Abstract
Female mammals are particularly sensitive to changes in food availability. The mechanisms that affect sexual behavior and food intake are closely related to one another; chief among the mechanisms that control sexual behaviors in females is estradiol. In order to understand how food deprivation results in inhibition of sexual behavior (attractivity, proceptivity, and receptivity), we measured the effects of food deprivation on circulating concentrations of estradiol. We also determined whether estradiol treatment was sufficient to restore sexual behaviors in food deprived female meadow voles. We found that estradiol titers of food-deprived female voles are significantly lower than those of ad lib-fed female voles. Further, we found that estradiol treatment was sufficient to restore proceptivity and receptivity in food-deprived, ovariectomized female voles. However, estradiol treatment was not able to overcome the food deprivation-induced inhibition of attractivity. Thus, decreases in estradiol titer of food-deprived female voles may be related to the suppression of their proceptive and receptive behaviors, and may be a mechanism that allows females to avoid mating when conditions are not propitious for their survival and that of their offspring.
1. Introduction
Reproduction involves a significant energetic investment, especially for female mammals. As a result, female mammals inhibit sexual behavior and change physiological parameters relating to sexual behaviors when a sufficient energy supply, in the form of foodstuff, is not present in suitable quantity or quality 1–3. The physiological mechanisms that directly or indirectly cause changes in sexual behaviors are of interest, as the mechanisms that control sexual behavior are closely associated with the frequency and amount of food intake 4.
The role that gonadal steroids play in mediating sex behaviors has been known for a quite some time. Additionally, considerable interest4–6 has focused on the effects of stressors, such as food deprivation play in mediating the synthesis of gonadatropins and gonadal hormones. For example, food deprivation or restriction interrupts the release of gonadatropin releasing hormone (GnRH) and subsequently, luteinizing hormone (LH) and follicle stimulating hormone (FSH) release; this, in turn, results in reduced secretion of gonadal steroid hormones 1, 4, 7. In a number of mammalian species including mice, rats, hamsters, musk shrews, and non-human primates changes in circulating titers of gonadal steroids are induced by changes in food availability cause 3, 8–10. For example, both food-restricted female mice and musk shrews have lower gonadal steroid titers than those that are ad lib-fed 1, 8, 11. Food-deprived intact golden hamsters have lower concentrations of circulating estradiol than do their ad lib-fed counterparts4, 12. These studies, however, did not report the titers of estradiol in steroid-primed animals.
The role of estradiol in mediating sexual behavior in food deprived and restricted females is not clear. Some studies have found that estradiol treatment reinstates short latency lordosis in food- restricted female golden hamsters12, 13. A more recent study, however, reported that priming females with a physiological amounts of estradiol was not sufficient to restore the amount of lordosis in food deprived ovariectomized (OVX) golden hamsters, compared to those that were ad lib-fed 14. Our lack of understanding the association among food deprivation, circulating estradiol titers, and the sexual behavior of females are made even murkier by the fact that many studies on food deprivation in female mammals focus on changes in a single component of sexual behavior, usually sexual receptivity or lordosis. However, sexual behavior is more complex than receptivity alone and measurement of lordosis does not reveal if a female is or is not showing an amount of lordosis sufficient for intromission and ejaculation. For example, females must first be attractive to males and display behaviors that elicit proceptive behaviors from males 15. In rodents, attractivity is associated with the production and secretion of odors by females that are attractive to males and proceptivity is associated with the male’s response or odor preferences for particular females 3, 15–22. We have shown that after acute food deprivation female voles were no longer attractive to males, did not display proceptive behaviors when they encounter scent marks of males, and did not copulate with male voles 22, 23.
Currently, we know little about the effects of acute food deprivation on more than a single component of sexual behavior in other mammals. Further, we do not know whether suppression of sexual behavior of food-deprived female voles is associated with a reduction in their circulating titers of estradiol. Estradiol has been found to be necessary and sufficient for the display of sexual behaviors in female meadow voles 16, 24, similar to that for females in other species 1. However, the amount of time required for females meadow voles to inhibit and subsequently recover sexual behaviors with re-feeding, were not the same were found with other species 22, 23. Such differences may be attributed to species differences in the endocrine and reproductive physiology that mediate sexual behavior of these small mammals. Nevertheless, the available data suggest that changes in circulating titer of estradiol may be a mechanism that underlies changes in sexual behavior in food-deprived or restricted female mammals 3, 10, 11. The goal of the present study was to address the following two questions. 1) Is acute food deprivation sufficient to lower circulating titers of estradiol of female voles? 2) Is exogenous estradiol sufficient for female meadow voles to overcome the inhibitory effects of food deprivation on the three components of sexual behavior? To address these questions we tested the hypothesis that the expression of sexual behavior of food-deprived female voles is associated with their circulating titers of estradiol. This hypothesis makes the following predictions. 1) Acute food deprivation is sufficient to induce female meadow voles to have lower circulating titers of estradiol relative to female voles that were not food deprived. 2) Exogenous estradiol is sufficient for female meadow voles to overcome the inhibitory effects of food deprivation on the three components of sexual behavior.
2. Materials & Methods
2.1 Animals
We used first and second generation laboratory born meadow voles descended from individuals captured at the Miami University Ecological Research Center (Oxford, OH, USA). Voles were maintained from birth under a long photoperiod (14:10h L:D, lights on at 0700h Central Standard Time (CST). At 21 days of age voles were weaned and housed with littermates in clear plastic cages (26 x 32 x 31 cm; l, w, h, respectively) containing wood chip bedding and cotton nesting material. We changed cotton nesting material and hardwood shavings weekly. At 42 days of age, animals were separated from littermates and singly housed in clear plastic cages (27 x 16.5 x 12.5cm; l, w, h, respectively) unless otherwise noted. Voles born and reared in long-photoperiod reach puberty between 50–60 days of age 22. We used male voles that were 90–150 days old and not previously food deprived. Males were sexually experienced, having previously sired a litter. We also used female voles that were 80–150 days old, sexually naïve, and not previously food deprived. Female meadow voles do not undergo estrous cycles and those born and reared under a long-photoperiod (please see above) readily mate when paired with long-photoperiod male conspecifics 22.
