in membrane trafficking suggest that Vps45 is implicated in Golgi-to-endosome or Golgi-to-vacuole membrane trafficking, as well as in the secretory pathway in fission yeast.
Moreover, the chromosome-borne Vps45-GFP localized at the dot-like structures within the cytosol (Figure 2E). So, we examined whether these fluorescent dots of Vps45-GFP colocalized with the endocytic tracer dye FM4-64 at an early stage of endocytosis. After 5 min of dye uptake, most of the FM4-64-positive structures showed colocalization with Vps45-GFP structures (Figure 2E), strongly suggesting that the structures showing the localization of Vps45-GFP represent Golgi/endosome compartments, consistent with the role of Vps45 in membrane trafficking.
The Δvps45 cells displayed an accumulation of abnormal vesicular structures:
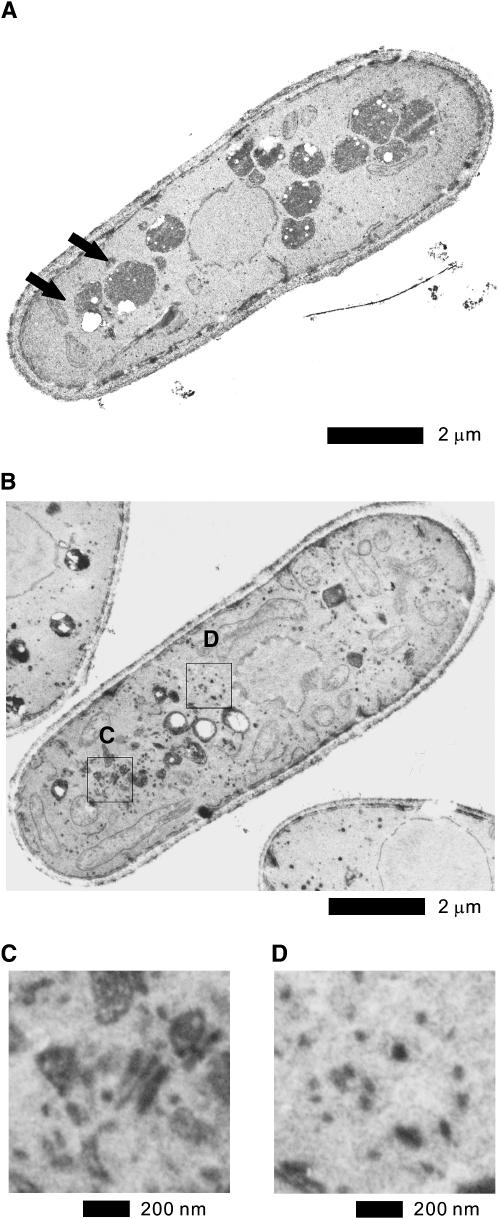
In general, electron microscopic analysis has shown that mutants defective in membrane trafficking accumulate an organelle or a vesicular intermediate of the secretory compartments that precede the step in which they first function (Novick et al. 1981; Kaiser and Schekman 1990; Cheng et al. 2002; Kita et al. 2004). To determine whether Δvps45 cells accumulate such structures, cells were examined by electron microscopy.
In Δvps45 mutant cells, Golgi structures were thick and swollen (Figure 3, B and C) while in wild-type cells these abnormalities were rarely seen (Figure 3A). Another striking feature of the Δvps45 cells was the accumulation of large numbers of vesicles. In Δvps45 cells, as shown in Figure 3, B and D, there is an accumulation of large vesicles (100–150 nm in diameter) and smaller vesicles (50–80 nm in diameter), suggesting that Vps45 is required for vesicle docking and/or fusion and that Vps45 participates in multiple steps of membrane trafficking. These vesicles seem to be dispersed throughout the cytoplasm and are not clustered. The large vesicles were 100–150 nm in diameter, falling within the range of post-Golgi secretory vesicles and the smaller vesicles were 50–80 nm in diameter, suggesting the involvement of Vps45 in ER-to-Golgi trafficking. In wild-type cells, however, these structures were negligible (Figure 3A). Another morphological consequence of depleting Vps45 is a fragmentation of the vacuole (Figure 3B), which is consistent with the above results obtained by FM4-64 that Δvps45 is defective in vacuolar fusion induced by hypo-osmotic stress (Figure 2D). Collectively, the findings observed in the electron micrographs suggest a role for Vps45 in the traffic between the Golgi-to-vacuole transport and/or in the secretory pathway in fission yeast.
Figure 3.—
Electron microscopic analysis of Δvps45 mutant cells and the effect of VPA treatment on wild-type cells. (A) The wild-type cells and (B) the Δvps45 mutant cells grown at 27° were analyzed by electron microscopy. Representative thin sections are shown. Arrows in A indicate vacuoles in wild-type cells. The boxed regions C and D in B are enlarged. Bar, 2 μm. (C) The enlargement of Golgi structures of Δvps45 mutant cells. Bar, 200 nm. (D) The enlargement of the accumulated vesicle structures in Δvps45 mutant cells. Bar, 200 nm.
