Abstract
Greater continuity in cognition between children and adults may exist than is usually appreciated. It was thought that after 3 to 4 years of age, the problem in switching on the dimensional-change card-sort task disappears. We show here, however, that if speed is used as the dependent measure, the effect of the first dimension is evident even in adults. Adults, like preschoolers, show difficulty in switching from a block of sorting by color or shape to a block of sorting by the other dimension. Notably, performance throughout the session was affected by the first dimension by which stimuli were sorted. We hypothesize that perhaps adults never fully outgrow any of the cognitive and perceptual biases of infancy and early childhood. Other examples of such biases that appear to still be present in adults are discussed. Conversely, the assumption that the optimal dependent measure for adults is the most sensitive measure for children is questioned.
Adult observers are often astonished to see 3-year-olds fail the simple dimensional-change card-sort task (DCCS; Zelazo, Frye, & Rapus, 1996), especially because the children routinely indicate accurate knowledge of the rules for sorting and then promptly sort incorrectly. In this task, each card contains a simple line drawing of a familiar object (such as a truck or star) colored entirely in a primary color. Children of 3 years generally sort the cards by either color or shape without a single error. However, when asked to switch the criterion for sorting, most 3-year-olds continue to sort by the initially correct criterion. This is particularly striking because before every trial the tester either reminds the child of the sorting rules (e.g., “We are playing the color game now, and in the color game, red ones go here and blue ones go there”) or asks the child where the red ones (or trucks) go and where the blue ones (or stars) go, and the child points correctly. This pattern of performance was first observed by Zelazo and colleagues (Zelazo et al., 1996) at Yale University and has been replicated in labs in five countries (United States: Kirkham, Cruess, & Diamond, 2003; Munakata & Yerys, 2001; Canada: Bialystok & Martin, 2004; Zelazo, Mueller, Frye, & Marcovitch, 2003; Austria: Kloo & Perner, in press; England: Riggs & Williams, 2003; Scotland: Rennie, Bull, & Diamond, 2004). If children know and understand the rules, remember the rules, and remember what each sorting bin stands for, why do they err?
Preschoolers err, we contend, because their cognitive system is characterized by a degree of inertia (Kirkham et al., 2003). Having put in motion a mind-set in which blue trucks go with red trucks and red stars go with blue stars, 3-year-olds have difficulty disengaging that mind-set and adopting one in which what had previously been relevant is now irrelevant, and in which the responses that had previously been wrong are now right. Switching to the color game means that blue trucks now belong with blue stars. Three-year-olds who have just pointed to indicate that blue things go with the blue star, when handed a stimulus that is not only blue but a truck, typically put the blue-truck stimulus card with the red-truck model card, occasionally verbalizing their reasoning, “But it’s a truck.” Thus, they obey the rules of the game they had been playing (the shape game) but violate the rules of the game they acknowledge they should now be playing (the color game). (Exactly analogous results obtain if children first sort by color and are then asked to sort by shape.) We (Kirkham et al., 2003) coined the term “attentional inertia” to try to capture this tendency of the cognitive system to stay focused on what it had been focused on. Developmental psychologists universally report that by the age of 4 to 5 years, children “solve” the DCCS task, as evidenced by correct switching from sorting by either shape or color to sorting by the other dimension.
Not so fast! Here we report results from testing adults on this task. As is done with children, we took pains to remind participants of the relevant sorting dimension on each trial before the stimulus appeared. In this computerized version, we also kept the response icons visible throughout, so no one had to remember which response key went with which stimulus attribute. As the results show, adults can indeed switch from sorting by either color or shape to sorting by the other, but attentional inertia is still evident. It remains evident in longer response times (RTs) when the sorting criterion switches and in the persistence of faster RTs throughout the testing session when participants are sorting by the initially relevant dimension.
METHOD
Participants
Fifty-three undergraduates (45% female, 18–22 years of age, predominantly Caucasian of European descent) participated in exchange for extra course credit. The final sample consisted of 49 participants. Data from 4 participants were dropped from analyses because their RTs were more than 3 standard deviations above the mean. All participants were recruited from Cornell University undergraduate psychology courses and through a department-wide automated sign-up system for study participation. All gave informed consent. All were right-handed.
Stimuli
Four picture files were presented on a Power Macintosh computer. Each picture was in full color, with a resolution of 800 × 600 pixels, and measured 5 × 5 cm. The picture files consisted of two response icons (a red truck and a blue star) and two stimuli (a blue truck and a red star). The response icons (or model pictures) were positioned at the bottom of the screen throughout testing, with the red truck in the bottom left-hand corner and the blue star at the bottom right. Note that no stimulus matched a model picture on both color and shape. Thus, the correct response when sorting by color was always the wrong response for sorting by shape (and the correct response when sorting by shape was the wrong response for sorting by color). During a trial, the word “color” or “shape,” indicating the relevant sorting criterion for that trial, remained on the screen in black bold font, centrally located between the two response icons.