2.2 Food Deprivations
We weighed female voles 24 h before they were used in the experiment. By doing so, we were able to assign female voles to treatment groups (see below for further details) using a randomization procedure based on body weight. Thus, we insured that female body weight was normally distributed across all treatment groups. Next, female voles were assigned to a group that had continuous access to food (ad lib-fed, AL) or to one of the groups that were food deprived for different intervals. Females in the ad lib-fed group received Purina Rodent Diet # 5008 (PMI Inc., St. Louis, MO, U.S.A.) and water. Females in the food-deprived groups received only water; we removed all the food from their cage tops and from the floor of their cages. Female voles that were food deprived were without food for either for 0 h , 6 h, 12 h, or 24 h. Females that were food-deprived for 6 h (FD 6) underwent tests for proceptivity and receptivity, whereas those that were food deprived for 12 h (FD 12) and 24 h (FD 24) underwent tests for attractivity, proceptivity, and receptivity. Females that were food deprived for 6 h did not undergo attractivity tests because this interval was not sufficient to reduce the attractiveness of their odors to male conspecifics22. Female voles were food deprived only once and returned to their home cages after testing. The University of Memphis Institutional Animal Care and Use Committee approved all procedures, and all procedures followed the guidelines set forth by the National Institutes of Health.
2.3 Experiment 1: Is acute food deprivation sufficient to lower circulating titers of estradiol of female voles?
2.3.1 Procedure
We food deprived female voles for 0 h (ad lib-fed), 6 h, 12 h, or 24 h intervals as described above. When the females had been food deprived for the designated interval, we anesthetized each one with isofluorane vapors and obtained a blood sample via cardiac puncture. All sampling took place between 0800 and 0900 CST. We analyzed the plasma samples via enzymatic immunoassay (EIA) using estradiol assay kits from Diagnostic Systems Laboratories (DSL, Webster, TX, USA). Sample sizes were 8, 10, and 9 for the 0 h (ad lib-fed), 6 h, 12 h, and 24 h food-deprivation intervals, respectively. Estradiol titers below the assay’s detectable concentrations were included in the analysis and assigned a value of the lower detectable limit of assay of 2 pg/ml of estradiol. We compared the estradiol concentrations of ad lib-fed females and food-deprived female voles using a one-way ANOVA followed by Student Newman-Keul’s post-hoc tests when appropriate. An alpha value of 0.05 indicated significant differences between groups.
2.4 Experiment 2 - Is exogenous estradiol sufficient for female meadow voles to overcome the inhibitory effects of food deprivation on the three components of sexual behavior?
2.4.1 Surgical procedure & hormone replacement
Forty female voles were anesthetized with a mixture of ketamine and xylazine (2.5 mg ketamine and 3 mg xylazine/kg body mass, IP) and ovariectomized via flank incisions. The ovariectomized (OVX) females were implanted with an empty 12-mm long Silastic capsule (Dow Corning, Midland, MI, U.S.A., o.d. 1.956 mm, i.d. 1.4732 mm) or one containing 5 mm active length of estradiol-17 β (Sigma-Aldrich Co., USA), which is sufficient to restore attractivity and proceptivity in meadow voles 16. All capsules were placed subcutaneously in the intrascapular region via a small incision.
2.4.2 Food deprivations and treatment groups
The 43 OVX voles were divided into two groups, 23 voles were implanted with a capsule filled with estradiol (OVX+ E2). The remaining 20 voles were treated with an empty capsule (OVX + blank). Fourteen to twenty days following surgery, both the OVX+ E2 treated females and the OVX + blank treated females were separated into two subgroups. The OVX + E2 subgroups were comprised of a group of 10 females that were ad lib-fed (OVX + E2 AL) and another group of 10 females that were food-deprived for 6, 12, or 24 h (e.g., OVX + E2 FD 6, OVX + E2 FD 12, etc.) . The OVX + blank subgroups consisted of one groups of females that were ad lib-fed (OVX + blank AL) and groups of 6–10 females that were food deprived for 6, 12, or 24 h (e.g., OVX + Blank FD 6, OVX + Blank FD 12 h, etc.). After the females were food deprived for the respective intervals, they were tested for their attractivity, proceptivity, and receptivity.
2.5 Attractivity Test
2.5.1 Subject and Scent Donors
All sexual behavior tests began at 0800h CST. Scent donors were the female voles in the different treatment groups as detailed above: OVX + E2 AL females, OVX + E2 FD females, the OVX + blank AL females, and the OVX + Blank FD females. All paired female donors were similar in weight (within 5 g), and unfamiliar and unrelated to the male that was investigating their odors. Subjects in the attractivity tests were 46 male voles (see above for further details). Each male was tested once with a unique pair of female odor.
2.5.2 Procedure
Fresh anogenital area scent marks were obtained for each trial from each female scent donor. We chose anogenital scent marks because they convey sex-specific information to conspecifics and their attractiveness to conspecifics is affected by the length of time that female voles have been food deprived 22, 23. Briefly, scent marks from the anogenital area were collected by rubbing a clean glass microscope slide against this area of the donor for 5–10 s. The resulting in an approximately 1.0 x 0.2 cm streak (scent mark) from each donor was placed randomly on either the right or left-side of the slide. Sixty seconds elapsed between placement of the scent marks from the first and second donor.
This type of attractivity test has been used in previous studies on voles and detailed elsewhere 22, 23. Briefly, each male was presented in its home cage with a clean glass microscope slide (2.5 x 7.6 cm) that was divided into three equal sections (each 2.5 cm long). For example, one end section contained the anogenital area scent mark from an OVX + E2 FD 12 female and the other end section contained the anogenital area scent mark from an OVX + E2 AL female. The middle section contained no stimulus odor. The placement of a particular scent mark either on the left or right side of the slide was random and unknown to the experimenter. The slide was suspended by a clasp and wire hanger approximately 1 cm above the substrate and against the wall opposite the male’s nest. We then recorded, during the 5-min test, the total amount of time that subjects investigated the marks from that pair of scent donors. Each slide was used only once and then discarded. During each 5-minute test, we recorded continuously the time each male investigated the two scented sections of the slide, and the middle section of the slide. Criteria for investigation of a mark were that: 1) the male was obviously licking or sniffing a stimulus odor or its nose was within approximately 1 cm of one end of the slide, 2) it investigated both of the two scented areas on the slide, and 3) it spent more time investigating the two scented areas of the slide than the clean middle section 22, 23.
We used paired t-tests for each comparison to determine if subjects spent significantly more time investigating a mark from the paired female scent donors; an ANOVA-type test would be inappropriate given the nature of the data, as there was no common denominator between all of the groups involved 23, 25. We considered the mark investigated for a longer duration as being more attractive.
