Abstract
Inactivation of the dead-end (Dnd1) gene in the Ter mouse strain results in depletion of primordial germ cells (PGCs) so that mice become sterile. However, on the 129 mouse strain background, loss of Dnd1 also increases testicular germ cell tumor incidence in parallel to PGC depletion. We report that inactivation of Dnd1 also affects embryonic viability in the 129 strain. Mouse Dnd1 encodes two protein isoforms, DND1-isoform α (DND1- α) and DND1-isoform β (DND1-β). Using isoform specific antibodies, we determined DND1-α is expressed in embryos and embryonic gonads whereas DND1-β expression is restricted to germ cells of the adult testis. Our data implicates DND1-α isoform to be necessary for germ cell viability and therefore its loss in Ter mice results in PGC depletion, germ cell tumor development and partial embryonic lethality in the 129 strain.
Keywords: testicular germ cell tumors, dead-end, Dnd1, isoform, antibodies
Introduction
The 129-Ter mouse strain develops testicular germ cell tumors (TGCTs) similar to congenital tumors which occur in the testes of human infants (testicular type I germ cell tumors) [1; 2; 3]. Tumors in the 129-Ter strain develop from primordial germ cells (PGCs) during embryonic development [4; 5; 6; 7]. A progressive loss of PGCs is observed in Ter mice starting at embryonic day (E) 8.5 [8]. Consequently Ter/Ter mice are sterile at birth. However, in males, some of the PGCs escape death and become transformed to embryonal carcinoma (EC) cells. Clusters of proliferating EC cells are first detected at E15.5 within the embryonic gonads [9; 10]. The proliferating EC cells disrupt the normal architecture of the gonads. Soon after birth, the EC cells differentiate into a random mix of differentiated tissues that constitute the tumors.
These effects of Ter have been identified to be due to inactivation of the dead-end (Dnd1) gene [11]. However, tumor development of Ter mice occurs in a strain specific manner such that 94% of 129-Ter/Ter mice develop testicular tumors. On other or mixed strain backgrounds, loss of functional Dnd1 results only in PGC depletion and consequently, sterility in Ter/Ter adults but no significant incidence of germ cell tumor development. The mechanism as to how the loss of Dnd1 leads to primordial germ cell death or tumor development is unknown.
Dnd1 is expressed in PGCs after E7.25 [12]. Widespread expression of Dnd1 transcript is also detected in the early embryo after E7.5 [11]. Here, we report that inactivation of Dnd1 also affects embryonic viability of 129-Ter mice.
The mouse Dnd1 gene encodes two protein isoforms, named DND1-isoform α and DND1-isoform β (or DND1-α and DND1-β, respectively, Fig.1A). They arise due to alternate splicing of transcripts (Fig.1A).
Figure 1. The mouse DND1-α and DND1-β protein isoforms.
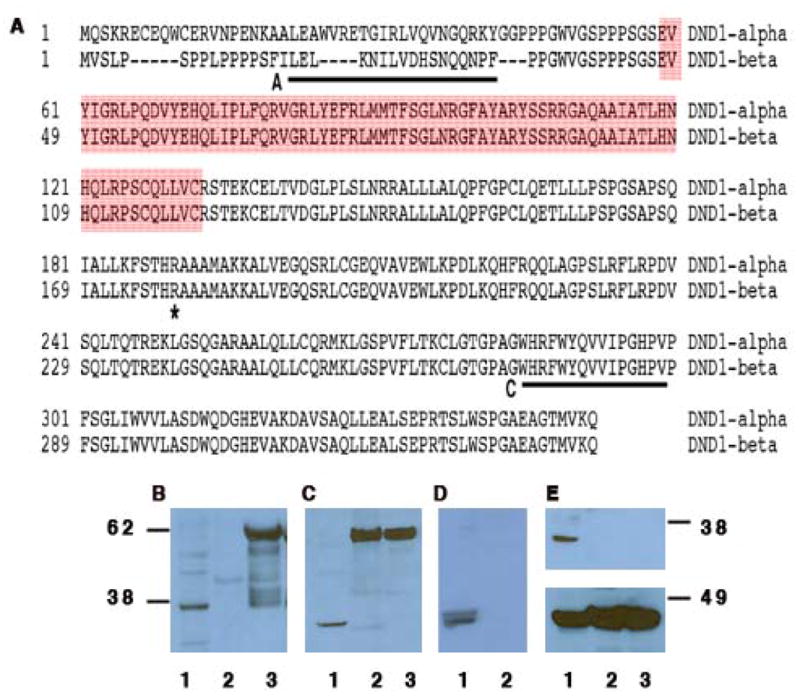
(A) Sequence comparison of DND1 isoforms (GenBank:AAQ63636 and AAH34897). A and C (underlined) mark the sequences to generate antibody A and C. The red box indicates the RNA recognition motif. The asterisk (*) marks the amino acid (R) that is mutated to a stop codon in Ter mice. (B) Western blotting using antibody A of testes lysate (lane 1); GST-DND1-α (lane 2) and GST-DND1-β (lane 3). (C) Western blotting using antibody C of testes lysate (lane 1); GST-DND1-α (lane 2) and GST-DND1-β (lane 3). (D) Western blotting using both antibody A and C of normal testes (lane 1) and spleen (lane 2). (E) (top panel) Western blotting using antibody C of normal testes (lane 1); germ cell deficient testes from Ter/Ter (lane 2); testicular tumor from Ter/Ter (lane 3). Rehybridization of the blot with anti-βactin antibody (bottom panel).