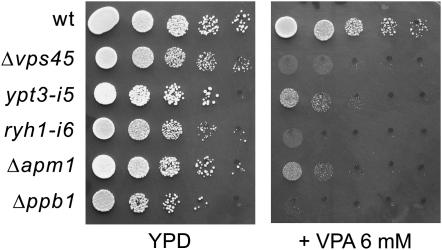
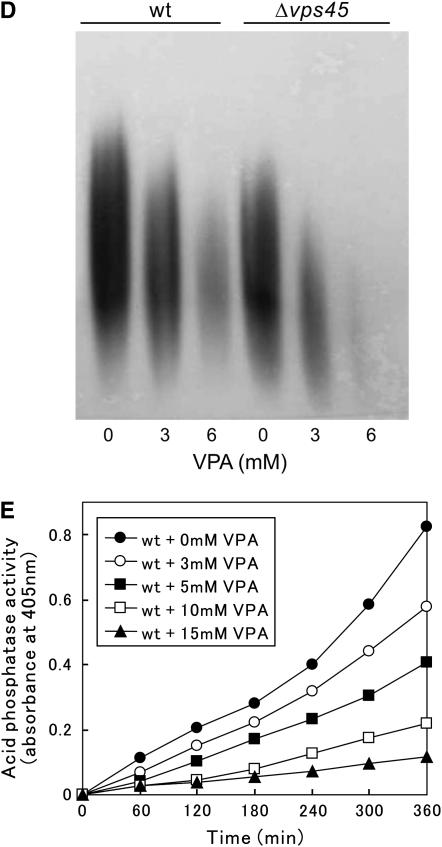
Various membrane trafficking mutants displayed hypersensitivity to VPA:
The identification of vps45+, which is implicated in membrane trafficking, as a VPA-sensitive mutant prompted us to examine whether other membrane trafficking mutants also show VPA hypersensitivity. Intriguingly, our previous genetic screen using the immunosuppressant drug FK506 has identified alleles of membrane trafficking genes, including ypt3+/its5+ (Cheng et al. 2002), ryh1+ (He et al. 2006) encoding a Rab small GTPase, and apm1+ (Kita et al. 2004) encoding a μ1 subunit of the AP-1 clathrin adaptor complex, respectively. We then examined the VPA sensitivity of these trafficking mutants. Notably, the growth of all the mutants examined was remarkably inhibited by the addition of 6 mm VPA to the medium, while the wild-type cells grew normally (Figure 4). In addition, Δppb1 cells, the calcineurin deletion in fission yeast, also displayed a severe growth defect in the presence of 6 mm VPA (Figure 4).
Figure 4.—
The Δvps45 mutant cells and various membrane trafficking mutants displayed hypersensitivity to VPA. Wild-type cells and the various mutant cells were spotted onto each plate containing YPD or YPD plus 6 mm VPA and then incubated for 3 days at 27°. Cells were spotted in serial 10-fold dilutions starting with OD660 = 0.3 of log-phase cells (5 μl).
VPA affects membrane trafficking:
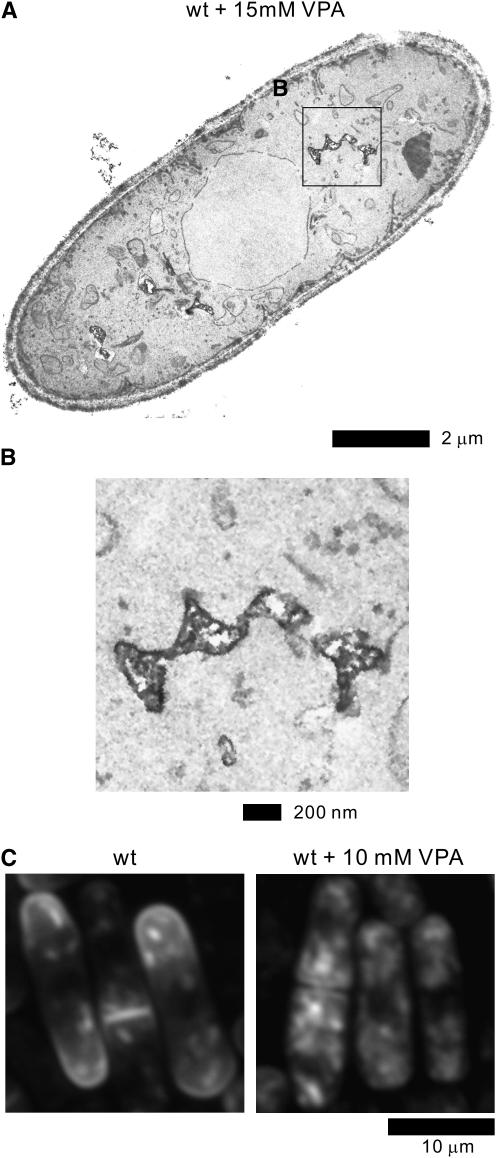
The above results suggested the possibility that the mechanisms of VPA action might be functionally related to membrane trafficking. To address this hypothesis, we first examined the effect of VPA on cell morphology by electron microscopic analysis. Notably, the wild-type cells treated with 15 mm VPA displayed highly fragmented vacuoles (Figure 5, A and B), in clear contrast to the large vacuoles observed in wild-type cells cultured in the absence of VPA (Figure 3A, arrows).