2.6 Proceptivity test
2.6.1 Procedure
This test is similar to the attractivity test detailed above except in the proceptivity test ad lib-fed females and food-deprived females were exposed to a glass slide for 5 min that contained an anogenital area scent mark of a male conspecific on one end of the slide and the anogenital area scent mark of a female conspecific on the other end of the slide. In this test, the male and female scent donors had continuous access to food 22, 23, 25. Thus, we measured the amount of time that OVX + E2 AL females, OVX + E2 FD females, OVX + blank AL females, and OVX + blank FD females investigated anogenital odors of a male and those of a female conspecific. We analyzed the differences in investigation time between groups with a one-way analysis of variance (ANOVA). In order to construct a single continuous variable from 2 investigation times, we calculated the proportion of time spent with male odors out of the total investigation time. To meet the equal variance assumption, we arcsine square-root transformed the proportions. Post-hoc tests were performed using Student’s Newman-Keuls post-hoc analyses when appropriate.
2.7 Receptivity test
2.7.1 Procedure
Immediately following their proceptivity test, each female was moved to a larger cage (37 x 21 x 15 cm; l x w x h) containing clean hardwood shavings, clean cotton bedding and continuous access to food and water. An additional 20 intact female voles (reference females) were also moved to larger cages. Ten of the reference females were ad lib-fed, whereas the remaining 10 reference females were food deprived for 12 h.
Fifteen min after the females were placed into the large cage, a sexually experienced, unfamiliar male conspecific was placed into the cage with them. Voles were paired between 0830 and 0900 h CST. We allowed pairs to interact for 4 h, and filmed them in real time using video cameras and recorders. During videotape playback we recorded the occurrence or non-occurrence of intra-vaginal ejaculation. We considered a female to display sexual receptivity if she allowed the male to ejaculate intra-vaginally one or more times during the 4 h encounter 22, 23. A Kruskal-Wallis non-parametric ANOVA was used to test for overall differences in receptivity among control and experimental groups. These were followed up with Mann-Whitney tests for pair-wise comparisons. An alpha value of 0.05 indicated that statistical differences existed among and between groups.
2.8 Experiment 3: Verification of estradiol treatment
2.8.1 Procedure
We measured the estradiol titers of five OVX-blank FD 12 h food-deprived females and five ad lib-fed- OVX-E2 females 10 days after their final sexual behavior test. We followed the procedures described in experiment 1 for blood sampling and analysis.
3.0 Results
3.1 Experiment 1
We found that concentrations of estradiol were different among ad lib-fed and food-deprived groups of female voles (F (3,28)= 16.076, P < 0.001). The estradiol titers of food-deprived female voles were lower than those of females in the ad lib-fed group (AL fed vs. FD 6 h, p < 0.001; AL fed vs. 12 h FD, p < 0.001; AL fed vs. 24 h FD, P < 0.001; Fig 1). Student Newman-Keul’s tests revealed that the estradiol titers of females in each of the food-deprived groups were similar (6 vs. 12 h FD, P = 0.997; 6 vs. 24 h FD, P = 0.996; 12 vs. 24 h FD, P = 0.999, Fig 1).
Figure 1.
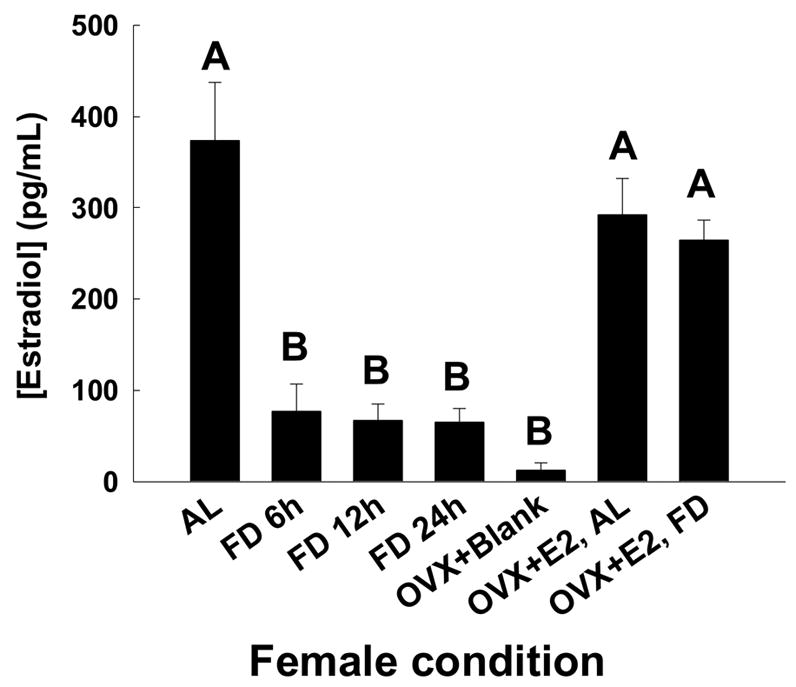
Mean ± SEM of estradiol titers of female voles in after being food deprived for different intervals. Also shown are the mean estradiol titers for the ad lib-fed and food deprived OVX-E2 treated female voles. There were no statistical differences between the groups of females.
3.2 Experiment 2
3.2.1 Attractivity
Male voles were exposed to the odors of two different female voles that differed in their hormonal state (presence of absence of estradiol), food deprivation state (food deprived or not food deprived), or both. We analyzed the different food deprivation intervals in separate analyses. All male voles investigated both of the two scented areas on the slide, and spent more time investigating the two scented areas of the slide than the clean middle section, and thus, were included in the analysis. Males preferred the odors of ad lib-fed OVX-E2-treated females to those of ad lib-fed OVX-blank treated females (t = 3.04, df = 7, P = 0.019, Fig. 2). Further, males preferred the odors of ad lib-fed OVX-E2-treated females to the odors of food-deprived OVX-E2-treated females (t = 3.0; df = 9, P < 0.05; Fig. 3a). Males preferred odors of 12 h food deprived OVX + E2 females to those of ad lib-fed OVX + blank females (t = 2.7, df = 6; P = 0.043; Fig. 4b). Additionally, males preferred the odors of OVX + E2 AL-fed voles to those of OVX + E2 FD 24 h females (t = 2.47, df = 6; P = 0.047). However, they did not show a preference for odors of OVX + E2 FD 24 h females over those of OVX +blank AL-fed females (t = 0.112, df = 7; P = 0.917). Finally, there was no effect of food availability or estradiol treatment on the latency of males to investigate the slides containing the female scent marks. The latency for investigation was similar for males investigating females in the different treatment groups (P > 0.1, all comparisons) with an average of 18.4 ± 7.1 s (mean ± SEM).
Figure 2.
