Summary
ClpB is a ring-shaped molecular chaperone, which has the remarkable ability to disaggregate stress-damaged proteins. Here we present the electron cryomicroscopy reconstruction of an ATP-activated ClpB trap mutant, along with reconstructions of ClpB in the AMPPNP, ADP, and in the nucleotide-free state. We show that motif 2 of the ClpB M-domain is positioned between the D1-large domains of neighboring subunits and could facilitate a concerted, ATP-driven conformational change in the AAA-1 ring. We further demonstrate biochemically that ATP is essential for high-affinity substrate binding to ClpB and cannot be substituted with AMPPNP. Our structures show that, in the ATP-activated state, the D1 loops are stabilized at the central pore, providing the structural basis for high-affinity substrate binding. Taken together, our results support a mechanism by which ClpB captures substrates on the upper surface of the AAA-1 ring before threading them through the ClpB hexamer in an ATP hydrolysis-driven step.
Introduction
ClpB and its eukaryotic orthologs Hsp101 and Hsp104 are ATP-dependent molecular chaperones essential for thermotolerance (Queitsch et al., 2000; Sanchez and Lindquist, 1990; Squires et al., 1991). ClpB belongs to the AAA+ ATPase superfamily of molecular machines that are found in all kingdoms (Neuwald et al., 1999). Despite their diverse functions, AAA+ proteins possess homologous Walker-type nucleotide-binding domains and share the propensity to form oligomeric ring structures (Ogura and Wilkinson, 2001; Ye et al., 2004). In light of their structural resemblance, it is conceivable that AAA+ proteins utilize similar mechanisms for converting the energy derived from ATP binding and hydrolysis into mechanical work (Hanson and Whiteheart, 2005; Wang, 2004; Ye et al., 2004).
ClpB is a multi-domain protein that consists of a globular N-terminal domain and two AAA+ modules in tandem (AAA-1 and AAA-2). Each AAA+ module consists of a RecA-like domain (D1/2-large) and a mostly α-helical domain (D1/2-small). The D1-small domain harbors the ClpB M-domain that is essential for chaperone activity (Cashikar et al., 2002; Kedzierska et al., 2003; Lee et al., 2003; Mogk et al., 2003). The M-domain is mobile (Lee et al., 2003; Watanabe et al., 2005) and consists of two shorter coiled-coil motifs (motif 1 and motif 2) that share a common 85 Å long α-helix (Lee et al., 2003). Unlike other Clp/Hsp100 proteins, however, ClpB neither associates with a chambered protease nor directs the degradation of its substrates (Maurizi and Xia, 2004; Sauer et al., 2004). Instead, ClpB has the remarkable ability to disaggregate stress-damaged proteins from a previously aggregated state, a process that requires the assistance of the DnaK chaperone system (Glover and Lindquist, 1998; Goloubinoff et al., 1999; Mogk et al., 1999; Motohashi et al., 1999; Zolkiewski, 1999).
We recently determined the hexameric structure of T. thermophilus ClpB (TClpB) by fitting the 3.0 Å resolution crystal structure of a TClpB monomer into a ∼21 Å resolution electron cryomicroscopy (cryo-EM) reconstruction of the AMPPNP-bound state (Lee et al., 2003). ClpB forms a two-tiered hexameric ring structure, with six copies of the AAA-1 and AAA-2 domains forming the top and bottom ring, respectively (Lee et al., 2003). Next, we have engineered a ClpB variant, known as BAP (ClpB-ClpA-P loop), which, unlike wild-type ClpB, can associate with the ClpP protease to form a novel disaggregating-degrading machine (Weibezahn et al., 2004). Using BAP, we have demonstrated that ClpB’s essential role in thermotolerance relies on its ability to disaggregate stress-damaged proteins via substrate translocation through the central pore of the hexamer (Weibezahn et al., 2004).
While ClpB utilizes the energy derived from ATP binding and hydrolysis to facilitate protein disaggregation, it remains unclear how the nucleotide-driven conformational changes are coupled to substrate recognition and binding. Determining the structure of a ClpB-substrate complex is very difficult due to the dynamic nature of substrate interaction, and because ClpB recognizes either aggregated or aggregation-prone proteins that are difficult to manipulate (Cashikar et al., 2002; Mogk et al., 1999). A major breakthrough was achieved by Bukau, Mogk and colleagues, who characterized an E. coli ClpB double Walker B mutant (E279A/E678A) that binds but does not hydrolyze ATP (Weibezahn et al., 2003). Most remarkably, this ClpB mutant functions as a physical trap that binds substrates in a stable, ATP-dependent manner.