Figure 5.—
Effect of VPA on membrane trafficking of wild-type cells. (A) Electron microscopy of wild-type cells treated with 15 mm VPA for 12 hr. The boxed region B in A is enlarged. Bar, 2 μm. (B) The enlargement of the fragmented vacuoles in wild-type cells treated with VPA. Bar, 200 nm. (C) Effect of VPA on the intracellular localization of GFP-Syb1 in wild-type cells. Wild-type strain expressing the chromosome-borne GFP-Syb1 was cultured in YPD medium and treated with 10 mm VPA for 10 hr at 27°. The localization of GFP-Syb1 was examined by fluorescence microscopy. Bar, 10 μm. (D) VPA-induced defects in glycosylation. Acid phosphatase glycosylation in the wild-type strain and the Δvps45 mutant cells. Effects of the addition of 3 or 6 mm VPA to the medium on these cellular processes were also examined. Immunoblot analysis and acid phosphatase staining were performed as described in materials and methods. (E) VPA treatment caused the defective secretion of acid phosphatase in wild-type cells. Wild-type strains treated with indicated concentrations of VPA were grown to an optical density at 660 nm of 0.3 and assayed for the secreted acid phosphatase activity as indicated. The data presented are representative of three independent experiments.
We next examined the effect of VPA on the intracellular localization of GFP-Syb1 in wild-type cells. As shown in Figure 5B in wild-type cells cultured in the presence of 10 mm VPA, the fluorescence of GFP-Syb1 was no longer visible at cell ends (Figure 5C). Instead, GFP-Syb1 was diffusely localized in the cytosol (Figure 5C), indicating that VPA treatment caused defects in the intracellular localization of GFP-Syb1.
We further examined the effects of VPA on the glycosylation of acid phosphatase (Maeda et al. 2004). On native gels, when 3 mm of VPA was added to the medium, acid phosphatase isolated from wild-type cells showed a band with a significantly lower molecular mass, and 6 mm of VPA had a dramatic impact on the mobility (Figure 5D). Acid phosphatase isolated from Δvps45 mutants migrated significantly faster than that from the wild-type strain even in the absence of VPA (Figure 5D), suggesting the impairment of protein glycosylation in Δvps45 mutants. VPA treatment also affected the mobility of acid phosphatase from the Δvps45 mutants and the effect was more marked as compared with that observed in wild-type cells (Figure 5D). Thus, the glycosylation of acid phosphatase is impaired upon VPA treatment.
Finally, we investigated the effect of VPA on secretion. For this, the secretion of acid phosphatase of wild-type cells was measured with or without the addition of various concentrations of VPA to the medium. The results clearly demonstrated that VPA inhibited the secretion of acid phosphatase in a dose-dependent manner (Figure 5E). The addition of VPA did not interfere with the activity of acid phosphatase in vitro (data not shown), indicating that VPA impaired the secretion of acid phosphatase in vivo. Together, these results suggest that VPA affects membrane trafficking in fission yeast.
Defects in cell-wall integrity lead to VPA hypersensitivity:
Since all the trafficking mutants that we identified were isolated in a screen for mutants that show sensitivity to FK506 (Cheng et al. 2002; Kita et al. 2004; He et al. 2006), we then hypothesized that Δvps45 mutants might also show FK506 sensitivity. Expectedly, the growth of Δvps45 mutants was significantly inhibited upon FK506 treatment, similar to that observed in other membrane trafficking mutants (Figure 6A, +FK506). As FK506 is a specific inhibitor of calcineurin phosphatase in both mammals and fission yeast (Liu et al. 1991; Sugiura et al. 1998), it is of interest to examine the VPA sensitivity of calcineurin deletion. As described above, Δppb1 cells, the calcineurin deletion in fission yeast, also displayed a severe growth defect in the presence of 6 mm VPA (Figure 4), thus indicating that loss of calcineurin function caused the VPA sensitivity in fission yeast. This further suggests the importance of calcineurin in the mechanisms of VPA hypersensitivity. As calcineurin regulates 1,3-β-d-glucan synthesis and FK506 treatment inhibits the glucan synthesis in budding yeast (Zhao et al. 1998), we hypothesized that the inhibition of calcineurin induced cell-wall integrity defects, which lead to hypersensitivity to VPA.