We wished to determine if both DND1 isoforms are involved in germ cell tumor development. Using antibodies that detect each DND1 isoform, we found DND1-α expression in embryonic cells and tissues whereas DND1-β expression is restricted to germ cells of the adult testis. We therefore pinpoint that loss of DND1-α in Ter mice is responsible for PGC loss, germ cell tumor development and partial embryonic lethality.
Materials and methods
Generation of antibodies
Rabbit polyclonal anti-peptide antibody-A (BioSource, MA) was against amino acids 16-33 of DND-β [11] (Ac-CILELKNILVDHSNQQNPF-amide) and Antibody-C against amino acids 285–299 of DND1-α or 273–287 of DND1-β (Ac-WHRFWYQVVIPGHPVC-amide). Antibodies were characterized by immunoblottting against tissue lysates known to express DND1, GST-DND1 and by peptide blocking of the antibody prior to hybridization.
Western blotting
This was carried out as described [11] using 25–100 μg protein electrophoresed on 4-12% NuPAGE gradient gels (Amersham-Pharmacia Biotech) before transfer onto membranes.
GST (glutathione S-transferase)-DND1 fusion proteins
Dnd1 cDNA (AAH34897 and AAQ63636, respectively) were cloned into pGEX-2TK (amersham pharmacia biotech) [11].
Mouse strains and tissue collection
129-Ter (129T1/Sv-+pTyrc-ch Ter/+@Na) and B6.129-Ter have been described [11]. To collect embryos, females were checked for plugs after timed matings (embryos of newly plugged females are denoted E 0.5). Pregnant females were sacrificed on the 13th and 15th day of pregnancy and dissected to obtain embryos. 4–6 embryos were pooled for protein extraction. E13.5 and E15.5 embryos were dissected to obtain embryonic testes. 4–8 pairs of embryonic testes were pooled for protein extraction.
Cell lines
Sertoli cell lines TM4 (ATCC number CRL-1715), 15P-1 (ATCC number CRL-2618) and MSC1 were cultured as described [13]. EG cells were maintained and passaged on Mitomycin-C arrested primary MEFs (PMEF-CF, Specialty Media, NJ) on media supplemented with LIF (1000 u/mL) and βFGF (1 ng/mL) [14]. EG cells were checked by staining with alkaline phosphatase chromogen (Fast Red tablets, abcam) (data not shown). G4 ES cell lines were passaged 2 times on feeder-free gelatin coated plates to remove MEF cells. COS-7 and HeLa were from ATCC.
Fluorescent tagged DND1
Dnd1 cDNA (AAH34897 or AAQ63636) were cloned into pEGFP-C1 (BD Biosciences Clontech). The expression plasmids, GPF-DND1-α or -β were transfected separately into cells and visualized 48 h later using Zeiss LSM 510 Confocal Microscope.
RT-PCR for Dnd1 transcripts
A 366 bp product from Dnd1-α whereas a 700 bp product Dnd1-β was amplified using primers Dnd10-F and Dnd10-R (5’-ATGCAGTCCAAACGGGAGTGCGAG-3’ and 5’-CTGGTGGTTGTGCAGCGTAGC-3’, respectively). Primers for hypoxanthine phosphoribosyltransferase (HPRT) were: HPRT-F (5’-GTTGAGAGATCATCTCCACC-3’) and HPRT-R (5’-AGCTATGATGAACCAGGTTA-3’).