To assess ClpB-substrate interaction in different nucleotide states, we used fluorescence polarization to measure the binding affinity of TClpB and a TClpB double Walker B mutant (E271A/E668A) for two model substrates. We show that, like the E. coli ClpB trap mutant, TClpB (E271A/E668A) functions as a substrate trap in the presence of ATP (Trap-ATP). Moreover, we found that tri-phosphates are essential for high-affinity substrate binding. Most remarkably, AMPPNP cannot substitute for ATP (Trap) or ATPγS (TClpB) to promote stable substrate binding, indicating that the TClpB-AMPPNP hexamer differs structurally from the ATP-activated state.
To illuminate the structural basis for high-affinity substrate binding, we determined the cryo-EM structures of Trap-ATP, TClpB-AMPPNP, TClpB-ADP, and nucleotide-free (apo) TClpB at resolutions ranging from 11.2 Å to 17.7 Å. Moreover, we generated an atomic model for both the Trap-ATP and TClpB-AMPPNP hexamer by fitting the crystal structure of a TClpB monomer into the corresponding cryo-EM reconstruction. Our structures show that motif 2 of the M-domain is positioned between the D1-large domains of neighboring subunits and could facilitate cooperative interactions in the AAA-1 ring. Comparison of the ATP- and AMPPNP-bound structures reveals that, in the ATP-activated state, the D1 loops of all six subunits are stabilized at the central pore of the ClpB hexamer. This increases the solvent-accessible, upper surface area of the AAA-1 ring and exposes Tyr243, a residue critical for substrate binding. These structural features are not observed in the AMPPNP, ADP and apo states, supporting the notion that stabilization of the D1 loops at the central pore is responsible for high-affinity substrate binding.
Taken together, our results suggest that ATP activation promotes stable substrate binding to the upper surface of the AAA-1 ring, followed by an ATP hydrolysis-driven translocation through the ClpB hexamer.
Results and Discussion
Cryo-EM Analysis of the TClpB Hexamer
TClpB and the TClpB Trap mutant were overexpressed and purified as described (Lee et al., 2003). For cryo-EM, TClpB hexamers were prepared by incubating TClpB with or without 2 mM nucleotide at 55 °C, the physiological temperature for T. thermophilus (Watanabe et al., 2002). Trap hexamers were prepared in a similar manner in the presence of 2 mM ATP. Once formed, the hexamers were stabilized by cross-linking with glutaraldehyde and further purified by sizeexclusion chromatography. Since we cross-linked a pre-formed TClpB and Trap hexamer, respectively, it is highly unlikely that our glutaraldehyde cross-linking procedure altered the final ClpB conformation. This is supported by X-ray crystallographic studies on other proteins, which showed that glutaraldehyde cross-linking did not perturb protein structure (Buhrman et al., 2003; Yonath et al., 1977).
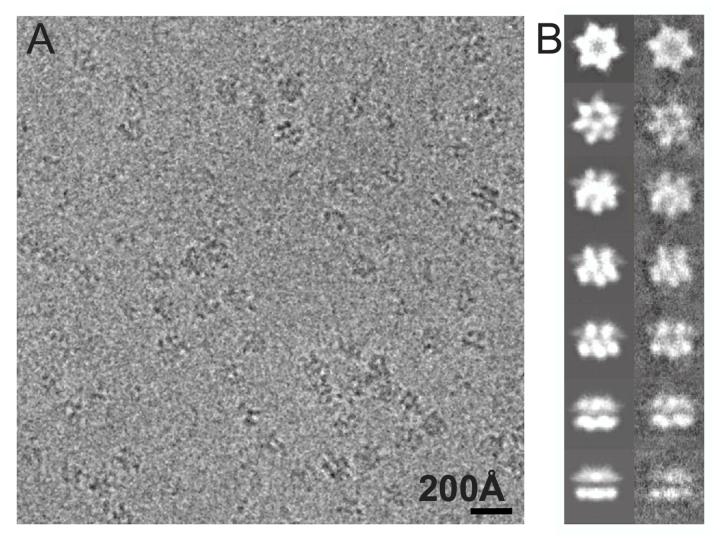
Examination of frozen hydrated specimens by cryo-EM shows that the TClpB hexamers were well distributed, showing no signs of aggregation and displaying a wide range of orientations in vitreous ice (Figure 1A). Table 1 summarizes the final statistics of our cryo-EM reconstructions which were determined to a nominal resolution of 11.2 Å (Trap-ATP), 12.1 Å (TClpB-AMPPNP), 16.7 Å (TClpB-ADP), and 17.7 Å (TClpB-apo) at a Fourier shell correlation (FSC) of 0.5 (Supplementary Figure 1).