Figure 6.—
The synergistic effect of VPA and cell-wall damage in fission yeast. (A) The Δvps45 mutant cells and various membrane trafficking mutants displayed sensitivity to the cell-wall-damaging agent micafungin and the immunosuppressant FK506. Wild-type cells and the various mutant cells as indicated were spotted onto each plate containing YPD plus 1 μg/ml micafungin or YPD plus 0.5 μg/ml FK506 and then incubated for 3 days at 27°. Cells were spotted in serial 10-fold dilutions starting with OD660 = 0.3 of log-phase cells (5 μl). (B) The temperature sensitivities of the vps45 mutants. Wild-type cells, vas1-1 cells, or Δvps45 cells were streaked onto each plate containing YPD or YPD plus 1.2 m sorbitol and then incubated for 3 days at 36°. (C) Synergism between VPA and cell-wall perturbation. Wild-type cells were spotted onto the plates with or without 5 mm VPA, 0.5 μg/ml FK506, or 1.0 μg/ml micafungin, individually or in combination, and then incubated for 4 days at 27°.
Intriguingly, all the membrane trafficking mutants that we have thus far identified displayed defects in cell-wall integrity, including the hypersensitivity to micafungin, an inhibitor of 1,3-β-d-glucan synthase (Carver 2004; Deng et al. 2005). We then examined the effect of micafungin on the Δvps45 cells as compared with other membrane trafficking mutants. Expectedly, micafungin markedly inhibited the growth of Δvps45 cells as well as ypt3-i5 cells and Δapm1 mutant cells as compared with those of wild-type cells (Figure 6A, left). Moreover, Δppb1 cells also displayed a severe sensitivity to micafungin similar to those of the membrane trafficking mutants (Figure 6A, left). Also, the septation index of Δvps45 cells is modestly higher (20 ± 3%, average ±SD of five independent experiments) than that of wild-type cells (15 ± 2%, average ±SD of five independent experiments), and Δvps45 cells displayed osmoremedial temperature sensitivity (Figure 6B), the characteristic phenotypes associated with cell-wall defective mutants. Thus, Δvps45 cells, like other trafficking mutants, appeared to be defective in cell-wall integrity.
Together, these observations suggest that defects in cell-wall integrity induced by these membrane trafficking mutants lead to hypersensitivity to VPA and micafungin as well as to FK506.
We next investigated whether the VPA sensitivity is enhanced upon cell-wall damage. We first tested the effect of a low concentration of VPA (5 mm) on cell growth in the presence or absence of micafungin (1 μg/ml). Neither drug alone had a significant effect on the cell growth of wild-type cells, whereas the synergistic effect of the two drugs was seen when VPA (5 mm) was added simultaneously with micafungin (1 μg/ml) (Figure 6C). As shown earlier, calcineurin deletion displayed severe sensitivity to both VPA and micafungin, and to confirm this, we examined the effect of calcineurin inhibition using FK506, a specific inhibitor of calcineurin activity. Consistently, FK506 treatment enhanced the sensitivity to VPA and micafungin, indicating the synergism between FK506 treatment and these drugs.
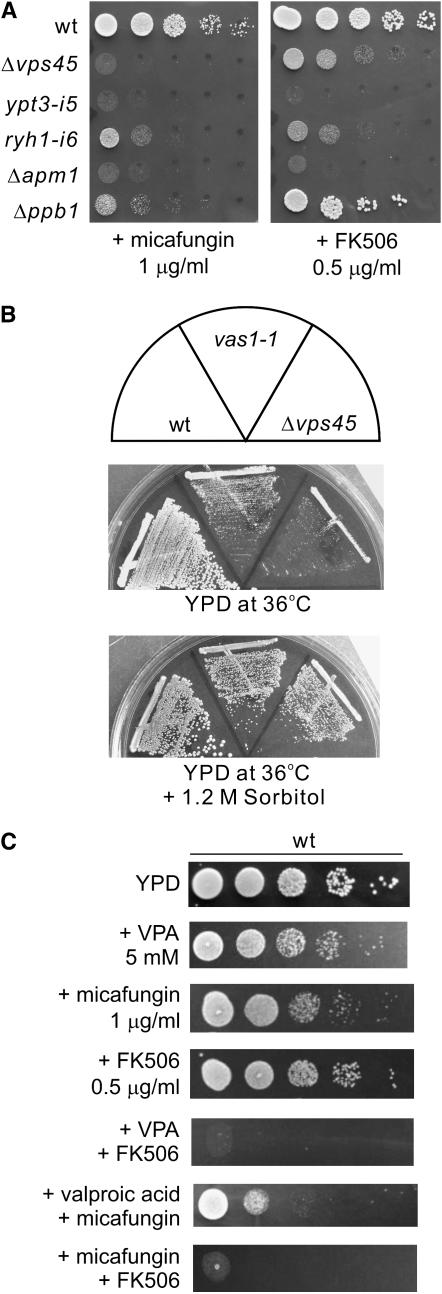
VPA stimulates calcineurin signaling:
We have recently demonstrated that cell-wall damage by micafungin treatment caused Ca2+ influx (Deng et al. 2006), thereby resulting in the activation of calcineurin followed by the nuclear translocation of Prz1, a calcineurin-dependent transcription factor (Hirayama et al. 2003). We hypothesized that VPA treatment would have a similar effect on Prz1 localization. We then examined the effects of VPA on the nuclear translocation of GFP-Prz1. Notably, the addition of VPA to the medium dramatically induced the translocation of GFP-Prz1 from the cytosol to the nucleus (Figure 7A). Upon treatment with 5 mm VPA, the translocation of GFP-Prz1 to the nucleus was significantly induced, and >60% of the cells displayed nuclear localization of Prz1 upon the addition of 10 mm VPA to the medium (Figure 7B).