Separation of Germ Cells
Germ cells were separated from cell suspensions prepared from adult testes of five C57BL/6J mice according to general procedures described previously [15]. Three fractions were obtained containing 84% pachytene primary spermatocytes, 90% round spermatids, and a mixture of late spermatids (14%) and cytoplasmic fragments (85%), 90% of which are detached from the late spermatids cells [16]. Hence this fraction can be considered as a relatively pure fraction of late spermatid nuclear and cytoplasmic material. The three fractions collected were concentrated and washed with PBS. Cells were lysed and 50 mg used for western blotting.
Results
Antibodies A and C detect mouse DND1-β and -α isoforms, respectively
We previously characterized, antibody A, a polyclonal antibody against DND1-β (Fig. 1A) [11]. We report here of a second antibody, antibody C (Fig. 1A) designed to detect both DND1-α and DND1-β.
Western blotting indicated that, as expected, antibody A detects only bacterially expressed, recombinant GST-DND1-β as well as a single band from normal testes, DND1-β (Fig.1B). Antibody C, as expected, detects both recombinant GST-DND1-α and GST-DND1-β (Fig.1C). However, antibody C detects a single band from normal mouse testes (Fig.1C) and which is slightly lower in size than the band detected by antibody A (Fig.1B). The two DND1 isoforms are apparent in testes lysate when the membrane is hybridized with both antibody A and C simultaneously (Fig.1D). Thus, although antibody C is able to detect both recombinant GST-DND1-α and −β proteins, it only detects one isoform of DND1 from the mouse testes. Antibody C likely detects the other DND1 isoform, DND1-α, because the band is close to but of different size compared to DND1-β as detected by antibody A.
Based on the amino acid composition, DND1-α is theoretically 39.1 KDa and DND1-β is 37.5 KDa. However, experimentally, electrophoresis of testes lysates on NuPAGE MES (Invitrogen) gels followed by western blotting with Antibody A or C shows a single distinct band near the 38 KDa marker (Fig. 1B and C). Moreover, the band for DND1-β is slightly larger in size compared to DND1-α (comparing lanes 1 of Fig. 1B to C) and thus DND1-β migrates anomalously on the NuPAGE MES (Invitrogen) gels. A likely explanation is that DND1-β is extensively post-translationally modified. This would explain its anomalously larger size compared to DND1-α on gels as well as why DND1-β cannot be detected by Antibody C.
Taking together the above observations, we conclude that antibody A detects DND1-β and antibody C detects DND1-α. Both antibodies were tested for specificity for DND1 by previously incubating the antibody with the cognate peptide (peptide blocking) so as to block recognition of recombinant GST-DND1 and testis DND1 (data not shown). In many instances (experiments described below), we hybridized each membrane with antibody A and rehybridized with antibody C. Neither of the antibodies detected DND1 from germ cell deficient testis or tumors from Ter/Ter mice (Fig.1E, top panel) or from normal spleen (Fig.1D) where Dnd1 is not expressed [11].
DND1-α is expressed in mouse embryos and embryonic gonads
To determine which isoform of DND1 is responsible for germ cell tumor development, we utilized the isoform specificity of the two antibodies, A and C, to determine the expression patterns of DND1 in the primordial gonads of the mouse. Western blotting carried out on lysates from embryonic testes at E13.5 and E15.5 detected DND1-α but not isoform β (Fig.2A, top and middle panels). Germ cell tumors start developing around E13.5 in the embryonic testes [9; 10]. As only DND1-α is detected in embryonic testes at these stages, this implies loss of DND1-α to be the cause of germ cell tumor development in Ter mice.
Figure 2. Expression of DND1-α and DND1-β in embryonic tissues.
