Abstract
Transgenic flies are generated by transposon-mediated transformation. A drawback of this approach is the size limit of transposable elements. Here, we propose a novel method that allows the extension of transgenes in vivo. This method is based on an incomplete transgene that has been constructed in vitro and integrated into the Drosophila genome by conventional transgenesis. The incomplete transgene contains two short stretches of DNA homologous to the 5′- and 3′-ends of a larger DNA segment of interest. Between the short stretches of homology an I-SceI recognition site is located. Once activated, I-SceI endonuclease introduces a DNA double-strand break, which triggers ectopic recombination between the stretches of homology and the endogenous locus. Through gap repair, the transgene obtains the complete region of interest in vivo. Our results show that this method is effective for copying up to 28 kb of genomic DNA into the transgene, thereby eliminating the technical difficulties associated with the in vitro construction of large transgenes and extending the size limits of current transgenesis protocols. In general, this method may be a useful technique for genetic engineering of eukaryotic model organisms.
IN Drosophila, transgenes can be integrated into the genome by mobile elements, such as P elements and piggyBacs (Rubin and Spradling 1982; Wimmer 2003). Transposon-mediated transgenesis is limited in size as the frequency of integration decreases dramatically with increasing size of the mobile element (Spradling 1986). It is inefficient to transform flies with mobile elements that are >20–30 kb. The largest P elements that have been directly introduced into the Drosophila genome were based on cosmid vectors, setting an arbitrary upper limit of ∼40 kb (Haenlin et al. 1985). A promising new approach for Drosophila transgenesis is to generate transgenic flies by ΦC31-, Cre- or Flp-mediated site-specific recombination (Groth et al. 2004; Horn and Handler 2005; Oberstein et al. 2005; Venken et al. 2006).
A remarkable ability of the cell is that its homologous recombination (HR) machinery can find a template DNA located anywhere in the whole genome. Areas of homology can be found and used as a template whether the homology resides on the sister chromatid, on the homologous chromosome, at a nonallelic ectopic position in the genome, or even on an injected DNA (Banga and Boyd 1992; Nassif et al. 1994; Keeler et al. 1996; Lankenau and Gloor 1998; Rong and Golic 2003). Several techniques that take advantage of this ability have been developed to modify the genome in a targeted manner in model organisms from yeast to mouse (Capecchi 1989; Jasin 1996; Rong and Golic 2000; Egli et al. 2004). HR is also used to generate recombinant constructs in yeast and bacteria, thus alleviating the difficulties associated with cloning using restriction enzymes (Zhang et al. 2000; Copeland et al. 2001; Muyrers et al. 2001).
Here, we propose a novel efficient method whereby we can introduce long DNA segments into transgene loci by HR. Furthermore, we show that HR may be coupled to nonhomologous end joining (NHEJ) even after extensive DNA repair synthesis at both ends of the break.
MATERIALS AND METHODS
DNA constructs:
Constructs were made using the P-element vector pTARG (GenBank accession no. DQ269206) and transformed by micro-injection. The 5′ part of the yellow gene (−2869–+1608, with numbers referring to the transcriptional initiation site of yellow) was cloned into the SalI–SphI position of pTARG. An I-SceI site was inserted at the SphI site and the resulting vector was termed pTARG-N. The following segments downstream of the yellow gene were amplified by PCR: segment F (+12,069–+15,762), segment G (+29,411–+34,985), segment K (+49,656–+53,914), and segment J (+87,554–+92,103). PCR products were blunted with T4 DNA polymerase, digested with SphI, and cloned into the SphI–StuI sites of the pTARG-N plasmid. All constructs were named according to their downstream segments of “F,” “G,” “K,” and “J,” respectively. The construct “F–R” is derived from “F” by the elimination of an EcoRI fragment and therefore contains a shorter segment from +12,069 to +12,627 from the transcriptional initiation site of yellow. The integration site of F–R2, F–R5, F1, G2, or J was determined by inverse PCR and found to be at 33B1, 99E4, 7E6/7, 80A1, or 55C2, respectively. Inverse PCR was performed using primer pairs, Plac1–Plac4 and Pry1–Pry2, as described in http://www.fruitfly.org/ and PCR products, were sequenced with primer P* as described in http://www.fruitfly.org/. The integration sites of other transgenes were not identified. The two segments of the Cg25C construct correspond to the region from −17091 to −15063 and from +4150 to +7103 from the transcription initiation site of the Cg25C gene. The 5′ segment was amplified and cloned into the SphI and NotI sites in pTARG. The 3′ segment of this construct was amplified and cloned into the MluI and SphI sites. An I-SceI site was inserted at the SphI site. The red fluorescent protein (RFP) (dsRed), was cloned into the MluI site of the Cg25C construct. The details of all primers are shown in supplemental Table 1 at http://www.genetics.org/supplemental/.
Fly stocks and genetics:
The stock y1 w1118; P[v+, 70I-SceI] is derived from y1 w1118;P[ry+, 70FLP]4 P[v+, 70I-SceI]2B Sco/S2 CyO, kindly provided by Y. Rong and K. Golic (Rong et al. 2002). Df(1)y3PLsc8R and Df(1)y3PLsc4R/Δ49 was provided by Juan Modolell (Campuzano et al. 1985). Flies carrying a heat-inducible I-SceI gene and one of the constructs—F, F–R, G, K, J, or N—as transgenes were generated by crossing. They were kept in vials to lay eggs for 6 hr at 26°. A heat shock (at 38° for 1 hr) was given to offspring at the time points indicated. Subsequently, all heat-shocked flies were singly mated to y1 w1118 to analyze gap repair efficiency in germline cells.