Figure 1.
Cryo-EM analysis of the TClpB hexamer.
(A) Representative area of a digital micrograph of the Trap-ATP hexamer in vitreous ice.
(B) The left panels show selected projection views of the Trap-ATP reconstruction. The corresponding class averages are shown in the right panels.
Table 1.
Summary of the data used forthe cryo-EM reconstructions.
| Cryo-EM Reconstruction | Total number of particles selected | Total number of particles used in the final refinement | Defocus (μm) | Final Resolution (Å) |
|---|---|---|---|---|
| Trap-ATP | 19008 | 15302 | 1.2-3.6 | 11.2 |
| TClpB-AMPPNP | 21105 | 16230 | 1.8-3.1 | 12.1 |
| TClpB-ADP | 17270 | 15300 | 1.4-3.2 | 16.7 |
| TClpB-apo | 10453 | 8163 | 1.9-3.9 | 17.7 |
Cryo-EM Structure of the TClpB Hexamer in Different Nucleotide States
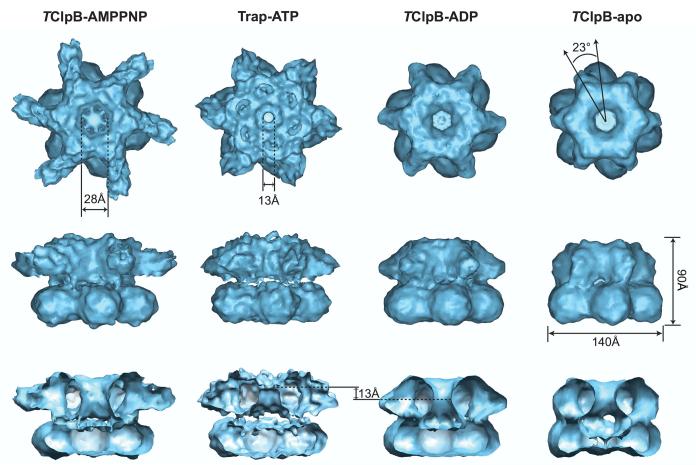
The gross structure of the ClpB hexamer is similar in all four states examined, even though the structures differ in detail (Figure 2). Our cryo-EM reconstructions consistently show that TClpB forms a two-tiered hexameric ring structure both in the presence and absence of nucleotides. Thisis in marked contrast to a recent report suggesting that the apo state of E. coli ClpB is a heptamer (Akoev et al., 2004). The nature of this discrepancy is unknown but may reflect differences in resolution, sample preparation, or species-specific disparities between ClpBs from mesophilic and thermophilic bacteria. In our current reconstructions, the two hexameric rings are stacked on top of each other with the top ring rotated ∼23° counterclockwise relative to the bottom ring (Figure 2). Twisting between the AAA-1 and AAA-2 rings is also seen in the cryo-EM reconstruction of ClpA (Ishikawa et al., 2004) but not in p97/VCP, the only AAA+ ATPase with two AAA+ domains for which a crystal structure of the hexameric assembly is currently available (DeLaBarre and Brünger, 2003; DeLaBarre and Brünger, 2005; Huyton et al., 2003).
Figure 2.
Cryo-EM reconstruction of the TClpB hexamer in different nucleotide states. The figure shows top down, side, and cut-away side views of the TClpB-AMPPNP, Trap-ATP, TClpB-ADP, and TClpB-apo hexamer obtained by cryo-EM and single particle reconstruction techniques. The TClpB hexamer has a height of 90 Å and a diameter of 140 Å (bottom ring). The AAA-1 and AAA-2 rings are stacked on top of each other with the top ring rotated ∼23° counterclockwise with respect to the bottom ring. While the central pore of the bottom ring is closed, the pore is open in the top ring and leads to a large internal cavity. The size of the central pore in the top ring varies in diameter and is the widest in the AMPPNP-bound state (28 Å) and the narrowest in the ATP-activated state (13 Å). It is noteworthy that, in the ADP-bound state, the narrowest part of the pore is located ∼13 Å below the narrowest part in the Trap-ATP hexamer.