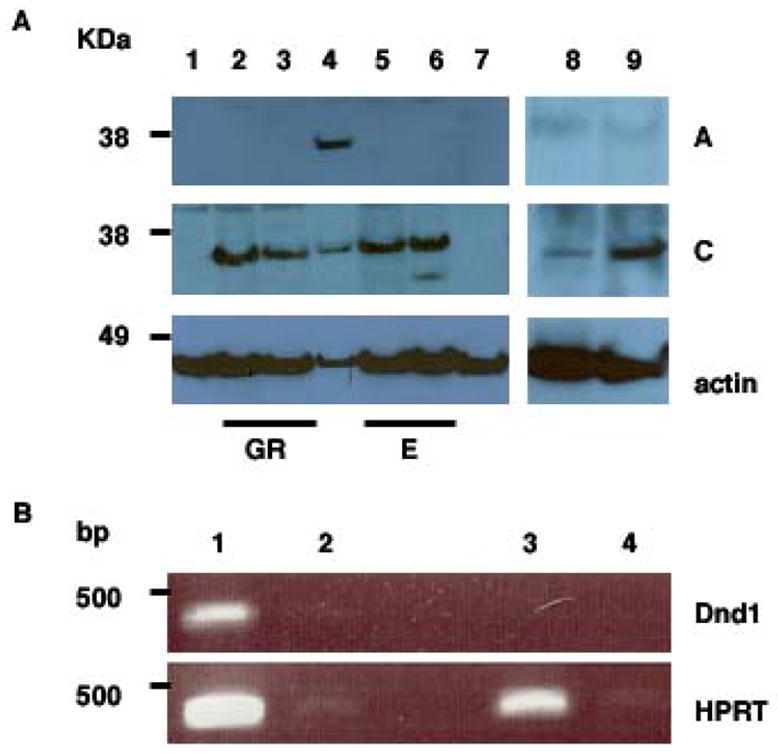
(A) Lysates from embryonic testes (GR) at E13.5 (lane 2) and E15.5 (lane 3), adult testes (lane 4), E13.5 embryo (lane 5), E15.5 embryo (lane 6), EG (lane 8) and ES cells (lane 9), were electrophoresed before western blotting with antibody A (top panel); antibody C (middle panel) and anti-β actin (bottom panel). Negative control: mouse spleen (lane 1) and germ cell tumor lysates from Ter/Ter mice (lane 7). GR = embryonic testis/genital ridges; E = embryos.
(B) (top panel) RT-PCR of total RNA from EG cells (lanes 1 and 2) grown on Mitomycin-C treated MEF cells and Mitomycin-C treated MEF cells alone (lanes 3 and 4). Lanes 2 and 4 are control lanes where no superscript was added during RT. (bottom panel) Control RT-PCR for HPRT.
To examine expression of DND1 in germ cells, we used embryonic germ (EG) cells (Fig. 2A) grown in culture. EG cells are derived from PGCs and resemble their founders in many respects [17; 18; 19; 20]. We detected low levels of DND1-α in EG cells (Fig. 2A, lane 8, middle panel). To facilitate detection of DND1, the protein concentration of the EG cell lysates was increased to 100 μg. However, because EG cells are grown on a feeder layer of Mitomycin-C arrested primary mouse embryo fibroblasts and lysates were made directly off the cell culture plate, it is likely that we are underestimating DND1 levels in EG cells. Therefore, we examined Dnd1 mRNA transcripts by RT-PCR on mRNA from EG cells grown on feeder layers and were able to readily detect expression of Dnd1-α transcripts (Fig. 2B). Dnd1 was not detected in mRNA derived from Mitomycin-C arrested primary mouse embryo fibroblasts (PMEF) alone. Thus, our data indicates that DND1 is expressed in EG cells. Additionally, quantitative single-cell gene expression analysis techniques have detected Dnd1 transcript in PGCs after E7.25 onwards [12]. Future work will examine protein expression levels of DND1 from purified isolated PGCs of mouse embryos.
Because ES (embryonic stem) cells share many common features including gene expression patterns with EG, PGCs and EC (embryonal carcinoma) cells [17; 18; 19; 20; 21; 22], we examined DND1 expression in one ES cell line (G4 line) and found DND1-α but no DND1-β expression (Fig.2A, lane 9). The ES cell lysates were extracted from G4 cells passaged twice on gelatin-coated plates to eliminate most of the feeder cells. Thus, DND1-α is also expressed in other types of pluripotent mouse cell lines.
Western blotting on lysates derived from mouse embryos at E13.5 and E15.5 detected expression of DND1-α but not DND1-β (Fig.2A).
Partial embryonic lethality of Ter/Ter on the 129 mouse strain background
Both in situ hybridization [11] and western blotting, as shown here, indicate that DND1 (DND-α ) is expressed in mouse embryos. However, although DND1 is expressed in the early embryo, no obvious effects on development have been reported when DND1 expression is inactivated in the Ter/Ter mice. We genotyped the progeny of 129-Ter/+ intercrosses and found that there was a 4-fold reduction in the expected numbers of Ter/Ter mice (P< 0.001) (Table 1). The expected number of 129/Sv-Ter/+ progeny were also decreased. We have not observed significant postnatal death of progeny after birth or upon weaning in the 129-Ter/+ colony and thus the death of a proportion of the Ter/Ter mice likely occurs before birth.