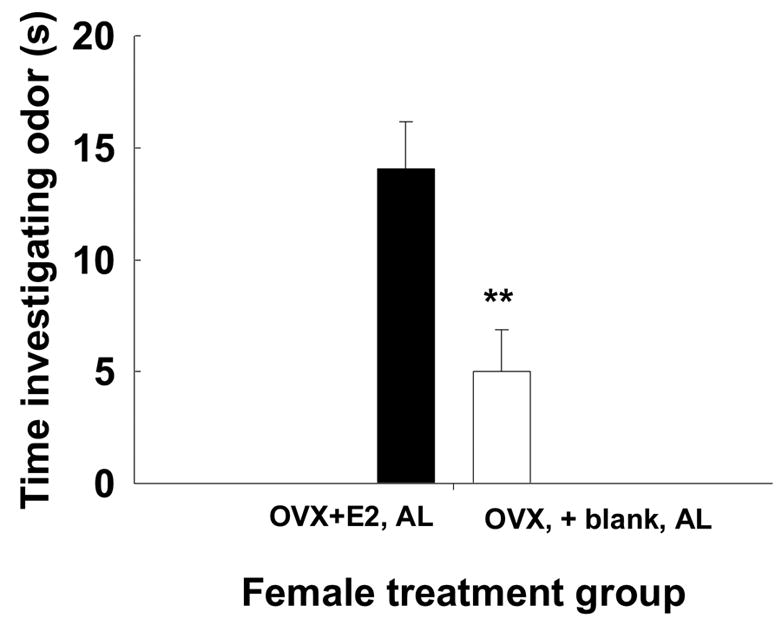
Mean ± SEM time (s) male voles spent investigating odors of OVX+E2, ad lib-fed vs. odors of OVX + blank, ad lib-fed female voles. Significant difference designated by ** indicates P < 0.01.
Figure 3.
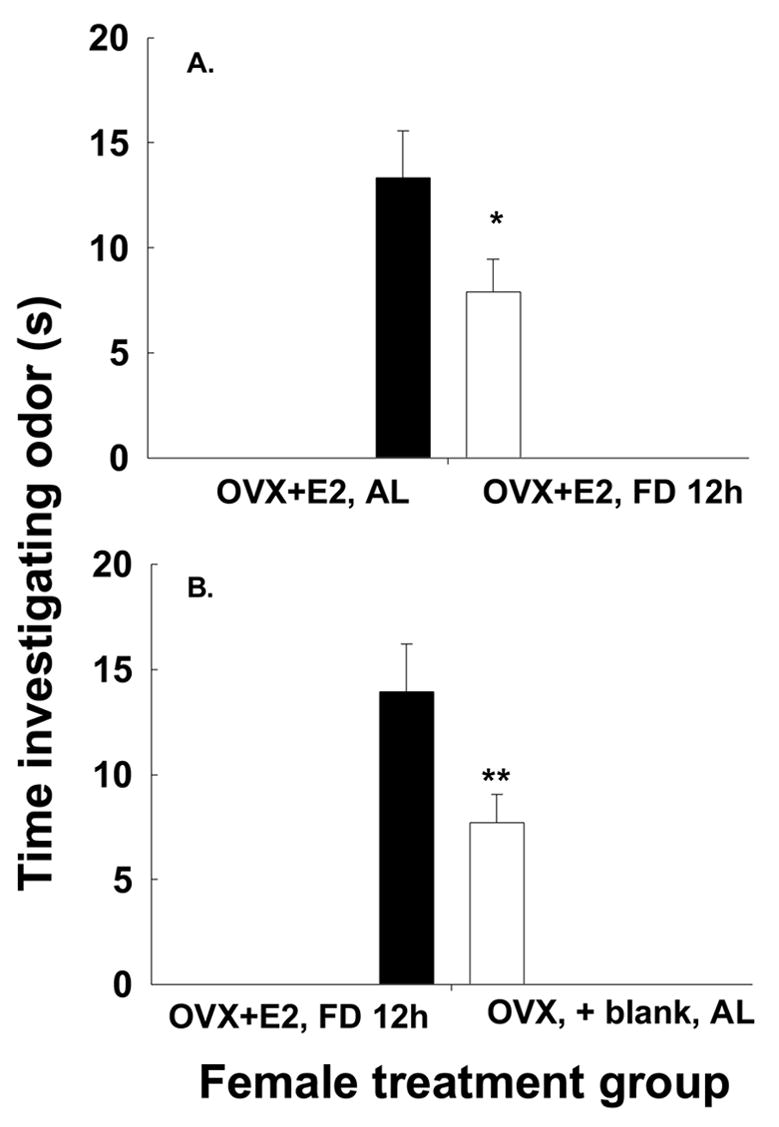
The mean ± SEM time (s) male voles spent investigating odors of (A) OVX+E2, ad lib-fed females compared to those of OVX+E2, FD 12 h females and the odors of (B) OVX+E2, 12 h food-deprived females compared to those of OVX + blank, ad lib-fed females. Significance differences signified by * indicates P <0.05, and ** indicates P < 0.01.
Figure 4.
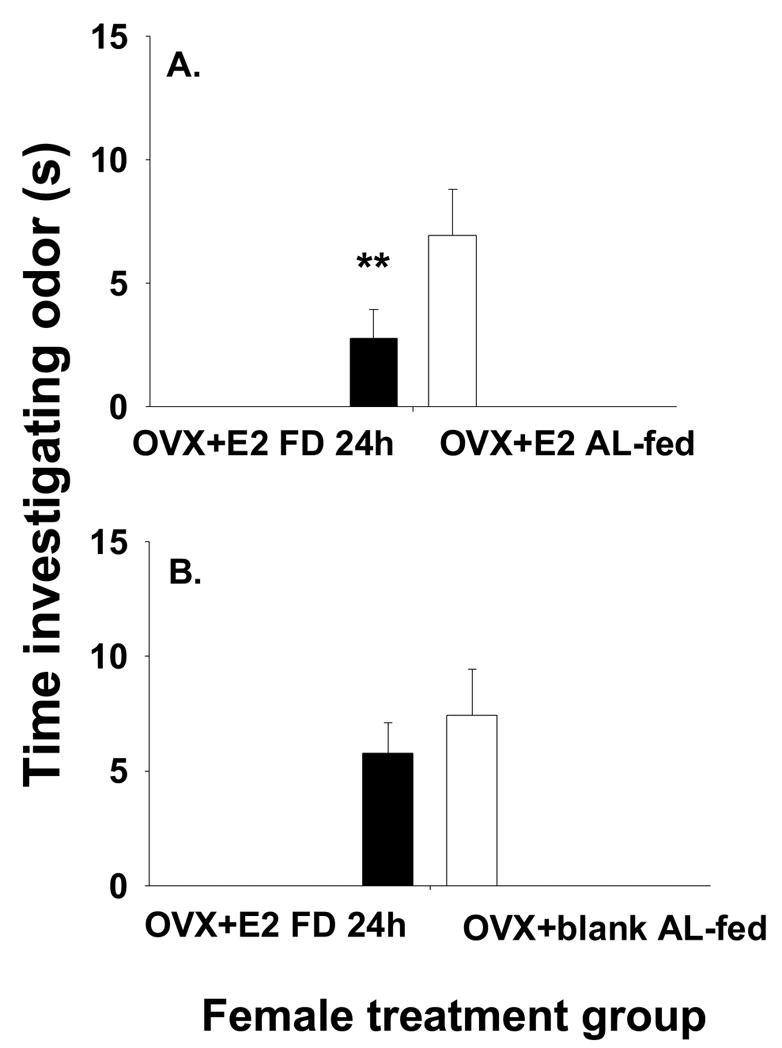
The mean time (s) ± SEM time (s) male voles spent investigating odors of (A) OVX+ E2, 24 h food-deprived compared to those of OVX+ E2, ad lib-fed females and odors of (B) OVX+ E2, 24 h food-deprived females compared to those of OVX + blank, ad lib-fed females. Significance differences signified by * indicates P <0.05, and ** indicates P < 0.01.