Molecular characterization of double-strand break repair events:
Using genomic DNA, gap repair events were analyzed by PCR using the primers shown in Figure 1C and supplemental Table 1 at http://www.genetics.org/supplemental/. Gap repair events of the Cg25C construct were analyzed by PCR using a primer located in RFP (5′-GTA CTG GAA CTG GGG GGA CAG-3′) and a primer in the endogenous Cg25 locus (5′-CAG GGC GCT GTC GGA GTA CC-3′) within the gap to be completed by HR. Events obtained by heat shocking at different developmental stages as well as with different numbers of heat shocks were included in the molecular analysis.
Figure 1.—
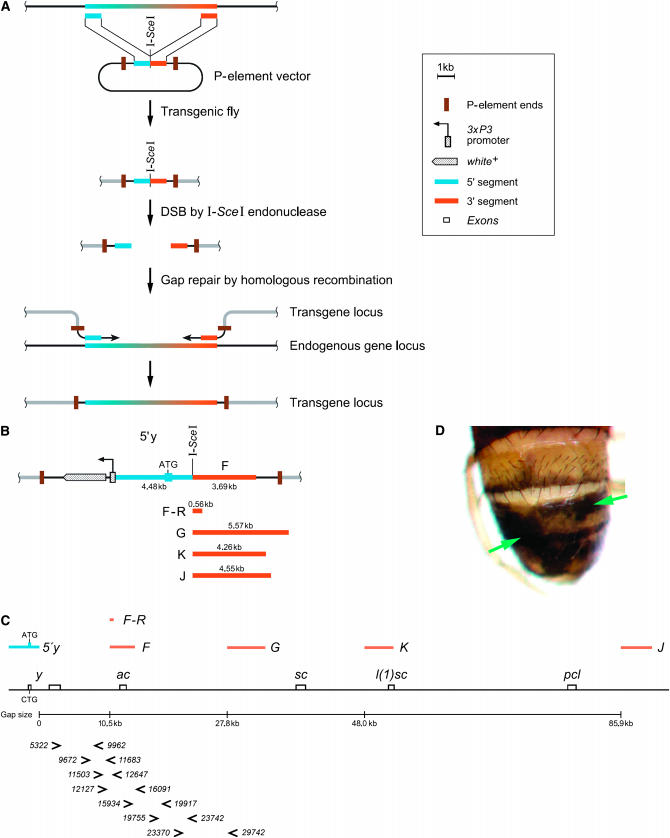
(A) Principle of the construction of transgenes in vivo. To copy a desired region from the endogenous locus into a transgene, two segments (blue and orange boxes) at both ends of the target DNA are cloned into an insect transformation vector. An I-SceI recognition site connects the two segments. This element is integrated into the Drosophila genome at a position different from the locus of interest. By crossing with flies expressing the endonuclease I-SceI, a DSB is introduced in the transgene between the two segments. Subsequently, the transgene acquires the missing region from the endogenous locus by gap repair. (B–D) In vivo construction of transgenes of the yellow–ASC locus. (B) Segments used for cloning are indicated by bars and labeled with 5′ yellow (blue), F, F–R, G, K, and J (orange). The size of each segment is indicated in kilobases. The w marker gene serves to identify transgenic flies. (C) yellow–ASC genomic region. The positions of each segment and primers for the molecular characterization of gap repair events are indicated. The y1 mutation is a point mutation in the yellow start codon; the mobile element constructs, however, contain a wild-type start codon. Primer numbers indicate the position relative to and downstream of the y transcription start. (D) DSB repair produced a mosaic phenotype. This fly carries both the F construct and the heat-shock-inducible I-SceI transgene. A heat shock has been applied at larval stages to induce DSBs. Dark patches (y+) are gap repair events (green arrows). The symbols in the boxed legend are also used in Figures 2 and 3.
RFP expression analysis and microscopy:
For the analysis of the expression pattern of Cg25C-RFP, second instar Drosophila larvae were photographed using a Leica DRB fluorescence stereomicroscope equipped with a Zeiss Axiocam.
RESULTS
In vivo assembly of transgenes by homologous recombination:
The in vivo assembly of transgenes requires an incomplete construct, made in vitro and containing two DNA segments derived from two distant sequences located in the chromosomal region of interest and an I-SceI recognition site between these segments (Figure 1A). The entire assembly is inserted within a mobile element vector for conventional transgenesis. In flies carrying these constructs, the expression of I-SceI endonuclease leads to a double-strand break (DSB). Each end at the breakpoint invades the homologous locus and initiates DNA synthesis. When synthesized DNA from each end reaches the complementary region, the two ends anneal to each other to restore a continuous strand, a process referred to as gap repair (see also Nassif et al. 1994). Thereby the sequence between the two pieces of DNA is copied from the endogenous locus into the mobile element (Figure1A).