Table 1. Comparing genotypes of progeny derived from 129-Ter to B6.129-Ter.
Comparing the number of Ter/Ter progeny derived from the 129-Ter strain to that from a non-129 strain (the B6.129-Ter strain). The B6.129-Ter strain contains a 5 Mb region from 129, containing the Ter mutation, which was made congenic on a C57Bl/6J strain background. Crosses were set up using heterozygote (Ter /+) parents. Adult progeny of both sexes were genotyped.
| Genotype
|
||||||
|---|---|---|---|---|---|---|
| +/+ | Ter/+ | Ter/Ter | Total no. examined | χ2 | P | |
| 129 -Ter | 62 | 98 | 17 | 177 | 23.6 | < 0.001 |
| B6.129 -Ter | 39 | 84 | 41 | 164 | 0.2 | 0.2 |
We also examined crosses of Ter/+ on other strain backgrounds (B6.129-Ter strain). In these crosses, normally expected numbers of Ter/Ter progeny were present (Table 1). Thus, partial embryonic lethality of Ter/Ter mice occurs only on the 129 strain background.
Differential expression of DND1-α and –β in testes of postnatal mice
To determine the pattern of expression of DND1 in postnatal testes, we performed western blotting of lysates from postnatal (PN) testes collected from mice at intervals from PN1 to PN 50. Results indicated that DND1-α is expressed continuously in PN testes although levels are low between post natal day 1 (PN1) to PN6. DND1-β is expressed from PN 20 onwards (Fig.3A and B).
Figure 3. Expression of DND1-α and DND1-β adult testis.
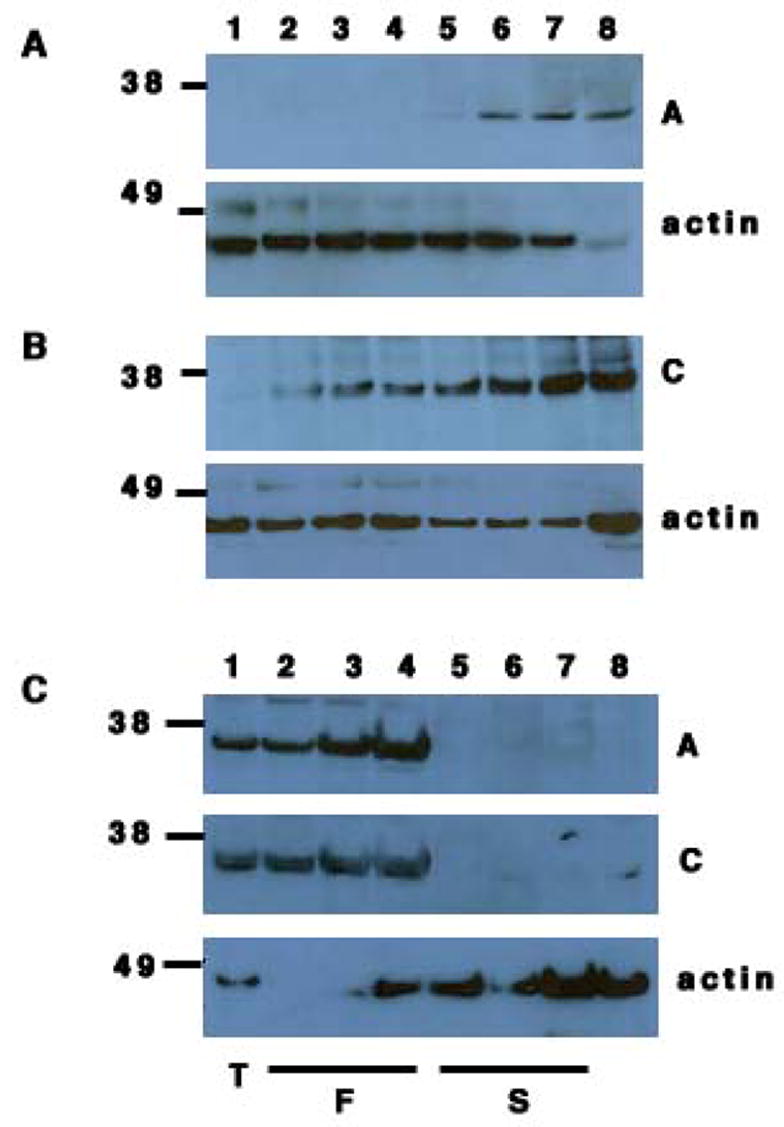
(A) Western botting of lysates from post-natal testes of day 1, 6, 10, 15, 20, 25, 30 and 50 (lanes 1, 2, 3, 4, 5, 6, 7 and 8 respectively) with antibody A (top panel) and anti-β actin (bottom panel).