Procedure
Each participant sat 40 cm from the screen of a Power Macintosh computer, on which was displayed the two model pictures (a red truck and a blue star). The participant was instructed to press a key as quickly as possible in response to a matching criterion. Instructions appeared on the screen prior to the experiment:
This is a matching game. You match the picture at the center of the screen by its color or by its shape. A cue word will appear on the screen, letting you know whether to match by color or shape. Whenever you see the word “COLOR,” you will be playing the Color Game, and should press either the Red (“S”) key or Blue (“L”) key, depending on the color of the picture on the screen. Whenever you see the word “SHAPE,” you will be playing the Shape Game, and should press either the Truck (“S”) key or the Star (“L”) key. Please respond as quickly as possible!!
The response keys were located directly below their corresponding response icons on the screen. Participants were instructed to keep their fingers on the keys.
Each trial began with the presentation of a cue word specifying the trial’s matching criterion (color or shape). After 500 ms, the stimulus (a blue truck or a red star) appeared in the middle of the screen, 4 cm above the model pictures. Key-press responses were recorded by Psyscope 1.25. RT from stimulus onset to key press was measured in milliseconds. As soon as a key was pressed, the stimulus and cue word disappeared, leaving only the model pictures. There was an 800-ms intertrial interval, after which the next cue word appeared. Thus, there was an 800-ms responsecue interval and a 500-ms cue-stimulus interval, though the cue continued to be displayed when the target appeared. See Figure 1 for a schematic diagram of the testing paradigm.
Fig. 1.
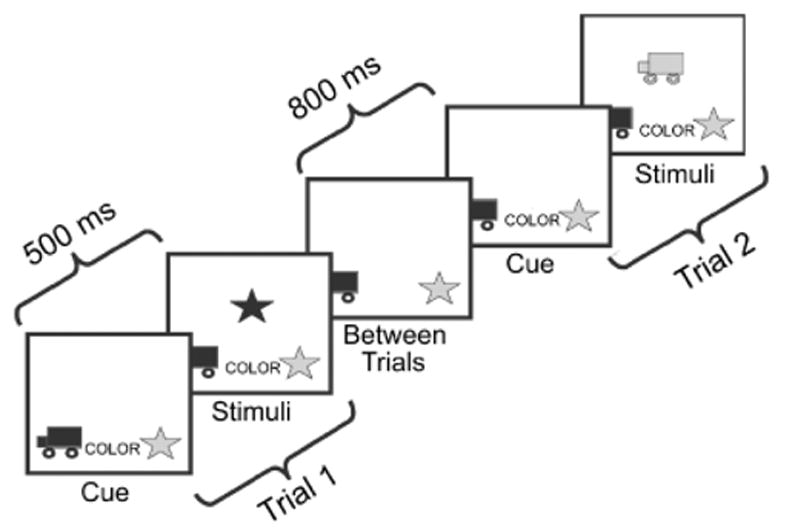
Schematic depiction of the dimensional-change card-sort test procedure used with adults in this study.
Given that the cue word remained on screen throughout each trial, participants did not have to remember the currently relevant rule; they could simply look at the word displayed. Similarly, because the response icons remained on screen throughout testing, participants did not have to remember which key corresponded to which stimulus properties. In this way, we attempted to minimize the memory demands of the task.
Participants completed 80 trials separated into blocks: 10 trials of the first dimension (color or shape; counterbalanced across participants), 10 trials of the second dimension (shape or color), 10 trials of the first dimension, 20 mixed-block trials (pseudorandomly intermixed color and shape trials with 13 nonswitch trials and 7 switch trials, a ratio of roughly 1½:1), 10 trials of the second dimension, 10 trials of the first dimension, and 10 trials of the second dimension.
The dependent measures were whether a response was correct or not and RT. Only trials on which subjects responded correctly were included in the RT analyses. The rare trials on which an RT was more than 2.5 standard deviations above the mean were dropped, as was the one trial on which the RT was less than 200 ms, too quick to have been in response to the stimulus.
RESULTS
Participants rarely erred. The mean percentage of correct responses was 94%. The range across participants was 89 to 100% across all trials, and 80 to 100% within individual blocks of trials. There was too little variation in accuracy for statistical analyses. Significant differences were found in RT, however. The opposite pattern of results has been found for children in task-switching studies (S. Cohen, Bixenman, Meiran, & Diamond, 2001), in which accuracy has been a more sensitive measure than RT, given the much greater RT variability in children than in adults.