The top ring of the ClpB hexamer undergoes most of the structural changes observed in different cryo-EM reconstructions (Figure 2). For instance, while the central pore of the bottom ring is closed in all nucleotide states examined, the pore of the top ring is open and leads to a large central cavity. The pore size of the top ring varies between 13 Å and 28 Å in diameter, depending on the nucleotide state. Together, our data support the notion that the top ring undergoes an ATP-driven conformational change, while the bottom ring remains essentially unchanged.
Our cryo-EM reconstruction of the TClpB-AMPPNP hexamer is similar to our previous ∼21 Å resolution reconstruction (Lee et al., 2003). The most striking structural differences are the six long spokes in the top ring, which splay out radially from the main body and are much shorter in all other nucleotide states examined (Figure 3). Other notable changes include a more compact quaternary structure, a better defined bottom ring, and a wider central pore (data not shown). The observed differences are likely due to the higher resolution of our current data and the larger number of particles used for the final cryo-EM reconstruction (see Experimental Procedures).
Figure 3.
Comparison of the cryo-EM reconstruction of TClpB in different nucleotide states. Top down and side views of the isosurface representation of different nucleotide states, which were superimposed pairwise via the AAA-2 ring. The TClpB-AMPPNP hexamer is shown in grey, the Trap-ATP hexamer in magenta, the TClpB-ADP hexamer in cyan, and the TClpB-apo hexamer in gold. The arrows point to the six protruding mass densities on the top surface of the AAA-1 ring in the Trap-ATP hexamer, which likely account for the beginning of the flexible linker that connects the N-terminal domain to the AAA-1 domain.
A pairwise superimposition of the ATP- and AMPPNP-bound states shows that the two cryo-EM structures differ in detail (Figure 3). This is surprising, since AMPPNP is often believed to mimic ATP, and can support some Clp/Hsp100 function (Huang and Goldberg, 1997; Lee et al., 2003; Maurizi, 1991; Shorter and Lindquist, 2004; Shorter and Lindquist, 2006; Thompson and Maurizi, 1994). Here, we show that the Trap-ATP hexamer adopts a more domeshaped structure, displaying a larger solvent-exposed surface, and a smaller central pore, 13 Å in diameter (Figures 2 and 3). Moreover, while the long spokes are less defined, additional 6-fold symmetrical mass densities are observed on the upper surface of the top ring (Figure 3).
The cryo-EM reconstructions of the ADP and apo states were determined at a lower resolution than the AMPPNP- and ATP-bound hexamers (Table 1). It is noteworthy that, in the apo state, the mass density in the central region of the bottom ring is considerably thinner, consistent with a less stable ClpB hexamer (Figure 2) (Watanabe et al., 2002; Zolkiewski et al., 1999).
Atomic Models of the Trap-ATP and TClpB-AMPPNP Hexamer
To generate an atomic model for the Trap-ATP and TClpB-AMPPNP hexamers, we fitted the crystal structure of a TClpB monomer (PDB entry code: 1QVR) into our cryo-EM maps. As shown previously (Lee et al., 2003), no additional mass density was observed that could account for the N-terminal domains, suggesting that they are mobile and were “averaged out” during single-particle reconstruction. The hexamer models were generated by applying 6-fold rotational symmetry to a fitted N-terminal domain-truncated monomer (see Experimental Procedures). Interestingly, the cryo-EM reconstruction of the Trap-ATP hexamer revealed six additional protruding mass densities on the top surface of the AAA-1 ring (Figure 3). These mass densities were not observed in any other nucleotide state examined. In our current model, the N-terminus of the D1-large domain is located near this protruding density. Hence, we surmised that the additional mass could represent the beginning of the flexible linker that connects the N-terminal domain to the D1-large domain (data not shown).