(B) Western blotting of lysates from post-natal testes of day 1, 6, 10, 15, 20, 25, 30 and 50 (lanes 1, 2, 3, 4, 5, 6, 7 and 8 respectively) with antibody C (top panel) and anti-β actin (bottom panel).
(C) Western blotting of lysates from adult testes (lane 1), fractionated cells from adult testes: pachytene spermatids (lane 2), round spermatids (lane 3) and elongated spermatids (lane 4); Sertoli cell lines, TM4 (lane 5), 15P-1 (lane 6), MSC1 (lane 7) and germ cell deficient Ter/Ter testis (lane 8) with antibody A (top panel); antibody C (middle panel) and anti-β actin (bottom panel).
To test the expression of DND1 in post-meiotic germ cells of adults, we examined elutriated pachytene spermatids, round spermatids and elongated spermatids from adult mouse testes of the C57Bl/6J strain. Higher expression of DND1-β was at the elongated spermatid stage (Fig.3C). This suggests a role of DND1-β in post-meiotic adult germ cells whereas DND1-α is present and likely required at almost all stages of germ cell development.
Neither DND1-α nor -β was detected by Western blotting of the Sertoli cell lines (Fig. 3C) TM4, 15P-1 and MSC1 [13]. Thus, expression of DND1 is restricted to the germ cells of adult testes.
Subcellular localization of DND1 in mammalian cells
We next examined the subcellular localization of mouse DND1 in mammalian cells. Expression constructs encoding GFP-DND1-α and -β were transfected into COS-7 and HeLa cells prior to examination by confocal microscopy. In COS-7 cells, both DND1-α and -β were localized to the cytoplasm (Fig.4A and B). However, in HeLa cells, both isoforms of the GFP-DND1 localized to the nucleus (Fig.5C and data not shown). DND1 shows homology with ACF (apobec-1 complementation factor). ACF possesses a canonical SV40-like nuclear localization signal as well as a novel 41-residue nuclear localization signal (ANS) [23] that allows it to actively shuttle between the cytoplasm and nucleus. However, DND1 lacks the ANS and shows no significant homology to the SV40-like nuclear localization signal. Thus, the mechanism as to how mouse GFP-DND1 migrates to the nucleus of some cell types is at present not understood.
Figure 4. Mammalian DND1 localizes to the cytoplasm or nucleus in different cell types.
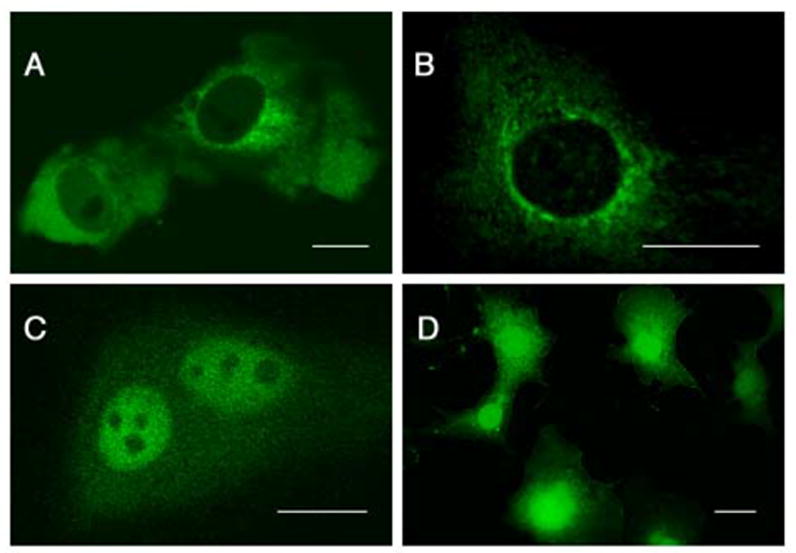
Subcellular localization of mouse GFP-DND1-α (A) and GFP-DND1-β (B) in COS-7 cells and He La cells (C). Control transfection of vector encoding GFP only (D) in COS-7 results in GFP expression in both cytoplasm and nucleus. Line indicates 20 μm.