Figure 7.—
VPA activates calcineurin signaling. (A) VPA and glucanase treatment stimulates translocation of GFP-Prz1 from the cytosol to the nucleus. The cellular localization of Prz1 was examined by immunofluorescence microscopy in wild-type cells carrying the chromosomally tagged GFP-Prz1 with or without the addition of 10 mm VPA for 6 hr or with the addition of 100 μg/ml of zymolyase 20T for 3 hr. Bar, 10 μm. (B) Quantification of the nuclear accumulation of GFP-Prz1 in wild-type cells with or without the addition of various concentrations of VPA for 6 hr or with the addition of 100 μg/ml of zymolyase for 3 hr to the medium. Data represent the average of four experiments. (C) Effect of VPA on cell-wall digestion by β-glucanase. Wild-type cells exponentially growing in YPD medium were treated with the indicated concentrations of VPA for 8 hr and incubated with 100 μg/ml of β-glucanase (zymolyase 20T) at 30° with vigorous shaking. Cell lysis was monitored by measuring optical density at 660 nm (the value before adding the enzyme was taken as 100%). The data shown are representative of multiple experiments.
We also examined the effect of glucanase (zymolyase 20T), a cell-wall-digesting enzyme, on the nuclear localization of GFP-Prz1. The results clearly showed that 100 μg/ml zymolyase 20T, the concentration used to digest cell wall, also stimulated the nuclear translocation of GFP-Prz1 (Figure 7A), although the frequency of nuclear accumulation was lower (45%) than that obtained with 10 mm VPA treatment (Figure 7B). Together, the nuclear accumulation of GFP-Prz1 by VPA treatment suggests the impairment of cell-wall integrity by VPA via its effect on membrane trafficking. Consistently, in the trafficking mutants that showed VPA sensitivity, including Δvps45 and Δapm1, the percentage of GFP-Prz1 located in the nucleus dramatically increased even without the addition of these drugs (data not shown).
We next examined whether VPA treatment can alter the resistance of wild-type cells to the cell-wall damage. For this, we used the glucanase sensitivity as a direct quantitative readout of cell-wall modifications. Wild-type cells were assayed for zymolyase sensitivity with or without the addition of VPA. The results clearly showed that VPA treatment enhanced the sensitivity of wild-type cells to zymolyase in a dose-dependent manner, suggesting that VPA treatment resulted in cell-wall alterations (Figure 7C).
DISCUSSION
Here, we have identified the mutations that exhibit VPA sensitivity and discovered a novel genetic interaction between VPA sensitivity and genes involved in membrane trafficking in fission yeast.
In our study, first, the vps45 mutants were identified in a screen to isolate VPA-sensitive mutants and it was found that the vps45 mutants displayed defects in membrane trafficking. Second, various membrane trafficking mutants and the Δvps45 mutants have several shared phenotypes, including sensitivity to the cell-wall-damaging agent micafungin, to the calcineurin inhibitor FK506, and to VPA. Third, VPA treatment affects membrane trafficking. Fourth, VPA sensitivity is enhanced upon cell-wall damage, including treatment with micafungin or FK506. Finally, similar to the cell-wall-damaging agents, VPA induced calcineurin activation as evidenced by the nuclear translocation of GFP-Prz1. Altogether, these results suggest that VPA primarily affects membrane trafficking, which leads to the enhanced sensitivity to cell-wall damage and the hyperactivation of calcineurin signaling in fission yeast.
It should be noted that among the membrane trafficking mutants, the Δvps45 mutants and ryh1-i6 mutants displayed similar sensitivities to various cell-wall-damaging agents examined. Both mutants showed more severe sensitivity to VPA as compared with that of the Δapm1 mutants and ypt3-i5 mutants (Figure 4). In contrast, the growth of the Δvps45 mutants and ryh1-i6 mutants was relatively weakly inhibited upon FK506 treatment compared with that of the Δapm1 mutants and ypt3-i5 mutants (Figure 6A).