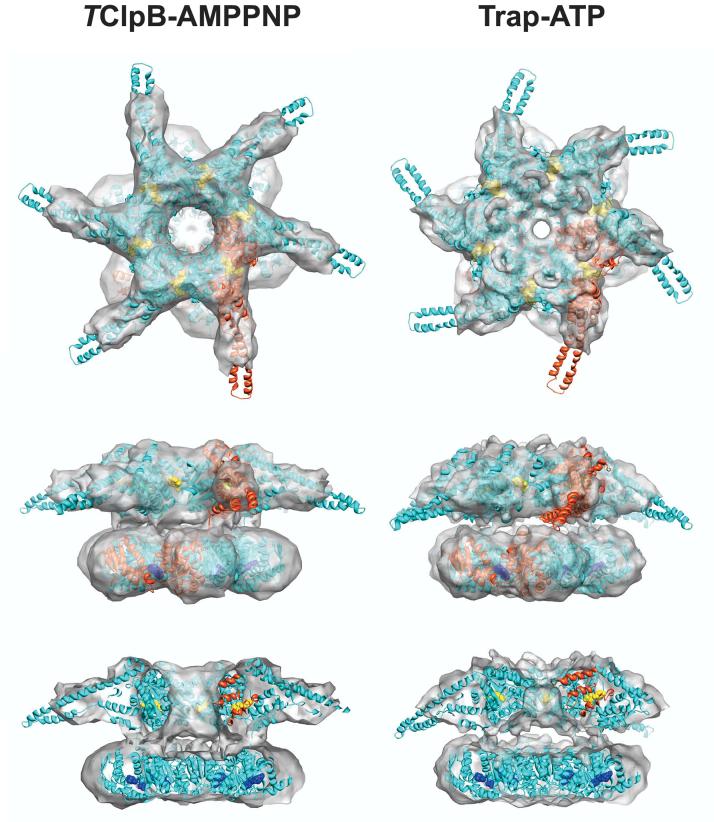
Figure 4 depicts the atomic models for the Trap-ATP and TClpB-AMPPNP hexamer. In our model, the D1-large and D2-large domains of the same TClpB subunit are in a staggered conformation. This tertiary arrangement is similar to the proposed structure of a ClpA hexamer (Guo et al., 2002), but differs from our earlier model (Lee et al., 2003), likely because of the limited resolution of our previous cryo-EM reconstruction.
Figure 4.
Atomic models of the fitted TClpB-AMPPNP and Trap-ATP hexamer. The cryo-EM reconstruction is shown as a semitransparent surface (top down, side, and cut-away side view), with the structure of an N-terminal domain-truncated TClpB hexamer docked in, demonstrating the goodness of fit. The hexamer model is depicted as a ribbon diagram. One subunit of the hexamer is highlighted in red, illustrating the staggered conformation of the AAA-1 and AAA-2 domains. The bound nucleotides are shown as CPK models and are colored yellow (AAA-1) and blue (AAA-2), respectively.
The most striking structural features are the six long spokes in the top ring of the TClpB-AMPPNP hexamer. The shape of each spoke is most consistent with an elongated coiled-coil (Lee et al., 2003), and less consistent with a globular and stably folded N-terminal domain (Tek and Zolkiewski, 2002). Hence, we surmised that each spoke accounts for a near intact M-domain that extends from the top ring in a close to perpendicular fashion (Figure 4). In the Trap-ATP hexamer model, the M-domains adopt a more leaning position, giving them a dynamic appearance (Figure 4). Moreover, while the structure of the AAA-2 ring is essentially the same in both models, the pore of the AAA-1 ring is much narrower in the ATP-activated state (Figure 4 and 5A). The additional mass in the pore can be attributed to the D1 loop (residues 235-245 in TClpB) (Hinnerwisch et al., 2005) that was disordered in the crystal structure of TClpB (Lee et al., 2003) but is ordered in the crystal structure of an Hsp104 fragment (S.L and F.T.F.T., unpublished data). This allowed us to model the ClpB D1 loop by fitting the analogous D1 loop from Hsp104 onto the structure of TClpB.
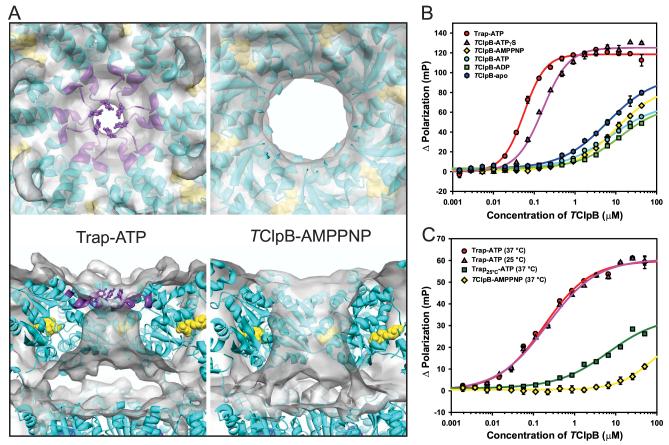
Figure 5.