A genetic selection system for the in vivo construction of transgenes in Drosophila:
To test this system, we used the yellow–ASC region on the Drosophila X chromosome because of its extensive genetic characterization and the availability of mutations causing visible phenotypes (Garcia-Bellido 1979; Ruiz-Gomez and Modolell 1987). Several fly strains with w+-marked mobile elements carrying the wild-type 5′ part of the yellow gene with one of several downstream segments—F, F–R, G, K, or J—were constructed (Figure 1B). Flies carrying one of those constructs within a y1 mutant background are y−w+ as the 5′ part of the yellow gene in the mobile element is truncated and therefore nonfunctional. The downstream segments of the construct are, when aligned with the X chromosome, separated from the 5′ yellow segment by 10.5 kb for F and F–R, 27.8 kb for G, 48.0 kb for K, and 85.9 kb for J. The difference between F and F–R is the size of the downstream segment (Figure 1C).
We induced DSBs by heat-shock-inducible I-SceI expression. DSB repair using the endogenous y1 locus as a template restored a functional yellow gene within the transgene, resulting in flies mosaic for the y+ gene. y+ patches indicate that the 3′ part of the yellow gene has been copied into the transgene (Figure 1D). The frequencies of y+ retrieval in the germline of these mosaic flies were determined by singly crossing them to y w flies and screening their offspring for y+ (Tables 1 and 2). These results can be summarized as follows:
y+ rescue events occurred in the constructs with an 11-, 28-, or a 48-kb gap but only rarely with an 86-kb gap.
The frequency of copying a y+ gene into the P-element locus decreases when the size of the gap increases. This is particularly clear when the y+ rescue frequencies obtained with insertions of F are compared with the ones obtained with insertions of G or K.
The frequency of y+ rescue varies considerably with respect to the insertion site of the mobile element in the genome.
A segment of as little as 560 bp on one side of the break together with a longer segment on the other side is able to support efficient copying of 11 kb of DNA from the endogenous locus. Such a decrease in homology is accompanied by a decrease in copying efficiency (compare the average frequency of F with the one for F–R).
The frequency of y+ rescue also depends on the developmental stage at which the DSBs were induced, especially in the male germline where a heat shock at the first instar appears to result in the highest frequency (Table 2). These variations may also reflect the efficiency of the heat shock in inducing I-SceI expression.
TABLE 1.
y+ reconstitution efficiency of different constructs and insertions
| Transgene | Fly line | Sex | % events/crosses | % y+ in total offspring | % average efficiency of the constructs (events/crosses) |
|---|---|---|---|---|---|
| F1 (11-kb gap) | F1 (X chromosome) | M | 100 (19/19) | 15.7 (147/932) | 60 |
| F | 69 (24/35) | 11.0 (438/3957) | |||
| F1–2 (second chromosome) | M | 28 (7/25) | 0.79 (13/1633) | ||
| F | 25 (1/4) | 0.37 (1/271) | |||
| F1–3 (third chromosome) | M | 52 (26/50) | 1.95 (59/2958) | ||
| F | 86 (6/7) | 3.66 (19/500) | |||
| F–R (11-kb gap) | F–R2 (second chromosome) | M | 12 (4/33) | 0.45 (9/1980) | 17 |
| F | 24 (8/33) | 0.50 (12/2395) | |||
| F–R3 (second chromosome) | M | 23 (22/95) | 0.58 (40/6870) | ||
| F | 27 (21/79) | 0.82 (54/6599) | |||
| F–R4 (second chromosome) | M | 6 (1/18) | 0.091 (1/1096) | ||
| F | 13 (2/16) | 0.50 (6/1173) | |||
| F–R5 (third chromosome) | M | 11 (9/85) | 0.16 (10/6218) | ||
| F | 22 (19/86) | 0.66 (40/6043) | |||
| G (28-kb gap) | G1 (third chromosome) | M | 15 (12/80) | 0.28 (21/7366) | 13 |
| F | 13.5 (10/74) | 0.29 (16/5564) | |||
| G2 (third chromosome) | M | 0 (0/38) | 0 (0/2119) | ||
| F | 23 (9/40) | 0.29 (9/3018) | |||
| K (48-kb gap) | K 3.8 (second chromosome) | M | 10 (12/118) | 0.29 (30/10225) | 9.5 |
| F | 9 (9/102) | 0.32 (28/8518) | |||
| J (87-kb gap) | J1 (second chromosome)a | ND | 2.5 (11/450) | 0.04 (11/30000) | 2.5 |
| N (one arm of homology) | N2–3 | ND | 0.2 (1/500) | 0.0022 (1/45000) | 0.2 |
DSBs were generated by the heat-shock-inducible expression (1 hr at 38°) of the I-SceI endonuclease at the early third instar stage. F1, F1–2, F1–3, F–R2, F–R3, F–R4, F–R5, G1, G2, K3.8, J1, and N2–3 are transgenic fly lines for the corresponding constructs F, F–R, G, K, J, and N. M, male; F, female. The average efficiency for each construct was obtained by averaging the efficiency of each insertion of a particular construct. A single tube contains ∼80–120 flies of the relevant genotype. ND, not determined.
For the J and the N construct, three instead of one heat shock (1 hr 38°) was applied on subsequent days. The difference between the sexes was not addressed for the insertions of the J or the N construct.