These differences in sensitivity to the drugs can be explained by the roles of these two proteins in membrane trafficking, as both Ryh1 and Vps45 are functionally closely involved in Golgi-to-endosome transport, and the defects in secretion in both mutants were relatively modest compared with those of ypt3-i5 mutants and Δapm1 mutants as judged by the severity of the accumulation of the secretory vesicles (Kita et al. 2004).
Unexpectedly, Δvps45 mutants, unlike other membrane trafficking mutants, displayed similar sensitivity to glucanases as compared with that of wild-type cells (data not shown), although Δvps45 mutants showed the osmoremedial temperature sensitivity, a phenotype characteristic to cell-wall-defective mutants. We also monitored the cell-wall architecture or septum of Δvps45 cells by electron microscopy. However, these structures in Δvps45 cells were not discernible from those of wild-type cells (data not shown). Therefore, the sensitivity of Δvps45 mutants to VPA can be interpreted as the consequence of the synergistic inhibition of membrane trafficking by the vps45 mutation and the drug. Consistently, upon VPA treatment, the inhibition of the glycosylation was more severely manifested in the Δvps45 mutants compared with that of wild-type cells (Figure 5D).
In the same line, we demonstrated synergistic inhibition of cell growth by VPA and micafungin, FK506 showing that VPA sensitivity is increased by the presence of cell-wall-damaging agents. We reasoned that the synergistic effect is most probably the consequence of inhibition of cell-wall synthesis by micafungin combined with a more general defect produced by secretory defects induced by VPA and/or calcineurin inhibition.
Alternatively, the cell-wall defects can also be a consequence of calcineurin misregulation in the vps45 mutants, as a relationship between calcium and cell-wall synthesis has been shown (Carnero et al. 2000; Cortes et al. 2004). Consistent with this hypothesis, the membrane trafficking mutants, including the vps45 mutant, displayed a higher frequency of the nuclear accumulation of GFP-Prz1, indicating that calcineurin activation is induced in these mutants. This hyperactivation of the calcineurin signaling in the trafficking mutants may be due to defects in cell-wall integrity that are caused by impaired secretion.
Another important feature of our study is the demonstration that VPA affects glycosylation in fission yeast. It has been described that defects in glycosylation caused several drug sensitivities, including hygromycin B, an aminoglycoside (Dean 1995). It is possible that defects in glycosylation might cause alteration in the cell-wall glycoproteins, and as a consequence, a defective cell wall alters the sensitivity to VPA (Cortes et al. 2004; Bates et al. 2005). Thus, the sensitivity of the vas1/vps45 mutant to micafungin or FK506 might be an indirect effect of the secretion and glycosylation defects in the vas1/vps45 mutant.
Recent progress in glycobiology has highlighted the importance of glycosylation in many key biological processes, such as molecular trafficking, signal transduction, and cell adhesion, and an increasing number of diseases of glycosylation are being discovered, including congenital disorders (Ohtsubo and Marth 2006). Thus, if VPA affects glycosylation in higher eukaryotes as well, VPA-induced alteration in glycosylation might explain the various side effects of VPA.
In summary, this study demonstrates for the first time that VPA affects membrane trafficking, thereby inducing calcineurin hyperactivation and cell-wall damage. Also, the genetic screen of VPA-sensitive mutants in fission yeast may contribute to the further identification of the new components involved in the membrane trafficking pathway, as well as to the understanding of the molecular basis of VPA action.
Acknowledgments
We thank Takashi Toda, Mitsuhiro Yanagida, Chikashi Shimoda, and the National Bio-Resource Project for providing strains and plasmids; Susie O. Sio for critical reading of the manuscript; and Fujisawa JAPAN for gifts of FK506. This work was supported by the 21st Century Center of Excellence Program, the Asahi Glass Foundation, the Uehara Memorial Foundation, and research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Achachi, A., A. Florins, N. Gillet, C. Debacq, P. Urbain et al., 2005. Valproate activates bovine leukemia virus gene expression, triggers apoptosis, and induces leukemia/lymphoma regression in vivo. Proc. Natl. Acad. Sci. USA 102: 10309–10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst, M., G. Odorizzi, E. J. Estepa and S. D. Emr, 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1: 248–258. [DOI] [PubMed] [Google Scholar]