When 3-year-old children perform the DCCS task with the same stimuli as used here, they perform brilliantly on the first single-task block (sorting by the first dimension), but have difficulty switching to the second dimension on the next single-task block. This is indicated by a marked increase in errors from Block 1 to Block 2 (see Fig. 2, top panel; Diamond, 2004; Kirkham et al., 2003). Although adults did not show this dramatic change in accuracy, they did show a drop in speed. Participants showed elevated RTs when switching from the first to the second dimension: RTs increased significantly from the last two trials of Block 1 to the first two trials of Block 2, F(1, 41) = 37.33, p < .001 (see Fig. 2). An effect-size analysis (J. Cohen, 1988) showed this to be a very large effect (d = 1.31). This RT switch cost parallels the switch cost that children of 3 years show in the accuracy of their responses.
Fig. 2.
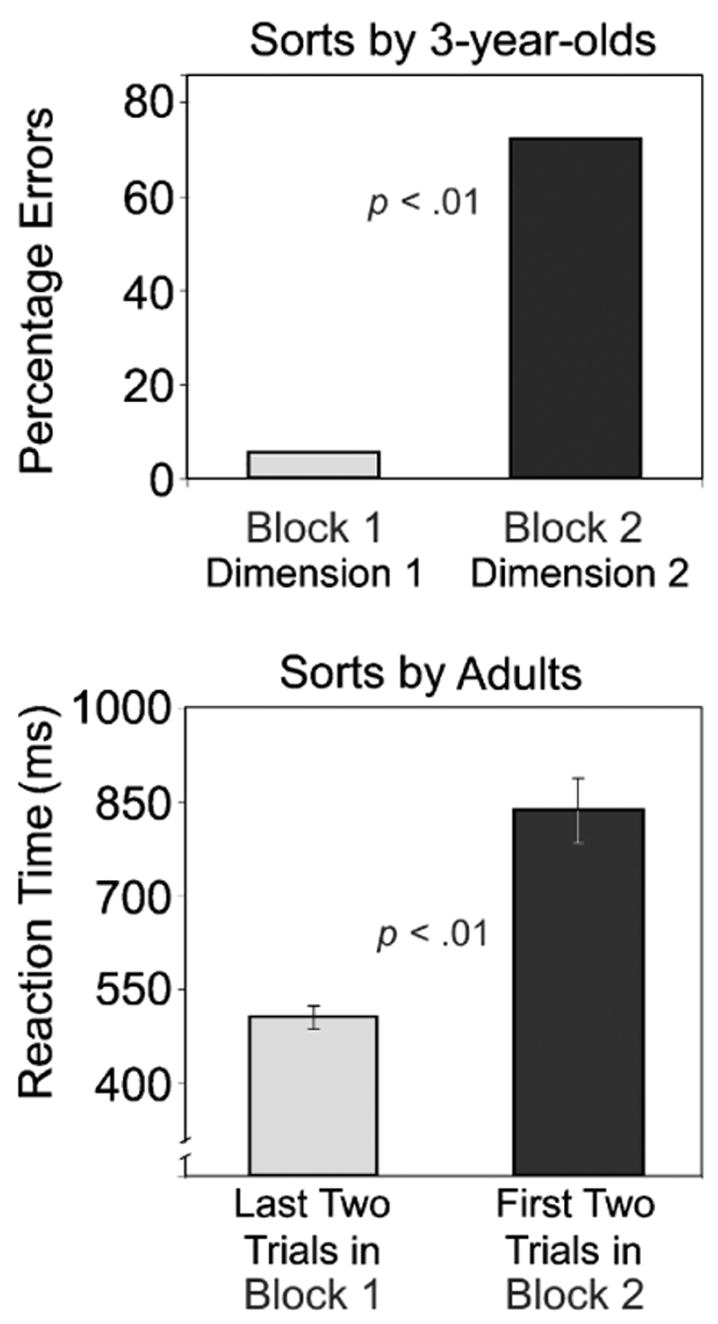
Cost in switching from sorting by color or shape in Block 1 to sorting by the other dimension in Block 2: percentage of 3-year-old children who sorted incorrectly in Block 1 versus Block 2 (top panel; from Diamond, 2004, a meta-analysis of all studies of children’s performance on the dimensional-change card-sort task) and reaction times for adults on the last 2 trials in Block 1 versus the first 2 trials in Block 2 (bottom panel).
Just as the same results are found for children whether the first dimension is color or shape, so, too, were RTs for our adult participants comparable on Block 1 whether the first dimension was color (mean RT = 571 ms) or shape (mean RT = 605 ms), and so were the RT costs of switching sorting criteria comparable whether the switch was to color or to shape.