3.2.2 Proceptivity
Females investigated male odors more than they did female odors if they were treated with estradiol, regardless of their feeding condition (Fig. 5). The proportions of time different categories of females spent with male vs. female odor were compared with a one-way ANOVA. Student’s Newman-Keuls post hoc tests revealed that the OVX + blank, AL and OVX + blank FD 12 h showed similar amounts of proceptive behavior, falling into a homogeneous subset (P = 0.125, Fig. 5). Newman-Keuls post-hoc tests also showed that OVX+ E2 AL, OVX+ E2 FD 6 h, OVX + E2 FD 12 h, and OVX+ E2 FD 24 h showed similar amounts of proceptive behavior, falling into the same homogeneous subset (P = 0.603, Fig. 5). Thus, females without estradiol replacement showed less proceptive behavior towards males than did females receiving estradiol replacement, regardless of their feeding condition.
Figure 5.
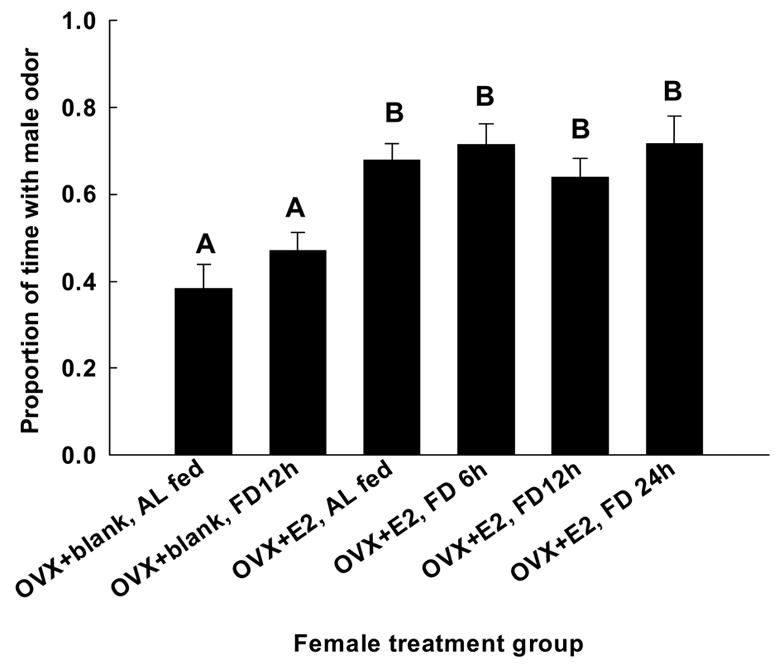
The proportion (mean ± SEM) of time (S) female voles spent investigating the odors of male conspecifics. Different letters designate significant differences at P < 0.05.
3.2.3 Receptivity
There were overall differences in receptivity amount the groups of females in the experiment (H = 50.213, df = 7; P < 0.01). Intact food-deprived females failed to display sexual receptivity towards males, when compared to those that were ad lib-fed (Fig. 6). Ad lib-fed females treated with estradiol displayed sexual receptivity towards males, whereas ad lib-fed females that were not treated with estradiol (OVX + Blank, AL) were not receptive to males. There were no differences in sexual receptivity shown by OVX + Blank AL female voles and intact food-deprived female voles (U = 50, P > 0.5); these females were not sexually receptive to male conspecifics (Fig. 6). There were no differences in the display of sexual receptivity by ad lib-fed OVX + E2 AL females and ad lib-fed intact females when they were paired with a sexually experienced male (U = 40.0, P =0.342, Fig. 6). For the estradiol-treated females, the interval of food deprivation did not affect their sexual receptivity displayed toward males (OVX+ E2 AL vs. OVX + E2 FD 6 h, U = 45.0, P = 0.542; OVX+ E2 AL vs. OVX + E2 FD 12 h, U = 37, P = 0.680; OVX+ E2 AL vs. OVX + E2 FD 24 h, U = 45.0, P = 0.615; OVX + E2 FD 6 h vs. OVX + E2 FD 12 h, U = 39.0, P > 0.5; OVX+ E2 FD 6 h vs. OVX + E2 FD 24 h; U = 40.0, P = 0.276; OVX + E2 FD 12 h vs. OVX + E2 FD 24 h, U = 33.0, P > 0.5, Fig. 6).
Figure 6.
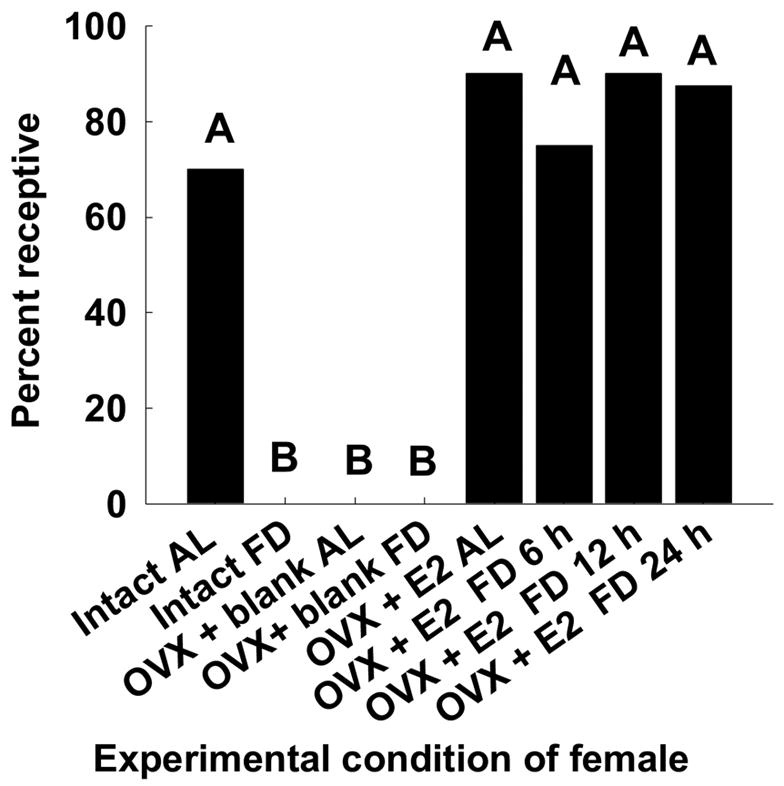
The proportion of female voles from the different treatment groups that displayed receptivity when paired with sexually experienced male voles. Different letters designate significant differences at P < 0.05.