TABLE 2.
y+ reconstitution efficiency at different developmental stages
| % events/crosses
|
% y+ in total offspring (y+ flies/total no.)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Construct | Sex | Embryo | First instar | Second instar | Third instar | Embryo | First instar | Second instar | Third instar |
| F–R3 | M | 62 (31/50) | 74 (37/50) | 59 (20/34) | 23 (22/95) | 4.6 (201/4390) | 6.5 (282/4319) | 4.7 (106/2269) | 0.6 (40/6870) |
| F | 32 (15/47) | 38 (13/34) | 40 (14/50) | 27 (21/79) | 0.8 (38/4736) | 1.3 (45/3437) | 1.5 (40/2687) | 0.8 (54/6599) | |
| F–R5 | M | 10 (5/50) | 48 (24/50) | 28 (14/50) | 11 (9/85) | 0.8 (35/4421) | 1.5 (66/4511) | 0.7 (21/2824) | 0.16 (10/6218) |
| F | 27 (14/51) | 30 (15/50) | 45 (20/44) | 22 (19/86) | 1.3 (54/4118) | 1.1 (50/4739) | 1.4 (47/3320) | 0.65 (40/6043) | |
DSBs were induced at various developmental stages as indicated (1 hr 38°). F–R3 and F–R5 are transgenic fly lines for the corresponding construct F–R.
We also wanted to know whether a single arm of homology is sufficient for invading the homologous template, initiating DNA synthesis, and copying an entire locus into the DSB. To address this question, we used a construct, termed “N,” with only the 5′ segment of the yellow gene but without a segment of downstream homology. In this case, a single arm would invade the yellow locus, start DNA synthesis, and possibly retrieve the entire y locus. Indeed, among 45,000 chromosomes or 500 tubes screened, we recovered a single y+ event (0.0022% of all chromosomes or 0.2% of all tubes) (Table 1).
A DNA segment of up to 28 kb can be integrated into a transgene in vivo:
We further analyzed the y+ events by genetic and molecular means (Figure 2, Table 3). Before inducing gap repair, none of the initial constructs were able to rescue a mutation in the achaete locus. Upon gap repair, the transgene obtained a part of the ASC locus whose size depends on the position of the downstream segment (Figure 1C). To test for phenotypic rescue of achaete, we crossed the y+ events to the deficiency Df(1)y3PLsc8R, which deletes the yellow and achaete loci. All the y+ events derived from the construct with an 11-kb gap (F) that were tested rescued achaete mutants, whereas 22 of 29 (76%) independent y+ events from the construct with a 28-kb gap rescued achaete. However, none of 6 tested y+ events derived from the construct with a 48-kb gap rescued achaete nor did the single y+ event obtained with the N construct that lacks a downstream homology segment. Interestingly, 3 of the 11 J-derived events were able to complement a mutation in the achaete locus. Events that did not rescue achaete were considered as incomplete.
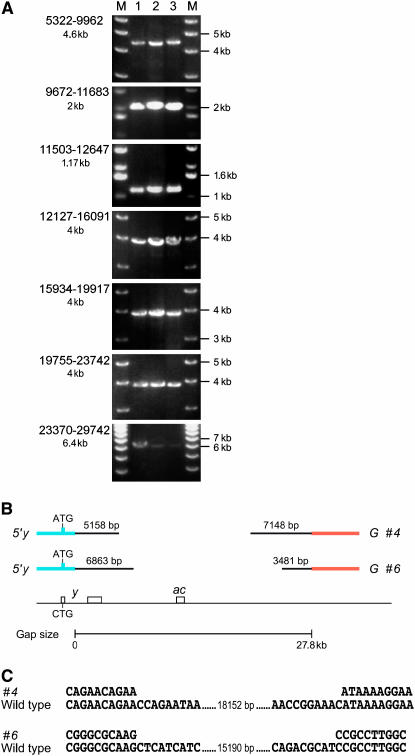
Figure 2.—
Examples of complete and partial gap repair events derived from the construct with a 28-kb gap (G). (A) PCR of three (lanes 1–3) complete events in the Df(1)y3PLsc8R background. The overlapping PCR products span a total of 24.4 kb corresponding to the downstream region of y. The primer pairs used are indicated on the left (see also Figure 1C). The size of the PCR product is indicated below the primer numbers. M, size marker. Primer numbers indicate the position relative to and downstream of the y transcription start. (B) Examples of two incomplete gap repair events (“#4” and “#6”). The in vivo-cloned DNA is in black with the size indicated above each segment. Orange and blue bars represent segments of the starting construct. The chromosomal region is shown below. Note that a considerable amount of DNA has been integrated at both ends of the DSB, but a gap of >15 kb remains. (C) Alignment of the DNA sequence at the junction of the events 4 and 6 with wild-type genomic DNA. The number indicates the length of the wild-type sequence not shown. Note the absence of (micro-)homology at the junction point.
TABLE 3.