Not surprisingly, adults were significantly slower during the mixed-task block, in which they had to switch back and forth between matching by color and by shape, than they were on the single-task blocks. RTs on the mixed block (mean = 727 ms) were significantly longer than RTs on blocks in which subjects sorted by a single dimension, F(1, 46) = 69.0, p < .001, d = 0.78. RTs on just the subset of trials within the mixed block that did not involve switching were still significantly longer than RTs on the preceding single-task blocks, F(1, 46) =18.81, p <.001, d =0.5. Figure 3 shows the RTs broken down by block. Post hoc comparisons showed that participants were significantly slower in the mixed block than in all other blocks (Tukey’s LSD, all ps < .05) except Block 2, the first instance when subjects had to switch tasks. The effect of having performed the mixed-task block continued into the single-task blocks that followed, only slowly dissipating.
Fig. 3.
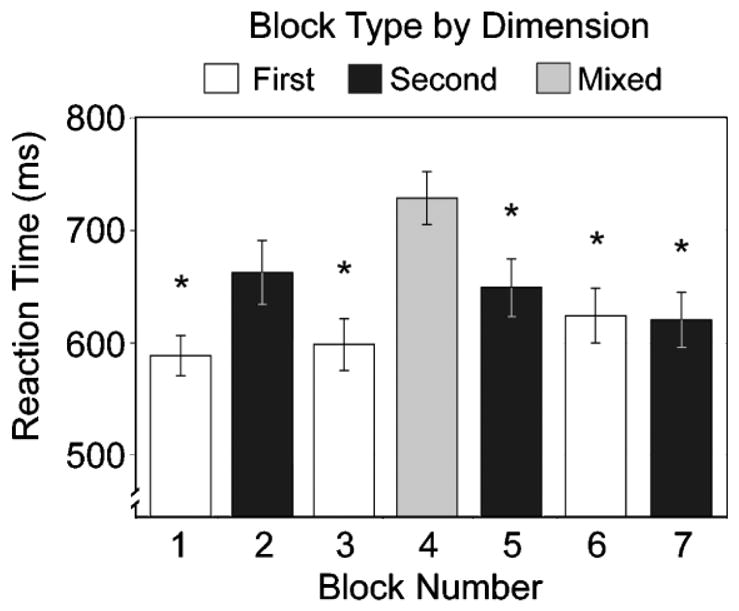
Reaction time (RT) on each block of trials. Stars indicate the single-task blocks on which RTs were significantly faster than the RTs on Block 4 (the mixed-task block), p < .05.
Testing adults for longer than children are usually tested revealed that the first dimension by which they sorted, or matched, the stimuli affected performance for the rest of the session. There was a significant interaction that showed differential performance between the two sorting conditions depending on which dimension was relevant first, F(1, 45) =4.21, p < .05 (see Fig. 4). Adults who started sorting by shape were slower when sorting by color than those who started with color. Similarly, those who started sorting by color were slower when sorting by shape than those who started with shape. RTs spiked for Block 2 (the introduction of the second dimension) and then went back down for Block 3 (when the first dimension was again correct). After a block of switching between the two sorting criteria (the mixed block, Block 4), it took participants awhile for their RTs to recover the speed evidenced before the mixed block. One would expect RTs to decrease progressively from Blocks 5 through 7. However, as the second dimension was relevant on Block 7, RTs were no faster there than on Block 6; the first-dimension advantage and recovery of speed after the mixed block counterbalanced one another.
Fig. 4.
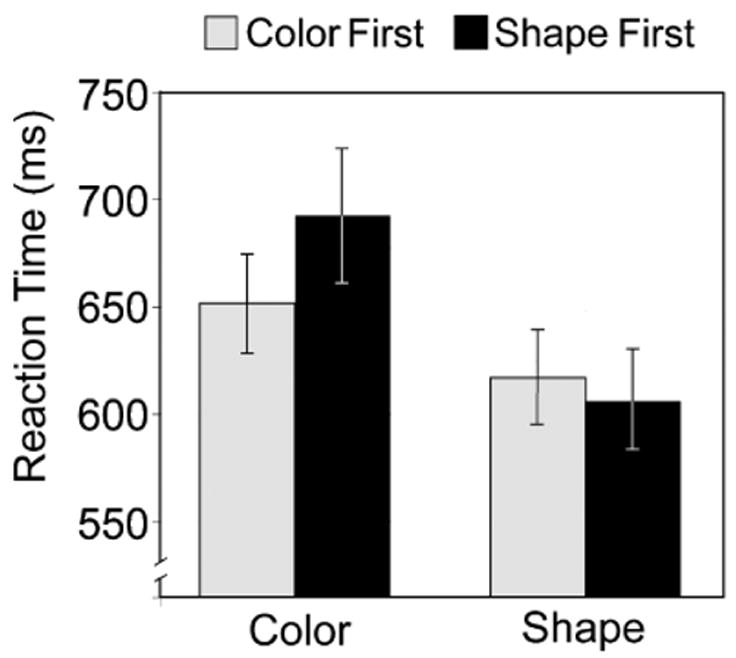
Effect of the first sorting dimension on performance throughout the session.