Discussion
We had previously identified that inactivation of Dnd1 causes PGC loss and germ cell tumor development in the Ter mouse strain. Two isoforms of Dnd1 are detected in adult testes [11]. We initially generated an antibody against DND1-β isoform [11]. Here, we report generation of antibody against DND1-α.
Although antibody C should theoretically recognize both isoforms of DND1, it was only able to recognize DND1-α from the tissue lysates. Moreover, DND1-β appears to be larger in size than expected. These two observations lead us to conclude that DND1-β is likely post-translationally modified, thus explaining its anomalous larger size and inability to be detected by antibody C. Future work will focus on the experimental identification of the post-translational modifications of DND1-β that blocks its recognition by Antibody C.
Mouse embryonic gonads at E13.5 and E15.5 express DND1-α. As germ cell tumors in mice develop at around E13.5, this observation implicates loss of DND1-α in Ter mice to be the cause of tumor development. We examined EG cells in culture [18] for DND1 expression and detected low levels of DND1-α but no DND1-β expression. Dnd1 transcripts were detected by RT-PCR of EG cell RNA and other investigators have reported presence of Dnd1 transcripts in PGCs at E7.25 [12]. PGC death starts at E8.5 in Ter mice [8] and PGC numbers start to decline gradually from this stage onwards. Taking these observations together implicates loss of DND1-α as the cause of PGC death at E8.5 onwards in the Ter mice.
We show that DND1-α is expressed in embryos at E13.5 and E15.5 thus confirming the previous in situ hybridization data on whole embryos [11]. However, the developmental consequences of lack of Dnd1 in embryos have not been examined rigorously. When we examined the 129-Ter colony, we found a deficit of Ter/Ter progeny due to partial embryonic lethality of Ter/Ter mice. On other strain backgrounds, lethality of Ter/Ter is not observed suggesting that modifiers or compensatory factors that rescue embryonic lethality are present in these strains. The embryonic lethality of 129- Ter/Ter mice is likely due the role of Dnd1 in critical organ systems in the developing embryo. For example, in situ hybridization indicated Dnd1 expression in the ventral neuroectoderm at E8.5 and in the head mesenchyme and first branchial arch at E9.5. The stage at which this partial embryonic lethality occurs remains to be determined.
Our studies show that DND1-α is expressed continuously in testes of postnatal (PN) mice. Although DND1 has been shown to be required for PGC viability in zebrafish, xenopus and mouse, our data suggests that DND1-α could be required for viability of postnatal and adult germ cells of the mouse as well. DND1-β is expressed from PN 20 onwards in meiotic and in post-meiotic adult germ cells of the testes with highest expression at the elongated spermatid stage.
We found that mouse DND1 localizes to the cytoplasm or the nucleus depending on the mammalian cell type. The sub-cellular localization of mouse DND1 may be a reflection of the rate of shuttling of GFP-DND1 between the two cellular compartments in different cell types or it may be an inherent consequence of the cell type. Future work will examine the subcellular distribution of DND1 in different cell types of the mouse embryo. This may provide clues about DND1 function in different tissues and cell types during development.
In summary, isoform specific antibodies show that DND1-α is expressed in the early embryo and gonads. Our data implicates loss of DND1-α to cause PGC death as well as testicular tumor development and partial embryonic lethality in the 129-Ter strain.