- Bankaitis, V. A., l. M. Johnson, and S. D. Emr, 1986. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc. Natl. Acad. Sci. USA 83: 9075–9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi, G., E. Schmid and K. Maundrell, 1993. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123: 131–136. [DOI] [PubMed] [Google Scholar]
- Bates, S., D. M. MacCallum, G. Bertram, C. A. Munro, H. B. Hughes et al., 2005. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J. Biol. Chem. 280: 23408–23415. [DOI] [PubMed] [Google Scholar]
- Beach, D., M. Piper and P. Nurse, 1982. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol. Gen. Genet. 187: 326–329. [DOI] [PubMed] [Google Scholar]
- Bone, N., J. B. Millar, T. Toda and J. Armstrong, 1998. Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr. Biol. 8: 135–144. [DOI] [PubMed] [Google Scholar]
- Bryant, N. J., R. C. Piper, S. R. Gerrard and T. H. Stevens, 1998. Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: a VPS45-dependent intracellular route and a VPS45-independent, endocytic route. Eur. J. Cell Biol. 76: 43–52. [DOI] [PubMed] [Google Scholar]
- Carnero, E., J. C. Ribas, B. Garcia, A. Duran and Y. Sanchez, 2000. Schizosaccharomyces pombe ehs1p is involved in maintaining cell wall integrity and in calcium uptake. Mol. Gen. Genet. 264: 173–183. [DOI] [PubMed] [Google Scholar]
- Carver, P. L., 2004. Micafungin. Ann. Pharmacother. 38: 1707–1721. [DOI] [PubMed] [Google Scholar]
- Chen, G., P. Yuan, D. B. Hawver, W. Z. Potter and H. K. Manji, 1997. Increase in AP-1 transcription factor DNA binding activity by valproic acid. Neuropsychopharmacology 16: 238–245. [DOI] [PubMed] [Google Scholar]
- Cheng, H., R. Sugiura, W. Wu, M. Fujita, Y. Lu et al., 2002. Role of the Rab GTP-binding protein Ypt3 in the fission yeast exocytic pathway and its connection to calcineurin function. Mol. Biol. Cell 13: 2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes, J. C., R. Katoh-Fukui, K. Moto, J. C. Ribas and J. Ishiguro, 2004. Schizosaccharomyces pombe Pmr1p is essential for cell wall integrity and is required for polarized cell growth and cytokinesis. Eukaryot. Cell 3: 1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles, C. R., S. D. Emr and B. F. Horazdovsky, 1994. Mutations in the VPS45 gene, a SEC1 homologue, result in vacuolar protein sorting defects and accumulation of membrane vesicles. J. Cell Sci. 107(Pt. 12): 3449–3459. [DOI] [PubMed] [Google Scholar]
- Dean, N., 1995. Yeast glycosylation mutants are sensitive to aminoglycosides. Proc. Natl. Acad. Sci. USA 92: 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, L., R. Sugiura, K. Ohta, K. Tada, M. Suzuki et al., 2005. Phosphatidylinositol-4-phosphate 5-kinase regulates fission yeast cell integrity through a phospholipase C-mediated protein kinase C-independent pathway. J. Biol. Chem. 280: 27561–27568. [DOI] [PubMed] [Google Scholar]
- Deng, L., R. Sugiura, M. Takeuchi, M. Suzuki, H. Ebina et al., 2006. Real-time monitoring of calcineurin activity in living cells: evidence for two distinct Ca2+-dependent pathways in fission yeast. Mol. Biol. Cell 17: 4790–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edamatsu, M., and Y. Y. Toyoshima, 2003. Fission yeast synaptobrevin is involved in cytokinesis and cell elongation. Biochem. Biophys. Res. Commun. 301: 641–645. [DOI] [PubMed] [Google Scholar]
- Gottlicher, M., S. Minucci, P. Zhu, O. H. Kramer, A. Schimpf et al., 2001. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20: 6969–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, K. L., S. Moreno, N. K. Tonks and P. Nurse, 1990. Complementation of the mitotic activator, p80cdc25, by a human protein-tyrosine phosphatase. Science 250: 1573–1576. [DOI] [PubMed] [Google Scholar]
- Gurvich, N., O. M. Tsygankova, J. L. Meinkoth and P. S. Klein, 2004. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 64: 1079–1086. [DOI] [PubMed] [Google Scholar]
- He, Y., R. Sugiura, Y. Ma, A. Kita, L. Deng et al., 2006. Genetic and functional interaction between Ryh1 and Ypt3: two Rab GTPases that function in S. pombe secretory pathway. Genes Cells 11: 207–221. [DOI] [PubMed] [Google Scholar]
- Hirayama, S., R. Sugiura, Y. Lu, T. Maeda, K. Kawagishi et al., 2003. Zinc finger protein Prz1 regulates Ca2+ but not Cl− homeostasis in fission yeast. Identification of distinct branches of calcineurin signaling pathway in fission yeast. J. Biol. Chem. 278: 18078–18084. [DOI] [PubMed] [Google Scholar]
- Johannessen, C. U., and S. I. Johannessen, 2003. Valproate: past, present, and future. CNS Drug Rev. 9: 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, C. A., and R. Schekman, 1990. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 61: 723–733. [DOI] [PubMed] [Google Scholar]