DISCUSSION
It is not surprising that adults show a cost in switching from one task to another; psychologists have known about that cost since it was first shown by Jersild (1927). However, the pervasive effect of the first task on subsequent performance in this experiment is noteworthy; there was a clear difference in performance between the two conditions that was created by the first dimension sorted. Even though accuracy was high on all blocks (as would be expected on such an easy task), adults’ RTs reflected the difficulty of switching the sorting criterion. The RT when sorting by the initial dimension (regardless of whether that was color or shape) remained faster than the RTwhen sorting by the other dimension, even when the data were averaged over the entire session.
The difficulty in switching sorting dimensions on the very simple DCCS task never completely disappears, contrary to what the literature has reported (which is that the difficulty disappears by 5 years of age). Certainly, adults perform better at switching sorting dimensions than do children, but the cognitive bias (the attentional inertia) seen in children does not completely disappear, even in adulthood, as the present results indicate. The effect of inertia can be seen (a) in adults’ slower RTs on the first trials after a switch than on the preceding trials, as well as (b) in the persistence of differential performance in sorting by each dimension depending on which dimension was used for sorting first (an effect perhaps related to another recently observed effect characterized by Waszak, Hommel, & Allport, 2003). The effect of inertia can also be seen (c) in the persistence of slower RTs after the mixed block. Subjects routinely perform more slowly in more difficult conditions, such as when they must switch back and forth between task sets within a block (e.g., Los, 1996; Rogers & Monsell, 1995). An inertial tendency is seen in the persistence of those slower RTs when participants are again presented with easy, single-task blocks after the mixed block (termed the “fade-out period” by Mayr & Liebscher, 2001). Seemingly, RTs are slower on single-task blocks following the mixed block than on single-task blocks preceding the mixed block because the mental setting that dictates slower RTs for the demanding mixed block takes time to be reset for the faster speed appropriate for single-task blocks.
In very few trials (typically three to eight trials are used, but the effect has been found even with one trial; Zelazo & Jacques, 1997), children get used to sorting according to a set of rules for color or shape, and they then have difficulty switching to another set of rules for the same stimuli. It is not that children are unaware when the sorting criterion changes, nor that they have forgotten the rules for sorting by the new criterion. Indeed, before they see the stimulus on any given trial, they can indicate clearly how to sort by the currently correct criterion. Whereas Rogers and Monsell (1995) argued that people need to see a stimulus to complete their “task-set reconfiguration,” we think that seeing a stimulus creates a problem in switching tasks rather than helping to complete the mental task switch. Children appear to be clear about what they should do before they see a stimulus. However, seeing a stimulus relevant to both the previous and the current sorting criteria in incompatible ways creates a problem. The pull to attend to, and act in accord with, the previously relevant dimension wins out for more than half the children at 3 years of age.1
Children quickly become used to focusing on the blueness or redness of a stimulus or on its object-kind property (that it is a truck or a star) and have great difficulty switching the way they think about the stimuli in the DCCS task. This difficulty is similar to what is observed in other paradigms:
(a) On appearance-reality tasks (Flavell, Green, & Flavell, 1986), children have difficulty thinking about one thing from two different perspectives (e.g., instead of accepting that something can appear to be a rock but really be a sponge, 3-year-olds tend to say the answer to both questions—What does it look like? What is it really and truly?—is the same). (b) On tests of spatial perspective, a 3-year-old often has difficulty thinking about a scene from two different perspectives, responding that what others see from a different vantage point (e.g., on the other side of a barrier) is what the child can see from his or her own vantage point (considered an aspect of egocen-tricism; Piaget & Inhelder, 1956). (c) When looking at an ambiguous figure, even when informed of the alternatives in the figure, 3-year-olds remain stuck in their initial way of perceiving the figure (Gopnik & Rosati, 2001). (d) False-belief tasks require holding two conflicting things about the same situation in mind. In one type of false-belief task (theory-of-mind tasks), the child needs to hold in mind the true state of affairs and the false belief of another person not privy to information the child knows. Many 3-year-olds fail this task by attributing the true belief not only correctly to themselves, but also incorrectly to the other person (Perner, Lang, & Kloo, 2002). In another type of false-belief task, the two things to be kept in mind are the true state of affairs (e.g., that pennies, rather than M & M’s, are in an M & M’s box) and the subject’s own reasonable earlier belief (e.g., that M & M’s would be in the box). Once 3-year-olds see what is in the box, they insist that the answer to what is actually in there and what they had earlier guessed is the same—they had thought all along that pennies were in the box (Perner, Leekam, & Wimmer, 1987).