Experiment 3.3
Most of the OVX females not given hormone replacement had estradiol titers below the detectable limits of the assay. OVX with titers below the detectable limit were assigned a value equal to the lower sensitivity of the assay, 2 pg/ml. The OVX + blank, both food-deprived and ad lib-fed females were pooled, as there were no significant differences between them, and they had an average of 4.6 ± 0.7 pg/ml of estradiol. The randomly selected sub-sample consisted of 5 OVX + E2, ad lib-fed females and 5 OVX E2, food-deprived females. The OVX + E2, ad lib-fed females had an average estradiol titer of 292.84 ± 39.36 pg/ml and the OVX + E2 food-deprived females had an average estradiol titer of 264.44 ± 22.55 pg/ml. Neither of these groups differed significantly from ad lib-fed intact females (Fig. 1). (One-way ANOVA, F(2,15)=1.146, P = 0.344), nor did they differ from one another (Student Neuman Kuels P = 0.385). The average intra-assay and the inter-assay coefficients of variation were 11.8% and 15.3%, respectively.
4. Discussion
In this study, we determined whether suppression of sexual behavior of food-deprived female voles was associated with a reduction in their circulating titers of estradiol. The results of the first experiment showed that food deprivation decreased the concentration of circulating estradiol in female meadow voles. That is, females that were food deprived for 6, 12 and 24 h had lower circulating estradiol titers relative to those of female voles that had continuous access to food (ad lib-fed). This finding supports our prediction that acute food-deprived females have lower estradiol titers than do female voles that had continuous access to food. Our data are also in agreement with the hypothesis that food deprivation lowers endogenous sex steroid concentration in food-deprived female mammals26, 27. Experiments conducted with mice, rats, hamsters, and primates reflect similar results. Lowering food availability or food intake reduces many physiological functions, among those that are relevant to decreasing estradiol titers, are decrease in function that the gonadatropin releasing hormone circuits that govern luteinizing hormone release 3, 4, 7, 10, 26–28. In the present study, we did not track changes in gonadotropin releasing hormone or luteinizing hormone. Yet, the net effect that we observed was a decline in estradiol titers of female voles that underwent acute food deprivation, and that the estradiol titers of these females were similar to those of female voles that were ovariectomized.
The results of experiment 2 showed that the decrease in their circulating estradiol titer was concomitant with the reduction or suppression of attractivity, proceptivity, and receptivity in food-deprived female voles. To our knowledge, this experiment is the first to address the necessary and sufficient role of a hormone in affecting olfactory cues, olfactory responses, and sexual behavior in the same animals. We assessed the attractivity of females in various states, relative to one another, by letting a male vole investigate their odors and measuring the time the spent investigating each odor. We manipulated the intervals of food deprivation and the estradiol treatments, and we found, that attractivity of the odors of food deprived female voles were significantly less attractive than the odors of ad lib-fed female voles. In addition, estradiol treatment was not sufficient to overcome negative effects of food deprivation on odor attractivity in female meadow voles that was caused by 24 h of food deprivation. That is, 24 h of food deprivation was sufficient to lower attractivity in OVX + E2 females to the level of attractivity to the level of OVX + blank, AL-fed females. This result is similar to our previous findings showing that 24 h of food deprivation was necessary to reduce the attractivity of odors of food-deprived female voles to male conspecifics 22. Our current results suggest that the decrease in attractivity among food-deprived females is independent of estradiol. These results are the first of their kind and of particular interest in that results from a previous study show long-term changes in attractivity among female voles are dependent on circulating estradiol titers 19.
We posit three possible mechanisms that may account for the difference in attractivity of odors to males produced by food-deprived female voles. First, food deprivation induces a decrease in overall metabolic rate29; that could slow down the metabolism of estradiol, decreasing the concentration of estrogen metabolites in the anogenital area secretions or affect the activity of sebaceous tissues in the anogenital area of food-deprived females. The concentration of estrogen metabolites in these secretions could also be changed by a decrease in estrogen receptor immunoreactivity in the periphery, similar to food deprivation-induced changes in the central estrogen receptor immunoreactivity30. Second, food deprivation may induce a rise in ketone body secretion resulting from increase oxidation of proteins during starvation. Ketosis generally produces unattractive body odors in humans31 and may induce food-deprived female voles to produce odors that are no longer attractive to male voles. Third, it is possible that metabolites from glucose, lipid, protein, or progesterone metabolism contribute to attractivity of female odors. In this case, estradiol treatment of OVX food deprived females would not be sufficient to fully restore the attractivity of odors.
We also determined whether the display of proceptive behavior of female voles was affected by the effects of estradiol and their access to food. We did so, by allowing female voles in different treatment groups to investigate the scent marks of both male and female conspecifics. We found that food deprivation induced females to suppress their proceptive behavior, in this case their preferences for the scent mark of male conspecifics to that of female conspecifics. A preference for the scent mark of male conspecifics over that of a female conspecific was induced after the food-deprived females received a physiological dose of estradiol. Our current findings were similar to those that show that estradiol replacement is sufficient to restore the odor preference of ovariectomized female voles for the odors of male conspecifics to that of female conspecifics 19. Our results are among the first in demonstrating that the proceptive behaviors of food-deprived female voles and ovariectomized voles depend on physiological concentrations of estradiol for their expression. Earlier work indicated that scent marking in musk shrews declined with metabolic fuel manipulation that is similar to food deprivation, but that study did not examine the role of gonadal hormones in mediating this effect 32.
In the present study, receptivity was restored in food-deprived female voles when they are treated with exogenous estradiol. Our findings augment a list of studies showing that food deprivation lowers estradiol titers and inhibits sexual receptivity in female mammals 3, 4, 7, 26–28, 33–35. Our results, however, are different from those reported for Syrian hamsters and musk shrews. We found that physiological doses of estradiol were sufficient to induce receptivity in food-deprived female voles. In contrast, gonadal steroids do not change in concentration as a result of food restriction11, although manipulating food availability also inhibits the sexual behavior of female musk shrews 32, 36, 37. In addition, food-deprived OVX hamsters treated with estradiol and progesterone displayed lordosis for shorter durations than did ad lib-fed intact females.