Completeness of gap repair
| Transgene | Fly line | % offspring with complete gap repair |
|---|---|---|
| F (11-kb gap) | F1 (X chromosome) | 100 (12/12) |
| F1–2 (second chromosome) | 100 (1/1) | |
| F1–3 (third chromosome) | 91 (10/11) | |
| F–R (11-kb gap) | F–R2 (second chromosome) | 83 (5/6) |
| F–R3 (second chromosome) | 100 (9/9) | |
| F–R4 (second chromosome) | 100 (1/1) | |
| F–R5 (third chromosome) | 100 (5/5) | |
| G (28-kb gap) | G1 (third chromosome) | 73 (11/15) |
| G2 (third chromosome) | 79 (11/14) | |
| K (48-kb gap) | K3.8 (second chromosome) | 0 (0/6) |
| J (87-kb gap) | J1 (X chromosome) | 0 (0/11) |
Numbers represent independent y+ events with a completely copied region in percentage of all y+ events. Completeness of gap repair was analyzed by both genetic and molecular means. F1, F1–2, F1–3, F–R2, F–R3, F–R4, F–R5, G1, G2, K3.8, and J1 are transgenic fly lines for the corresponding constructs F, F–R, G, K, and J. Incomplete in vivo cloning events carry a single large deletion (Figures 2 and 4).
For the molecular analysis, we checked several y+ events by PCR using primer pairs indicated in Figure 1C and supplemental Table 1 at http://www.genetics.org/supplemental/. PCR was performed in a Df(1)y3PLsc8R background, which deletes the endogenous chromosomal PCR template. Events obtained with the 48-kb gap and 86-kb gap constructs K and J were analyzed by quantitative PCR. Of 35 events that rescued both the yellow and the achaete gene derived from both the 11-kb gap construct (F) and the 28-kb gap construct (G), 34 carried a completely copied yellow–ASC region in the transgene (Figure 2A, Table 3). Sequencing of in vivo-cloned DNA did not show any evidence for small deletions or point mutations (data not shown). However, none of the yellow or even the yellow–achaete rescue events obtained with either the 48-kb gap or the 86-kb gap construct were complete. Accordingly, both genetic and molecular data show that DNA sequences up to 28 kb can be faithfully copied into transgenes by HR.
A two-side invasion can be resolved by NHEJ to yield incomplete gap repair events:
Gap repair events classified as incomplete by genetic and molecular analysis contained a single large deletion of several kilobases. All of these events, however, had copied DNA from the endogenous locus at both ends of the break (examples in Figure 2B). To examine the nature of partial gap repair events, junctions of two independent events derived from the construct with a 28-kb gap were amplified using PCR and sequenced. Alignment with the wild-type genomic sequence revealed a junction without homology at the junction point (Figure 2C). Apparently, both ends invaded the homologous template and, after extensive DNA synthesis, they ligated by NHEJ. Extensive DNA synthesis followed by NHEJ was also found for events obtained with the 86-kb gap construct. Three of these were able to rescue the achaete locus, suggesting that >10 kb was added to one end of the break before ligation of the nonhomologous ends.
The endogenous template locus usually remains unchanged:
Recombination between the endogenous locus and the DNA sequence contained in the transgene could potentially result in the modification of the endogenous locus. While this could be useful for other applications, it would complicate the selection system for the construction of transgenes in vivo. We therefore wanted to know how frequent modifications of the endogenous locus occur in our system. To this end, we screened for the correction of the CTG point mutation at the endogenous y1 locus by the correct ATG start codon sequence contained in the transgene. In two independent cases among a total of 134,000 flies screened, a wild-type y+ gene was restored at the endogenous locus, demonstrating that genetic information can flow from the broken strand to the intact DNA strand, albeit at a very low efficiency. This finding is in agreement with previous observations in both mammals and flies that the template strand for DNA repair usually remains unchanged (Engels et al. 1990; Richardson et al. 1998). We conclude that the inefficiency of this process is unlikely to confound the use of HR for the completion of transgenes in vivo.
A complete transgene can be selected for by reinstating a fluorescent protein reporter:
To test whether this method can be applied to a gene located elsewhere in the genome, we generated transgenic flies carrying a copy of a partial Cg25C gene, the endogenous copy of which is located on the second chromosome. Unlike with the y locus, we used a wild-type copy of Cg25C as a template and therefore could not directly select for a genetic rescue by the transgene. Instead, in this partial construct, the segment corresponding to the 3′-end of the coding region was fused in frame to an RFP (Figure 3, A and B). Due to the incompleteness of the transgene, RFP was not expressed before the gap repair. Induction of DSBs, however, gave rise to flies expressing RFP in the larval fat body, as reported previously for the expression of the endogenous Cg25C gene (Figure 3C) (Yasothornsrikul et al. 1997). Using two different P elements inserted on the second chromosome, RFP-expressing flies were recovered in 21.5% (9/42) of the crosses or at a frequency of 2.5% (31/1249) of total flies for the first line and in 0.92% (3/325) of total flies for the second line. Another line on the third chromosome did not yield RFP-expressing flies among 600 flies screened. Molecular analysis of these gap repair events at the breakpoint revealed the integration of DNA from the endogenous Cg25C locus into the gap, thereby excluding the possibility that integration of DNA from another nonhomologous locus by nonhomologous recombination resulted in the expression of RFP (Figure 3D). As the correct expression of a Cg25C–RFP fusion protein requires the integration of the promoter as well as the maintenance of the reading frame and the correct splicing of six exons, it appears very likely that the entire Cg25C gene was faithfully integrated into the transgene.