Of course, adults generally pass all of those tasks, but a discomfort with ambiguity and difficulty in seeing both sides of an issue or two perspectives on the same thing remain forever (Van Hiel & Mervielde, 2003). Thus, even adults show some difficulty accepting that good people (or good nations) sometimes act wrongly or that people who disagree with them might be right about something. Even adults have difficulty representing more than one interpretation of an ambiguous figure at a time (Chambers & Reisberg, 1992). Epley, Morewedge, and Keysar (2004) showed that when another person refers to something, adults’ first inclination in determining the referent is based, not on the speaker’s knowledge, but on what they themselves know, even when they know the speaker is unaware of that information. Epley et al. argued that the difference between adults and children lies “in the ability to correct an initial egocentric interpretation, rather than differences in the tendency to form one,” and that therefore “egocentricism isn’t outgrown so much as it is overcome” (p. 12). Thus, they argued, the initial egocentric bias is as true of adults as it is of children.
Birch and Bloom (2003) have pointed out the similarity between 3-year-olds’ errors on theory-of-mind tasks and adults’ curse-of-knowledge errors (e.g., Hinds, 1999; Nickerson, 1999); both involve a tendency to attribute what one knows to someone less knowledgeable. Similarly, adults do not claim that they earlier said that pennies would be in an M & M’s box, but in analogous situations they claim that they earlier rated similarly unlikely outcomes that actually occurred as more probable than they actually had (this bias is dubbed “knew it all along” by Fischhoff, e.g., Fischhoff & Beyth, 1975; see also related work on hindsight bias, e.g., by Hawkins & Hastie, 1990). Adults are astonished that 3-year-olds can show that they know something one moment (e.g., how cards should be sorted) and the next moment fail to use that knowledge when performing a task. Yet Keysar, Lin, and Barr (2003) found similar behavior in adults.
Indeed, it might be possible that adults do not fully grow out of any of the cognitive or perceptual biases of infancy and early childhood. In adults, these biases are surely more subtle. With preschoolers and infants, one can see these with the naked eye and by gross measures such as success or failure. In adults, one needs more subtle measures, such as RT measured in milliseconds, or sometimes contrived or unusual situations, but we hypothesize that adults are not as cognitively different from infants and preschoolers as adults would like to believe, and that all biases found in young children can be found in adults.
Consider a few examples in addition to those we have already discussed. Infants do not always grasp the relation between two objects (such as the relation between a stimulus object and its associated reward), even when that relation is obvious to adults. When infants need to use reward feedback from acting on stimulus objects to deduce an abstract rule (choose the item that does not match the sample), a physical connection between the stimulus and reward objects appears to be key to the infants’ success. In recent studies (Diamond, Churchland, Cruess, & Kirkham, 1999; Diamond, Lee, & Hayden, 2003), when a physical connection was present (by the objects being attached to each other or attached to the same larger unit), the two objects did not need to be physically close, nor did the reward need to appear immediately after the infant acted on the stimulus. However, without the perception that stimulus and reward were components of a single thing, even both close spatial and temporal proximity were insufficient for infants in the first year to grasp the nonmatching rule (Diamond et al., 1999; Diamond et al., 2003). Physical connection appears to continue to hold a special potency even in adulthood. For example, when Baker (2003) asked adult observers to report only individual parts of a visual display, they spontaneously encoded and learned the combinations of parts only if the parts were physically connected, but not otherwise.
Three-year-olds readily succeed on the DCCS task if the color and shape dimensions are separated (Kloo & Perner, in press), though both color and shape appear on all stimulus and model cards. In this version of the task, children see stimulus cards with the black outline of a banana (or cherry) alongside a blue (or yellow) patch. Such results are analogous to those for adults on the Stroop task. In the standard version of the task, in which color words are written in ink of another color, adults find it difficult to switch between naming the ink color and saying the word (MacLeod, 1991), but adults find the task far easier if a color word (printed in black ink) and a patch of a conflicting color are presented simultaneously (MacLeod, 1998).
Similarly, Barrett and Shepp (1988) found that children 4 to 5 years of age tended to stay stuck in perceiving integrated stimuli as wholes, unable to focus on just one dimension (e.g., color or shape), but when the dimensions were spatially separated, children could focus on just one, ignoring irrelevant variation in the other. There is mounting evidence that when adults attend to one aspect of an integrated stimulus, they, too, are unable to avoid processing its irrelevant features (e.g., Schoenfeld et al., 2003). Indeed, Pratt and Hommel (2003) have shown not only that an irrelevant feature (color) of an integrated stimulus is processed, but also that if the irrelevant feature (the same color) then appears as part of a wholly irrelevant stimulus (an arrow), that wholly irrelevant stimulus then influences adults’ performance.