Although we found that estradiol treatment was sufficient to restore proceptive and receptive behavior in food-deprived female, the present study did not examine other possible mechanisms that may mediate the effects of food availability on sexual behavior. First, procuring and ingesting food stimulates the mouth and then causes distention of the gut; these signals reach the brain directly via the glossopharangeal nerve or the vagus nerve, respectively, and may impart information that food is available4. This stimulation may, in turn, stimulate cells in the hypothalamus to release gonadatropin releasing hormone, causing the pituitary to release luteinizing hormone, and then the ovaries would synthesize and release estradiol4. Perhaps, food deprivation disrupts this pathway, and results in decreased circulating estradiol (c.f. 13, 39). Second, the amount of metabolic fuels available to cells may mediate sexual behaviors30. In hamsters, hindbrain neurons in the area postrema are responsible for processing the presence of metabolic fuels sending signals to the reproductive centers of the brain to be active or not 3, 13, 32, 39, 40 3, 41. Third, other hormones that change with food intake may mediate sexual behavior. Decreases in these anorexogenic hormones (leptin and insulin) or increases in orexogenic hormones (e.g., ghrelin, neuropeptide Y, agouti related protein, cholecystokinin) induced by fasting may be responsible for signaling the brain to suppress sexual behavior41–43. Leptin’s effects appear to be dose dependent and paradoxical, and cholecystokinin appears to have little effect on mediating sexual behavior in hamsters44, 45. Last, the amount of body fat an individual has may mediate their sexual behavior 46, but several studies, including some with voles, do not support this hypothesis47, 48.
Acknowledgments
We thank Javier delBarco-Trillo for comments on an early draft of this manuscript and three anonymous reviewers for their insightful comments in helping improve the quality of this paper. The authors were supported by funding from NSF UMEB grant to University of Memphis, and by NSF grant IOB-0444553 and NIH grant HDO 49525 to MHF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bronson FH. Mammalian Reproductive Biology. University of Chicago Press; 1989. pp. 47–185. [Google Scholar]
- 2.Seymour PL, et al. Corticotropin-releasing factor receptor subtypes mediating nutritional suppression of estrous behavior in Syrian hamsters. American Journal of Physiology. 2005;289:R418–R423. doi: 10.1152/ajpregu.00168.2005. [DOI] [PubMed] [Google Scholar]
- 3.Wade GN, Jones JE. Neuroendocrinology of nutritional infertility. American Journal of Physiology. 2004;287:R1277–R1296. doi: 10.1152/ajpregu.00475.2004. [DOI] [PubMed] [Google Scholar]
- 4.Wade GN, et al. Control of fertility by metabolic cues. American Journal of Physiology. 1996;270:E1–E19. doi: 10.1152/ajpendo.1996.270.1.E1. [DOI] [PubMed] [Google Scholar]
- 5.Jones JE, et al. Disinhibition of female sexual behavior by a CRH receptor antagonist in Syrian hamsters. Am J Physiol. 2002;283:R591–R597. doi: 10.1152/ajpregu.00233.2002. [DOI] [PubMed] [Google Scholar]
- 6.Rissman EF, Bronson FH. Role of the ovary and adrenal gland in the sexual behavior of the musk shrew, Suncus murinus. Biology of Reproduction. 1987;36:664–668. doi: 10.1095/biolreprod36.3.664. [DOI] [PubMed] [Google Scholar]
- 7.Bronson FH, Marsteller FA. Effect of short-term food deprivation on reproduction in female mice. Biology of Reproduction. 1985;33:660–667. doi: 10.1095/biolreprod33.3.660. [DOI] [PubMed] [Google Scholar]
- 8.Kauffman AS, Rissman EF. A Critical Role for the Evolutionarily Conserved Gonadotropin-Releasing Hormone II: Mediation of Energy Status and Female Sexual Behavior. Endocrinology. 2004;145:3639–3646. doi: 10.1210/en.2004-0148. [DOI] [PubMed] [Google Scholar]
- 9.Loucks AB, et al. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol. 1998;84:37–46. doi: 10.1152/jappl.1998.84.1.37. [DOI] [PubMed] [Google Scholar]
- 10.Schneider JE. Energy balance and reproduction. Physiology & Behavior. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Temple JL, Rissman EF. Brief refeeding restores reproductive readiness in food-restricted female musk shrews (Suncus murinus) Hormones and Behavior. 2000;38:21–28. doi: 10.1006/hbeh.2000.1596. [DOI] [PubMed] [Google Scholar]
- 12.Morin LP. Environment and hamster reproduction: responses to phase-specific starvation during estrous cycle. American Journal of Physiology. 1986;251:R663–R669. doi: 10.1152/ajpregu.1986.251.4.R663. [DOI] [PubMed] [Google Scholar]
- 13.Dickerman RW, et al. Decreased availability of metabolic fuels suppresses estrous behavior in Syrian hamsters. American Journal of Physiology. 1993;264:R568–R572. doi: 10.1152/ajpregu.1993.264.3.R568. [DOI] [PubMed] [Google Scholar]
- 14.Jones JE, et al. Food deprivation inhibits estrous behavior in hormone-treated Syrian hamsters despite elevated estradiol levels. Hormones and Behavior. 2002;41:316–320. doi: 10.1006/hbeh.2002.1764. [DOI] [PubMed] [Google Scholar]
- 15.Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Hormones and Behavior. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- 16.Ferkin MH, et al. Ovarian hormones influence odor cues emitted by female meadow voles, Microtus pennsylvanicus. Hormones and Behavior. 1991;25:572–581. doi: 10.1016/0018-506x(91)90022-a. [DOI] [PubMed] [Google Scholar]
- 17.Ferkin MH, Johnston RE. Role of gonadal hormones in five sexually attractive odors of meadow voles (Microtus pennsylvanicus) Horm Behav. 1993;27:523–538. doi: 10.1006/hbeh.1993.1038. [DOI] [PubMed] [Google Scholar]
- 18.Ferkin MH, et al. Seasonal changes in scents and responses to them in meadow voles: evidence for the co-evolution of signals and response mechanisms. Ethology. 1995;100:89–98. [Google Scholar]
- 19.Ferkin MH, Zucker I. Seasonal control of odour preference of meadow voles (Microtus pennsylvanicus) by photoperiod and ovarian hormones. J Reprod Fertil. 1991;92:433–441. doi: 10.1530/jrf.0.0920433. [DOI] [PubMed] [Google Scholar]