Substrate binding to the TClpB hexamer is ATP- and temperature-dependent.
(A) Structural basis for high-affinity substrate binding. The figure shows an enlarged top down and cut-away side view of the pore region of the fitted Trap-ATP and TClpB-AMPPNP hexamer. The cryo-EM reconstruction is shown as a semitransparent surface, the hexamer model as a ribbon diagram, Tyr243 as a ball-and-stick model, and the bound nucleotides as CPK models. Residues 238-250 in the pore region, which lie outside the mass density in the TClpB-AMPPNP reconstruction, are colored purple. The figure shows that ATP-activation stabilizes the D1 loop at the central pore. The positions of the Tyr243 side-chains are shown for clarity.
(B) Binding isotherm of FITC-casein and TClpB. To measure fluorescence polarization, the Trap and TClpB hexamers were pre-assembled at 55 °C in the presence or absence of nucleotide, and mixed with FITC-casein. All measurements were carried out at 55 °C. The binding curve for Trap-ATP is shown in red, TClpB-ATPγS in purple, TClpB-AMPPNP in yellow, TClpB-ATP in cyan, TClpB-ADP in green, and TClpB-apo in blue. The curves represent least-square nonlinear regression fits of the change in fluorescence polarization obtained from three independent measurements. Standard deviations, if larger than the size of the symbols, are shown. The apparent KD was calculated where possible and is 0.147 ± 0.010 μM for Trap-ATP and 0.378 ± 0.009 μM for TClpB-ATPγS, corresponding to a calculated KD of 0.025 μM and 0.063 μM for the respective hexamers.
(C) Binding isotherm of FAM-TrfA and TClpB. Trap-ATP and TClpB-AMPPNP hexamers were generated as described in (B). The Trap25°C-ATP sample was prepared by pre-incubation of Trap mutant at 25 °C in the presence of 1 mM ATP. To measure fluorescence polarization, the pre-assembled Trap-ATP and TClpB-AMPPNP samples were mixed with FAM-TrfA, incubated for 5 min at either 37 °C or 25 °C as shown, and measured at the corresponding temperature. The binding curve for Trap-ATP at 37 °C is shown in red, Trap-ATP at 25 °C in purple, Trap25°C-ATP at 37 °C in green, and TClpB-AMPPNP at 37 °C in yellow. The curves represent leastsquare nonlinear regression fits of the change in fluorescence polarization from three independent measurements. Standard deviations, if larger than the size of the symbols, are shown. The apparent KD for the binding of FAM-TrfA to Trap-ATP was calculated to be 0.267 ± 0.022 μMat 37 °C and 0.308 ± 0.014 μM at 25 °C, corresponding to 0.045 μM and 0.051 μM for the respective hexamers.
Taken together, our atomic models suggest that the TClpB-AMPPNP and Trap-ATP hexamer structures represent different conformational states.
ClpB Binds Substrates in an ATP-dependent Manner
To investigate the ability of TClpB to bind substrates in different nucleotide states, we measured the binding affinity of pre-assembled Trap and TClpB hexamers for fluorescein-5-isothiocyanate-conjugated casein (FITC-casein). Our data show that high-affinity substrate binding strictly requires tri-phosphates (Trap-ATP or TClpB-ATPγS) ure (Fig 5B). As anticipated, TClpB-ATP did not bind substrates in a stable manner presumably because TClpB hydrolyzes ATP readily (data not shown), concomitant with substrate release. TClpB bound FITC-casein with apparent equilibrium dissociation constants (KD) of 0.147 ± 0.010 μM (Trap-ATP) and 0.378 ± 0.009 μM (TClpB-ATPγS) , corresponding to a calculated KD of 0.025 μM and 0.063 μM for the respective hexamers. To our surprise, high-affinity substrate binding was not observed in the presence of AMPPNP (Figure 5B). A KD value for this nucleotide state could not be determined due to low-affinity binding of FITC-casein to the TClpB-AMPPNP hexamer, which made measurements at saturating concentrations impossible. This was also the case for the other nucleotide states examined.