In sum, we propose that adults never fully outgrow the cognitive and perceptual biases that are so striking in infants and preschoolers. That is a humbling thought, much as it was humbling to discover that humans are not the center of the universe or as rational and “in control” as once thought. However, if clarity can come from investigating extreme cases, then perhaps studying children, who show these biases more blatantly than adults, might be a rich source of insight and future hypotheses about adult cognition.
Acknowledgments
This research was supported by grants to A.D. from the National Institute of Child Health and Human Development (Grant No. R01 #HD35453) and the McDonnell Foundation (JSMF Grant No. 21002016). Part of this research was reported at the meeting of the Cognitive Development Society, October 2001, Virginia Beach, VA. We gratefully acknowledge Daniel Richardson for his immeasurable help with programming and data analysis and Jessica Rosekrans for helping with participant recruitment and testing.
Footnotes
Note areas of overlap between our perspective and “task set inertia” (Allport & Wylie, 2000) and “stimulus-triggered retrieval” (Wylie & Allport, 2000). Also, note that our perspective is not inconsistent with that of Munakata and Yerys (2001), who, like Allport and Wylie, emphasized memory more and inhibition less than do we.
References
- Allport A, Wylie G. Task switching, stimulus-response bindings, and negative priming. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance XVII. Cambridge, MA: MIT Press; 2000. pp. 35–70. [Google Scholar]
- Baker C. Learning and temporal cortex: Neurons, behavior and hi-resolution functional imaging; Paper presented at MIT, Department of Brain and Cognitive Sciences colloquium; Cambridge, MA. 2003. Sep, [Google Scholar]
- Barrett SE, Shepp BE. Developmental change in attentional skills, the effect of irrelevant variations on encoding and response selection. Journal of Experimental Psychology. 1988;45:382–399. doi: 10.1016/0022-0965(88)90038-0. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Martin MM. Attention and inhibition in bilingual children: Evidence from the Dimensional Change Card Sort Task. Developmental Science. 2004;7:325–339. doi: 10.1111/j.1467-7687.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Birch S, Bloom P. Children are cursed: An asymmetric bias in mental-state attribution. Psychological Science. 2003;14:283–286. doi: 10.1111/1467-9280.03436. [DOI] [PubMed] [Google Scholar]
- Chambers D, Reisberg D. What an image depicts depends on what an image means. Cognitive Psychology. 1992;24:145–174. doi: 10.1016/0010-0285(92)90006-n. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cohen S, Bixenman M, Meiran N, Diamond A. Task switching in children; Paper presented at the South Carolina Bicentennial Symposium on Attention; University of South Carolina, Columbia, SC. 2001. May, [Google Scholar]
- Diamond A. Strength in numbers: Pooling data to investigate the effects of age, dimension, and gender on young children’s ability to switch sorting dimensions. 2004. Manuscript submitted for publication. [Google Scholar]
- Diamond A, Churchland A, Cruess L, Kirkham N. Early developments in the ability to understand the relation between stimulus and reward. Developmental Psychology. 1999;35:1507–1517. doi: 10.1037//0012-1649.35.6.1507. [DOI] [PubMed] [Google Scholar]
- Diamond A, Lee EY, Hayden M. Early success in using the relation between stimulus and reward to deduce an abstract rule: Perceived physical connectedness is key. Developmental Psychology. 2003;39:825–847. doi: 10.1037/0012-1649.39.5.825. [DOI] [PubMed] [Google Scholar]
- Epley N, Morewedge CK, Keysar B. Perspective taking in children and adults: Equivalent egocentricism but differential correction. 2004. Manuscript submitted for publication. [Google Scholar]
- Fischhoff B, Beyth R. I knew it would happen”: Remembered probabilities of once-future things. Organizational Behavior and Human Decision Processes. 1975;13:1–16. [Google Scholar]
- Flavell JH, Green FL, Flavell ER. Development of knowledge about the appearance-reality distinction. Monographs of the Society for Research in Child Development. 1986;51(1):1–87. [PubMed] [Google Scholar]
- Gopnik A, Rosati A. Duck or rabbit? Reversing ambiguous figures and understanding ambiguous representations. Developmental Science. 2001;4:175–183. [Google Scholar]
- Hawkins SA, Hastie R. Hindsight: Biased judgments of past events after the outcomes are known. Psychological Bulletin. 1990;107:311–327. [Google Scholar]