- 20.Gray GD, Dewsbury DA. A quantitative description of the copulatory behaviour of meadow voles (Microtus pennsylvanicus) Animal Behaviour. 1975;23:261–267. [Google Scholar]
- 21.Johnston RE. Olfactory preferences, scent marking and “proceptivity” in female hamsters. Hormones and Behavior. 1979;13:21–39. doi: 10.1016/0018-506x(79)90032-1. [DOI] [PubMed] [Google Scholar]
- 22.Pierce AA, et al. Food deprivation-induced changes in sexual behavior of meadow voles, Microtus pennsylvanicus. Animal Behaviour. 2005;70:339–348. [Google Scholar]
- 23.Pierce AA, Ferkin MH. Re-feeding and the restoration of odor attractivity, odor preference, and sexual receptivity in food-deprived female meadow voles. Physiol Behav. 2005;84:553–561. doi: 10.1016/j.physbeh.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Ferkin MH, et al. Influence of gonadal hormones on odours emitted by male meadow voles, Microtus pennsylvanicus. J Reprod Fertil. 1992;25:729–736. doi: 10.1530/jrf.0.0950729. [DOI] [PubMed] [Google Scholar]
- 25.Ferkin MH, Johnston RE. Effects of pregnancy, lactation, and postpartum estrous on odour signals and the attraction to odours in female meadow voles, Microtus pennsylvanicus. Animal Behaviour. 1995;49:1211–1217. [Google Scholar]
- 26.Bronson FH, Heideman PD. Short-term hormonal responses to food intake in peripubertal female rats. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 1990;259:R25–R31. doi: 10.1152/ajpregu.1990.259.1.R25. [DOI] [PubMed] [Google Scholar]
- 27.Bucholtz D, et al. Metabolic interfaces between growth and reproduction. V. Pulsatile luteinizing hormone secretion is dependent on glucose availability. Endocrinology. 1996;137:601–607. doi: 10.1210/endo.137.2.8593808. [DOI] [PubMed] [Google Scholar]
- 28.Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neuroscience and Biobehavioral Reviews. 1992;16:235–272. doi: 10.1016/s0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- 29.Nagy TR, Pistole DH. The effects of fasting on some physiological parameters in the meadow vole, Microtus pennsylvanicus. Comparative Biochemistry and Physiology A. 1988;91:679–684. doi: 10.1016/0300-9629(88)90948-6. [DOI] [PubMed] [Google Scholar]
- 30.Li HY, et al. Manipulations of metabolic fuel availability alter estrous behavior and neural estrogen-receptor immunoreactivity in Syrian hamsters. Endocrinology. 1994;135:240–247. doi: 10.1210/endo.135.1.8013358. [DOI] [PubMed] [Google Scholar]
- 31.Lori L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes/Metabolism Research and Reviews. 1999;15:412–426. doi: 10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Temple JL, et al. Mating behavior is controlled by acute changes in metabolic fuels. American Journal of Physiology. 2000;282:R782–R790. doi: 10.1152/ajpregu.00383.2001. [DOI] [PubMed] [Google Scholar]
- 33.Bronson F. Food-restricted, prepubertal, female rats: rapid recovery of luteinizing hormone pulsing with excess food, and full recovery of pubertal development with gonadotropin-releasing hormone. Endocrinology. 1986;118:2483–2487. doi: 10.1210/endo-118-6-2483. [DOI] [PubMed] [Google Scholar]
- 34.Loucks AB, Verdun M. Slow restoration of LH pulsatility by refeeding in energetically disrupted women. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1218–1226. doi: 10.1152/ajpregu.1998.275.4.R1218. [DOI] [PubMed] [Google Scholar]
- 35.Loucks AB, et al. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol. 1998;84:37–46. doi: 10.1152/jappl.1998.84.1.37. [DOI] [PubMed] [Google Scholar]
- 36.Gill CJ, Rissman EF. Female sexual behavior is inhibited by short- and long-term food restriction. Physiology and Behavior. 1997;61:387–394. doi: 10.1016/s0031-9384(96)00449-0. [DOI] [PubMed] [Google Scholar]
- 37.Temple JL. The musk shrew (Suncus murinus): a model species for studies of nutritional regulation of reproduction. Institute for Laboratory Animal Research Journal. 2004;45:25–34. doi: 10.1093/ilar.45.1.25. [DOI] [PubMed] [Google Scholar]
- 38.Pfaff DW, et al. X-ray cinematographic analysis of lordosis in female rats. Journal of Comparative Physiological Psychology. 1978;92:937–941. [Google Scholar]
- 39.Schneider JE, Wade GN. Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science. 1989;244:1326–1328. doi: 10.1126/science.2734610. [DOI] [PubMed] [Google Scholar]
- 40.Jones JE, Lubbers LS. Suppression and recovery of estrous behavior in Syrian hamsters after changes in metabolic fuel availability. American Journal of Physiology. 2001;280:R1093–R1098. doi: 10.1152/ajpregu.2001.280.5.R1393. [DOI] [PubMed] [Google Scholar]
- 41.Panicker AK, et al. AP lesions block suppression of estrous behavior, but not estrous cyclicity, in food-deprived Syrian hamsters. American Journal of Physiology. 1998;275:R158–R164. doi: 10.1152/ajpregu.1998.275.1.R158. [DOI] [PubMed] [Google Scholar]
- 42.Panicker AK, Wade GN. Insulin-induced repartitioning of metabolic fuels inhibits estrous behavior in Syrian hamsters: Role of area postrema. American Journal of Physiology. 1998;274:R1094–R1098. doi: 10.1152/ajpregu.1998.274.4.R1094. [DOI] [PubMed] [Google Scholar]
- 43.Schneider JE, et al. Glucoprivic treatments that induce anestrus, but do not affect food intake, increase FOS-like immunoreactivity in the area postrema and nucleus of the solitary tract in Syrian hamsters. Brain Research. 1995;698:107–113. doi: 10.1016/0006-8993(95)00860-s. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz MW, et al. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 45.Ariyasu H, et al. Stomach Is a Major Source of Circulating Ghrelin, and Feeding State Determines Plasma Ghrelin-Like Immunoreactivity Levels in Humans. Journal of Clinical Endocrinology and Metabolism. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 46.Jones JE, et al. Effects of naltrexone and CCK on estrous behavior and food intake in Syrian hamsters. Peptides. 2001;22:601–606. doi: 10.1016/s0196-9781(01)00370-9. [DOI] [PubMed] [Google Scholar]
- 47.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. 89. 1992:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z, et al. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Research. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]