Acknowledgments
We thank M. Wilkinson for the Sertoli cell lines, P.Donovan for mouse EG cell line and R. Behringer for the ES cells. We thank A. Matera for verifying the localization of GFP-DND1 in mammalian cells. This work was supported by NIH RO1CA93754 and David M. Carmines Cancer Research Fund to AM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oosterhuis JW, Looijenga LHJ. Testicular germ-cell tumours in a broader perspective. Nature Rev Cancer. 2005;5:210–222. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- 2.Rescorla FJ. Pediatric germ cell tumors. Semin Surgical Oncology. 1999;16:144–158. doi: 10.1002/(sici)1098-2388(199903)16:2<144::aid-ssu6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367:754–765. doi: 10.1016/S0140-6736(06)68305-0. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi T, Noguchi M. A recessive mutation (ter) causing germ cell deficiency and a high incidence of congenital testicular teratomas in 129/Sv-ter mice. J Natl Cancer Inst. 1985;75:385–392. [PubMed] [Google Scholar]
- 5.Stevens LC. A new inbred subline of mice (129/terSv) with a high incidence of spontaneous congenital testicular teratomas. J Natl Cancer Inst. 1973;50:235–242. doi: 10.1093/jnci/50.1.235. [DOI] [PubMed] [Google Scholar]
- 6.Pierce GB, Stevens LC, Nakane PK. Ultrastructural analysis of the early development of teratocarcinoma. J Natl Cancer Inst. 1967;39:755–773. [PubMed] [Google Scholar]
- 7.Jiang LI, Nadeau JH. 129/Sv mice - a model system for studying germ cell biology and testicular cancer. Mamm Genome. 2001;12:89–94. doi: 10.1007/s003350010257. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai T, Iguchi T, Moriwaki K, Noguchi M. The ter mutation first causes primordial germ cell deficiency in ter/ter mouse embryos at 8 days of gestation. Develop Growth Differ. 1995;37:293–302. doi: 10.1046/j.1440-169X.1995.t01-2-00007.x. [DOI] [PubMed] [Google Scholar]
- 9.Stevens LC. Testicular teratomas in fetal mice. J Natl Cancer Inst. 1962;28:247–267. [PubMed] [Google Scholar]
- 10.Stevens LC. The origin and development of testicular, ovarian and embryo-derived teratomas. Cold Spring Harbor Conferences on Cell Proliferation: Teratocarcinoma. Stem Cells. 1983;10:23–36. [Google Scholar]
- 11.Youngren KK, Coveney D, Peng X, Bhattacharya C, Schmidt LS, Nickerson ML, Lamb BT, Deng JM, Behringer RR, Capel B, Rubin EM, Nadeau JH, Matin A. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature. 2005;435:360–364. doi: 10.1038/nature03595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yabuta Y, Kurimoto K, Ohinata Y, Seki Y, Saitou M. Gene expression dynamics during germline specification in mice identified by quantitative single-cell gene expression profiling. Biol Reprod. 2006;75:705–716. doi: 10.1095/biolreprod.106.053686. [DOI] [PubMed] [Google Scholar]
- 13.Selva DM, Hirsch-Reinshagen V, Burgess B, Zhou S, Chan J, McIsaac S, Hayden MR, Hammond GL, Vogl AW, Wellington CL. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J Lipid Res. 2004:1040–1050. doi: 10.1194/jlr.M400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.De Miguel MP, Donovan PJ. Isolation and culture of embryonic germ cells. Methods Enzymology. 2003;365:353–363. [PubMed] [Google Scholar]
- 15.Meistrich ML. Separation of spermatogenic cells and nuclei from rodent testes. Methods Cell Biol. 1977;15:15–54. doi: 10.1016/s0091-679x(08)60207-1. [DOI] [PubMed] [Google Scholar]
- 16.Meistrich ML, Longtin J, Brock WA, Grimes SR, Mace ML. Further purification of rat testicular cells and preliminary biochemical analysis of these cells. Biol Reprod. 1981;25:1065–1077. doi: 10.1095/biolreprod25.5.1065. [DOI] [PubMed] [Google Scholar]
- 17.Matsui Y, Zsebo K, Hogan LMB. Derivation of pluripotent embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 18.Resnick JL, Bixler LS, Cheng L, Donovan PJ. Long-term proloferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- 19.Stewart CL, Gadi I, Bhatt H. Stem cells from primordial germ cells can reenter the germ line. Dev Biol. 1994;161:626–628. doi: 10.1006/dbio.1994.1058. [DOI] [PubMed] [Google Scholar]
- 20.Tada T, Tada M, Hilton K, Barton SC, Sado T, Takagi N, Surani MA. Epigenotype switching of imprintable loci in embryonic germ cells. Dev Genes Evol. 1998;207:551–561. doi: 10.1007/s004270050146. [DOI] [PubMed] [Google Scholar]
- 21.Rohwedel J, Sehlmeyer U, Shan J, Meister A, Wobus AM. Primordial germ cell-derived embryonic germ (EG) cells in vitro resemble undifferentiated stem cells with respect to differentiation capacity and cell cycle distribution. Cell Biol Int. 1996;20:579–587. doi: 10.1006/cbir.1996.0076. [DOI] [PubMed] [Google Scholar]
- 22.Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, Jones SB, Brooks JD, Andrews PW, Brown PO, Thomson JA. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci U S A. 2003;100:13350–13355. doi: 10.1073/pnas.2235735100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanc V, Kennedy S, Davidson NO. A novel nuclear localization signal in the auxiliary domain of Apobec-1 complementation factor regulates nucleocytoplasmic import and shuttling. J Biol Chem. 2003;278:41198–41204. doi: 10.1074/jbc.M302951200. [DOI] [PubMed] [Google Scholar]