Figure 3.—
A fluorescent-protein-based selection system for the construction of transgenes in vivo. (A) A partial P-element construct. RFP is fused to a 3′ segment (blue) of the target gene. (B) Cg25C genomic region. Black boxes indicate the coding region and the left arrow indicates the transcription start of Cg25C. An orange and a blue bar indicate the genomic position of each segment used for cloning. (C) Expression pattern of the in vivo-completed Cg25C–RFP fusion transgene. Shown are images of second instar larvae carrying either a copy of the in vivo-completed Cg25C–RFP construct (left) or a copy of the original transgene showing only auto-fluorescence of the intestinal tract (right). (D) Molecular analysis of gap repair events by PCR using the primers indicated by arrowheads in A and B. Lanes 1 and 2 represent independent gap repair events and lane 3 represents the transgenic fly before gap repair. The product is of the expected size of 3.6 kb. M, size marker. From top to bottom: 5, 4, 3, and 2 kb.
DISCUSSION
Several loci in the Drosophila genome are large, extending 40 kb in the case of Notch (Artavanis-Tsakonas et al. 1983) and 50 kb in the case of broad (DiBello et al. 1991), or very large, extending 90 kb in the case of Cadherin-N (Iwai et al. 1997). We demonstrated that gap repair permits the efficient introduction of an ∼30-kb DNA segment into a transgene that already contains part of the locus of interest. Together with preexisting segments whose length is limited by the limits of mobile-element-mediated transgenesis to ∼30 kb, a fly carrying a transgene of at least 60 kb may be constructed. This would allow the genetic analysis of large genes and their regulatory regions. Possibly, the current size limit of the in vivo extension of transgenes can be overcome by the use of three or more segments that are linked by different meganuclease restriction sites that can be used in successive rounds of extension. The availability of an increasing number of molecularly and phenotypically characterized Drosophila mutants permits a genetic selection strategy for complete transgenes, similar to the one demonstrated in this study for y1, where the transgene contains part of the gene spanning the region harboring a mutation in the endogenous gene. In other cases, the generation of a fluorescent fusion protein may be desirable, requiring, however, a strong promoter for detectable fluorescence. Our system therefore allows the generation of rescue and transcriptional reporter constructs and of fusion proteins. Also, the method presented could simplify cloning of long DNA segments by PCR and restriction enzymes that may be associated with time-consuming difficulties when DNA segments reach a size of several kilobases. Rather than cloning a single large segment into the transgene vector, two short and incomplete segments can be cloned and the cloning process can then be completed in vivo. A major challenge to this technique may be to conveniently sort out incomplete from complete gap repair events especially when the segment of interest is large. A clever choice of the segments used for the construction of the partial transgene certainly can facilitate the secondary and necessary selection step for full-length transgenes. Such an assay can be genetic, PCR based, or a Southern blot.
In addition to introducing a new technique to Drosophila, this study also contributes to the understanding of DNA repair mechanisms. It appears that in Drosophila, repair DNA synthesis tracts are limited to <50 kb. This finding is also reported by an independent study that shows efficient filling of an 11-kb gap, inefficient filling of a 43-kb gap, and no complete filling of a 210-kb gap (Johnson-Schlitz and Engels 2006). Such a limitation appears to be unlike that in yeast, where an entire chromosomal arm can be copied into a double-strand break by break-induced replication (Morrow et al. 1997). Probably a low processivity of DNA repair synthesis accounts for such an upper limit. It appears that repair synthesis proceeds through multiple cycles of strand invasion and dissociation (McVey et al. 2004). Each cycle may be an opportunity to complete the repair event by either a homologous or a nonhomologous repair pathway. Such a coupling of HR to NHEJ has been reported previously (Richardson and Jasin 2000). If this coupling were efficient, we would expect similar frequencies of y+ recovery at different gap sizes, assuming that strand invasion does not depend on the distance between the different targets. We find, however, a dramatic reduction in y+ rescue events with increasing gap size, possibly because NHEJ is not as efficient in sealing the break as when the entire gap is filled and sealing can occur by homologous end joining. Interestingly, the incomplete gap repair events that we analyzed have all invaded and initiated DNA synthesis at both ends of the break. Most likely, these incomplete gap repair events reflect how complete repair events are initiated. We therefore suggest a two-end invasion for both complete and incomplete events (Figure 4). This two-end invasion model is also supported by the finding that a single-end invasion by a single arm of homology can only very rarely copy a larger DNA segment into the break. Remarkable is the length of the DNA segments and the sequence of the junction that we found in the incomplete gap repair events. At least in one case, a tract of >10 kb has been added to one side of the break. In the two cases where we sequenced the junction, a microhomology was absent in one case and a possible microhomology of a single C nucleotide was found in the other case (see also Staveley et al. 1995; Adams et al. 2003). It appears unlikely that two noncomplementary, very long, leading single strands are generated and then ligated without the presence of homology, especially since the Drosophila genome appears to lack family X group DNA polymerases that could initiate DNA synthesis on nonhomologous ends (Sekelsky et al. 2000; McElhinny et al. 2005). Therefore, we speculate that lagging-strand synthesis occurs during gap repair of longer tracts. In this scenario, lagging-strand synthesis restores two double strands that may be ligated by homologous end joining or nonhomologous end joining after resection of the single-stranded ends (Figure 4). The involvement of lagging-strand synthesis in DSB-induced gene conversions of even very short tracts has been suggested in yeast, but was later again revised (Holmes and Haber 1999; Wang et al. 2004). However, several models have been suggested for DSB repair events with long tracts of new DNA synthesis, such as a synthesis-dependent strand-annealing model (SDSA) with lagging-strand synthesis or a break-induced replication model that involves a true replication fork (for review see Paques and Haber 1999). Our data appear to fit an SDSA model involving lagging-strand synthesis (Figure 4). The SDSA model is used frequently to explain mitotic recombination events in Drosophila, as it can explain the absence of crossover in most mitotic recombination events and the unidirectional flow of information from the template strand to the broken strand (Nassif et al. 1994). Also in this study, the flow of genetic information in almost all cases is from the intact to the broken strand, with two remarkable exceptions where the endogenous y1 point mutation was corrected to wild type upon DSB induction in the transgene. This reversion depends on DSB formation in the transgene, as we have never observed spontaneous reversion of the y1 mutation in any of our transgenic fly strains. These two cases can be readily explained by a model involving Holliday junction migration with heteroduplex formation and with the correction of the mismatch.