- Hinds PJ. The curse of expertise: The effects of expertise and debiasing methods on prediction of novice performance. Journal of Experimental Psychology: Applied. 1999;5:205–221. [Google Scholar]
- Jersild AT. Mental set and shift. Archives of Psychology. 1927;89:5–82. [Google Scholar]
- Keysar B, Lin S, Barr DJ. Limits on theory of mind use in adults. Cognition. 2003;89:25–41. doi: 10.1016/s0010-0277(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Kirkham NZ, Cruess L, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6:449–467. [Google Scholar]
- Kloo D, Perner J. Disentangling dimensions in the dimensional change card sorting task. Developmental Science. doi: 10.1111/j.1467-7687.2005.00392.x. in press. [DOI] [PubMed] [Google Scholar]
- Los SA. On the origin of mixing costs: Exploring information processing in pure and mixed blocks of trials. Acta Psychologica. 1996;94:145–188. [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Training on integrated versus separated Stroop tasks: The progression of interference and facilitation. Memory & Cognition. 1998;26:201–211. doi: 10.3758/bf03201133. [DOI] [PubMed] [Google Scholar]
- Mayr U, Liebscher T. Is there an age deficit in the selection of mental sets? European Journal of Cognitive Psychology. 2001;13:47–69. [Google Scholar]
- Munakata Y, Yerys BE. All together now: When dissociations between knowledge and action disappear. Psychological Science. 2001;12:335–337. doi: 10.1111/1467-9280.00361. [DOI] [PubMed] [Google Scholar]
- Nickerson RS. How we know—and sometimes misjudge—what others know: Imputing one’s own knowledge to others. Psychological Bulletin. 1999;125:737–759. [Google Scholar]
- Perner J, Lang B, Kloo D. Theory of mind and self-control: More than a common problem of inhibition. Child Development. 2002;73:752–767. doi: 10.1111/1467-8624.00436. [DOI] [PubMed] [Google Scholar]
- Perner J, Leekam SR, Wimmer H. Three-year-olds’ difficulty with false belief: The case for a conceptual deficit. British Journal of Developmental Psychology. 1987;5:125–137. [Google Scholar]
- Piaget J, Inhelder B. The child’s conception of space. London: Routledge & Kegan Paul; 1956. [Google Scholar]
- Pratt J, Hommel B. Symbolic control of visual attention: The role of working memory and attentional control settings. Journal of Experimental Psychology: Human Learning and Performance. 2003;29:835–845. doi: 10.1037/0096-1523.29.5.835. [DOI] [PubMed] [Google Scholar]
- Rennie D, Bull R, Diamond A. Executive functioning in preschoolers: Reducing the inhibitory demands of the dimensional change card sort task. Developmental Neuropsychology. 2004;26:423–443. doi: 10.1207/s15326942dn2601_4. [DOI] [PubMed] [Google Scholar]
- Riggs KJ, Williams O. Dimensional-change card-sorting task performance in preschoolers. London Metropolitan University; London, England: 2003. Unpublished manuscript. [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology. 1995;124:207–231. [Google Scholar]
- Schoenfeld MA, Tempelmann C, Martinez A, Hopf JM, Sattler C, Heinze HJ, Hillyard SA. From the cover: Dynamics of feature binding during object-selective attention. Proceedings of the National Academy of Sciences, USA. 2003;100:11806–11811. doi: 10.1073/pnas.1932820100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hiel A, Mervielde I. The need for closure and the spontaneous use of complex and simple cognitive structures. Journal of Social Psychology. 2003;143:559–568. doi: 10.1080/00224540309598463. [DOI] [PubMed] [Google Scholar]
- Waszak F, Hommel B, Allport A. Task-switching and long-term priming: Role of episodic stimulus-task bindings in task-shift costs. Cognitive Psychology. 2003;46:361–413. doi: 10.1016/s0010-0285(02)00520-0. [DOI] [PubMed] [Google Scholar]
- Wylie G, Allport A. Task switching and the measurement of “switch costs. Psychology Research. 2000;63:212–233. doi: 10.1007/s004269900003. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Frye D, Rapus T. An age-related dissociation between knowing rules and using them. Cognitive Development. 1996;11:37–63. [Google Scholar]
- Zelazo PD, Jacques S. Children’s rule use: Representation, reflection, and cognitive control. Annals of Child Development. 1997;12:119–176. [Google Scholar]
- Zelazo PD, Mueller U, Frye D, Marcovitch S. The development of executive function in childhood. Monographs of the Society for Research in Child Development. 2003;68(3):1–137. doi: 10.1111/j.0037-976x.2003.00260.x. [DOI] [PubMed] [Google Scholar]