- Kita, A., R. Sugiura, H. Shoji, Y. He, L. Deng et al., 2004. Loss of Apm1, the μ1 subunit of the clathrin-associated adaptor-protein-1 complex, causes distinct phenotypes and synthetic lethality with calcineurin deletion in fission yeast. Mol. Biol. Cell 15: 2920–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, O. H., P. Zhu, H. P. Ostendorff, M. Golebiewski, J. Tiefenbach et al., 2003. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 22: 3411–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., J. D. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman et al., 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66: 807–815. [DOI] [PubMed] [Google Scholar]
- Loscher, W., 1999. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog. Neurobiol. 58: 31–59. [DOI] [PubMed] [Google Scholar]
- Maeda, T., R. Sugiura, A. Kita, M. Saito, L. Deng et al., 2004. Pmr1, a P-type ATPase, and Pdt1, an Nramp homologue, cooperatively regulate cell morphogenesis in fission yeast: the importance of Mn2+ homeostasis. Genes Cells 9: 71–82. [DOI] [PubMed] [Google Scholar]
- Marchion, D. C., E. Bicaku, A. I. Daud, D. M. Sullivan and P. N. Munster, 2005. Valproic acid alters chromatin structure by regulation of chromatin modulation proteins. Cancer Res. 65: 3815–3822. [DOI] [PubMed] [Google Scholar]
- Maundrell, K., 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123: 127–130. [DOI] [PubMed] [Google Scholar]
- Moreno, S., A. Klar and P. Nurse, 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 795–823. [DOI] [PubMed] [Google Scholar]
- Novick, P., S. Ferro and R. Schekman, 1981. Order of events in the yeast secretory pathway. Cell 25: 461–469. [DOI] [PubMed] [Google Scholar]
- Ohtsubo, K., and J. D. Marth, 2006. Glycosylation in cellular mechanisms of health and disease. Cell 126: 855–867. [DOI] [PubMed] [Google Scholar]
- Perego, P., F. Zunino, N. Carenini, F. Giuliani, S. Spinelli et al., 1998. Sensitivity to cisplatin and platinum-containing compounds of Schizosaccharomyces pombe rad mutants. Mol. Pharmacol. 54: 213–219. [DOI] [PubMed] [Google Scholar]
- Perego, P., G. S. Jimenez, L. Gatti, S. B. Howell and F. Zunino, 2000. Yeast mutants as a model system for identification of determinants of chemosensitivity. Pharmacol. Rev. 52: 477–492. [PubMed] [Google Scholar]
- Pevsner, J., S. C. Hsu, P. S. Hyde and R. H. Scheller, 1996. Mammalian homologues of yeast vacuolar protein sorting (vps) genes implicated in Golgi-to-lysosome trafficking. Gene 183: 7–14. [DOI] [PubMed] [Google Scholar]
- Phiel, C. J., F. Zhang, E. Y. Huang, M. G. Guenther, M. A. Lazar et al., 2001. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276: 36734–36741. [DOI] [PubMed] [Google Scholar]
- Piper, R. C., E. A. Whitters and T. H. Stevens, 1994. Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, post-Golgi transport vesicles. Eur. J. Cell Biol. 65: 305–318. [PubMed] [Google Scholar]
- Robinson, J. S., D. J. Klionsky, L. M. Banta and S. D. Emr, 1988. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell Biol. 8: 4936–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J. H., and T. H. Stevens, 1986. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell 47: 1041–1051. [DOI] [PubMed] [Google Scholar]
- Rothstein, R. J., 1983. One-step gene disruption in yeast. Methods Enzymol. 101: 202–211. [DOI] [PubMed] [Google Scholar]
- Rusnak, F., and P. Mertz, 2000. Calcineurin: form and function. Physiol. Rev. 80: 1483–1521. [DOI] [PubMed] [Google Scholar]
- Sugiura, R., T. Toda, H. Shuntoh, M. Yanagida and T. Kuno, 1998. pmp1+, a suppressor of calcineurin deficiency, encodes a novel MAP kinase phosphatase in fission yeast. EMBO J. 17: 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura, R., S. O. Sio, H. Shuntoh and T. Kuno, 2002. Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes Cells 7: 619–627. [DOI] [PubMed] [Google Scholar]
- Tabuchi, M., O. Iwaihara, Y. Ohtani, N. Ohuchi, J. Sakurai et al., 1997. Vacuolar protein sorting in fission yeast: cloning, biosynthesis, transport, and processing of carboxypeptidase Y from Schizosaccharomyces pombe. J. Bacteriol. 179: 4179–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda, T., S. Dhut, F. G. Superti, Y. Gotoh, E. Nishida et al., 1996. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 16: 6752–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toonen, R. F., and M. Verhage, 2003. Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol. 13: 177–186. [DOI] [PubMed] [Google Scholar]
- Vida, T. A., and S. D. Emr, 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128: 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., R. Sugiura, Y. Lu, M. Asami, T. Maeda et al., 2000. Phosphatidylinositol 4-phosphate 5-kinase Its3 and calcineurin Ppb1 coordinately regulate cytokinesis in fission yeast. J. Biol. Chem. 275: 35600–35606. [DOI] [PubMed] [Google Scholar]
- Zhao, C., U. S. Jung, P. Garrett-Engele, T. Roe, M. S. Cyert et al., 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18: 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]