Figure 4.—
Model of gap repair involving two-end invasion and lagging-strand synthesis. (A) A double-strand break is generated by I-SceI, followed by the processing of DNA ends, strand invasion, and DNA synthesis. Whether or not the branches migrate following DNA synthesis has not been addressed in this study. Ligation of the ends may occur by either homologous end joining (B) or nonhomologous end joining (NHEJ) after resection of the single-stranded ends (C). The model in B is a modification of the SDSA model proposed by Nassif et al. (1994). An alternative model involving Holliday junction formation and resolution without associated crossing over is also compatible with our data.
Taken together, these results open promising new applications for the modification of the Drosophila genome. Transgenes can probably also be constructed in vivo in other model organisms with a relatively small genome and with efficient ectopic recombination, such as Arabidopsis or Caenorhabiditis elegans.
Acknowledgments
We are grateful to Antonia Manova and Bruno Schmid for technical assistance, to Fritz Ochsenbein for the preparation of figures, and to Primo Schär (University of Basel), Konrad Basler (University of Zurich), and Serge Gangloff (Commissariat à l'Énergie Atomique de Fontenay-aux-Roses) for stimulating discussions, and two anonymous reviewers for many helpful comments on the manuscript. This work was supported by grants from the project Mechanisms of Gene Integration (LSHG-CT-2003-503303) of the European Union, the Schweizer Staatssekretariat für Bildung und Forschung, the Kanton Zürich, and the Swiss National Science Foundation.
References
- Adams, M. D., M. McVey and J. J. Sekelsky, 2003. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science 299: 265–267. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas, S., M. A. Muskavitch and B. Yedvobnick, 1983. Molecular cloning of Notch, a locus affecting neurogenesis in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 80: 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga, S. S., and J. B. Boyd, 1992. Oligonucleotide-directed site-specific mutagenesis in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 89: 1735–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano, S., L. Carramolino, C. V. Cabrera, M. Ruiz-Gomez, R. Villares et al., 1985. Molecular genetics of the achaete-scute gene complex of Drosophila melanogaster. Cell 40: 327–338. [DOI] [PubMed] [Google Scholar]
- Capecchi, M. R., 1989. Altering the genome by homologous recombination. Science 244: 1288–1292. [DOI] [PubMed] [Google Scholar]
- Copeland, N. G., N. A. Jenkins and D. L. Court, 2001. Recombineering: a powerful new tool for mouse functional genomics. Nat. Rev. Genet. 2: 769–779. [DOI] [PubMed] [Google Scholar]
- DiBello, P. R., D. A. Withers, C. A. Bayer, J. W. Fristrom and G. M. Guild, 1991. The Drosophila Broad-Complex encodes a family of related proteins containing zinc fingers. Genetics 129: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli, D., E. Hafen and W. Schaffner, 2004. An efficient method to generate chromosomal rearrangements by targeted DNA double-strand breaks in Drosophila melanogaster. Genome Res. 14: 1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels, W. R., D. M. Johnson-Schlitz, W. B. Eggleston and J. Sved, 1990. High-frequency P element loss in Drosophila is homolog dependent. Cell 62: 515–525. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido, A., 1979. Genetic analysis of the achaete-scute system of Drosophila melanogaster. Genetics 91: 491–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth, A. C., M. Fish, R. Nusse and M. P. Calos, 2004. Construction of transgenic Drosophila by using the site-specific integrase from phage ΦC31. Genetics 166: 1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenlin, M., H. Steller, V. Pirrotta and E. Mohier, 1985. A 43 kilobase cosmid P transposon rescues the fs(1)K10 morphogenetic locus and three adjacent Drosophila developmental mutants. Cell 40: 827–837. [DOI] [PubMed] [Google Scholar]
- Holmes, A. M., and J. E. Haber, 1999. Double-strand break repair in yeast requires both leading and lagging strand DNA polymerases. Cell 96: 415–424. [DOI] [PubMed] [Google Scholar]
- Horn, C., and A. M. Handler, 2005. Site-specific genomic targeting in Drosophila. Proc. Natl. Acad. Sci. USA 102: 12483–12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai, Y., T. Usui, S. Hirano, R. Steward, M. Takeichi et al., 1997. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron 19: 77–89. [DOI] [PubMed] [Google Scholar]
- Jasin, M., 1996. Genetic manipulation of genomes with rare-cutting endonucleases. Trends Genet. 12: 224–228. [DOI] [PubMed] [Google Scholar]
- Johnson-Schlitz, D. M., and W. R. Engels, 2006. The effect of gap length on double-strand break repair in Drosophila. Genetics 173: 2033–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler, K. J., T. Dray, J. E. Penney and G. B. Gloor, 1996. Gene targeting of a plasmid-borne sequence to a double-strand DNA break in Drosophila melanogaster. Mol. Cell. Biol. 16: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankenau, D. H., and G. B. Gloor, 1998. In vivo gap repair in Drosophila: a one-way street with many destinations. BioEssays 20: 317–327. [DOI] [PubMed] [Google Scholar]
- McElhinny, S. A. N., J. M. Havener, M. Garcia-Diaz, R. Juarez, K. Bevenek et al., 2005. A gradient of template dependence defines distinct biological roles for family X polymerases in nonhomlogous end joining. Mol. Cell 19: 357–366. [DOI] [PubMed] [Google Scholar]
- McVey, M., D. Radut and J. J. Sekelsky, 2004. Evidence for multiple cycles of strand invasion during repair of double-strand gaps in Drosophila. Genetics 167: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow, D. M., C. Connelly and P. Hieter, 1997. “Break copy” duplication: a model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics 147: 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyrers, J. P., Y. Zhang and A. F. Stewart, 2001. Techniques: recombinogenic engineering—new options for cloning and manipulating DNA. Trends Biochem. Sci. 26: 325–331. [DOI] [PubMed] [Google Scholar]
- Nassif, N., J. Penney, S. Pal, W. R. Engels and G. B. Gloor, 1994. Efficient copying of nonhomologous sequences from ectopic sites via P-element-induced gap repair. Mol. Cell. Biol. 14: 1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstein, A., A. Pare, L. Kaplan and S. Small, 2005. Site-specific transgenesis by Cre-mediated recombination in Drosophila. Nat. Methods 2: 583–585. [DOI] [PubMed] [Google Scholar]
- Paques, F., and J. E. Haber, 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63: 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, C., and M. Jasin, 2000. Coupled homologous and nonhomologous repair of a double-strand break preserves genome integrity in mammalian cells. Mol. Cell. Biol. 20: 9068–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, C., M. E. Moynahan and M. Jasin, 1998. Double-strand break repair by interchromosomal recombination: suppression of chromosomal translocations. Genes Dev. 12: 3831–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, Y. S., and K. G. Golic, 2000. Gene targeting by homologous recombination in Drosophila. Science 288: 2013–2018. [DOI] [PubMed] [Google Scholar]
- Rong, Y. S., and K. G. Golic, 2003. The homologous chromosome is an effective template for the repair of mitotic DNA double-strand breaks in Drosophila. Genetics 165: 1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, Y. S., S. W. Titen, H. B. Xie, M. M. Golic, M. Bastiani et al., 2002. Targeted mutagenesis by homologous recombination in Drosophila melanogaster. Genes Dev. 16: 1568–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gomez, M., and J. Modolell, 1987. Deletion analysis of the achaete-scute locus of Drosophila melanogaster. Genes Dev. 1: 1238–1246. [DOI] [PubMed] [Google Scholar]
- Sekelsky, J. J., M. H. Brodsky and K. C. Burtis, 2000. DNA repair in Drosophila: insights from the Drosophila genome sequence. J. Cell Biol. 150: F31–F36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling, A. C., 1986. Drosophila: A Practical Approach. IRL Press, Oxford.
- Staveley, B. E., T. R. Heslip, R. B. Hodgetts and J. B. Bell, 1995. Protected P-element termini suggest a role for inverted-repeat-binding protein in transposase-induced gap repair in Drosophila melanogaster. Genetics 139: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken, K. J., Y. He, R. A. Hoskins and H. J. Bellen, 2006. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314: 1747–1751. [DOI] [PubMed] [Google Scholar]
- Wang, X., G. Ira, J. A. Tercero, A. M. Holmes, J. F. X. Diffley et al., 2004. Role of DNA replication proteins in double-strand break-induced recombination in Saccharomyces cerevisiae. Mol. Cell. Biol. 24: 6891–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer, E. A., 2003. Innovations: applications of insect transgenesis. Nat. Rev. Genet. 4: 225–232. [DOI] [PubMed] [Google Scholar]
- Yasothornsrikul, S., W. J. Davis, G. Cramer, D. A. Kimbrell and C. R. Dearolf, 1997. viking: identification and characterization of a second type IV collagen in Drosophila. Gene 198: 17–25. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., J. P. Muyrers, G. Testa and A. F. Stewart, 2000. DNA cloning by homologous recombination in Escherichia coli. Nat. Biotechnol. 18: 1314–1317. [DOI] [PubMed] [Google Scholar]