ATP-dependent substrate binding was also observed with TrfA, a physiological ClpB substrate (Konieczny and Liberek, 2002; Weibezahn et al., 2004), which in our experiments was labeled with 5-carboxyfluorescein succinimidyl ester (FAM-TrfA) (Figure 5C). Like FITCcasein, high-affinity substrate binding was only observed with a pre-assembled Trap-ATPhexamer but not with TClpB-AMPPNP. This was neither due to AMPPNP hydrolysis (data not shown) nor to trace amounts of ADP present in AMPPNP solutions, since our cryo-EM reconstructions of the AMPPNP- and ADP-bound states are different (Figure 3). Lack of substrate binding in the presence of AMPPNP was also reported recently for HslU (Burton et al., 2005), a distant relative of ClpB, suggesting that the functional correlation between ATPbinding/hydrolysis and substrate binding may be conserved among AAA+ proteins.
To test whether substrate binding is temperature dependent, we measured the binding affinity of Trap-ATP and TClpB-AMPPNP for FAM-TrfA at 25 °C and 37 °C, respectively (Figure 5C). Binding experiments at the physiological temperature of 55 °C were not possible since TrfA precipitated at higher temperatures (data not shown). While the Trap-ATP hexamer bound FAM-TrfA with similar KD values at either 37 °C or 25 °C (0.045 μM and 0.051 μM, respectively), high-affinity substrate binding was strictly dependent on pre-assembly of the Trap-ATP hexamer at 55 °C (Figure 5C: compare Trap-ATP versus Trap25°C-ATP).
Taken together, our data suggest that ClpB binds substrates with both high- and low- affinity depending on the nucleotide state. High-affinity substrate binding requires hexamer assembly at the physiological temperature and is strictly ATP-dependent, but cannot be substituted with AMPPNP. Consequently, the immobilization of the M-domains, as seen in the TClpB-AMPPNP hexamer structure (Figure 4), cannot contribute to stable substrate binding. The function of the immobilized M-domain is currently unknown.
ATP-activation Stabilizes the Critical Tyr243 at the Pore of the ClpB Hexamer
Our cryo-EM reconstructions show that the pore size of the AAA-1 ring is the narrowest in the ATP-activated state (Figure 2). The additional mass in this nucleotide state accounts for the D1 loop that harbors Tyr243, a residue critical for substrate binding and translocation (Lum et al., 2004; Schlieker et al., 2004b; Weibezahn et al., 2004). In the Trap-ATP hexamer model, the critical Tyr243s are positioned at the center of the pore (Figure 5A), where they are accessible to substrates, as suggested in UV cross-linking studies (Hinnerwisch et al., 2005; Schlieker et al., 2004b). This additional mass in the pore was not observed in all other nucleotide states examined, suggesting that the stabilization of the D1 loop at the pore and the resulting larger, solvent-exposed, upper surface area of the top ring are critical for high-affinity substrate binding. It is noteworthy that, in the ADP-bound state, the narrowest part of the pore is located ∼13 Å below the narrowest part in the Trap-ATP hexamer (Figure 2 and Supplementary Figure 2), indicating a possible downward motion of the D1 loops as would be required for substrate translocation.
ATP-driven Conformational Changes of the M-domain
The M-domains are most ordered in the AMPPNP-bound state and become increasingly more flexible in the ATP- and ADP-bound states, and are no longer visible in the apo state (Figure 3). Hence, we propose that the hinge region between the D1-small domain and the M-domain is flexible in the absence of nucleotide. Surprisingly, none of our atomic models reveal an M-domain conformation seen in the crystal structure of TClpB (Figure 6C). Yet, we have previously shown, by sulfhydryl cross-linking of the M-domain to the D1-large domain via G167C/R475C, that the M-domain conformation in the crystal structure also occurs in solution, and is independent of the crystal lattice (Lee et al., 2003; Watanabe et al., 2005).
Figure 6.
The M-domain undergoes a large motion.
(A) Top down view of the AAA-1 ring of an N-terminal domain-truncated Trap-ATP hexamer fitted into the corresponding cryo-EM reconstruction shown as a semitransparent surface. The figure illustrates the position of the ClpB M-domain in the ATP-activated state. The Trap-ATP hexamer model is depicted as a ribbon diagram and is colored cyan, with the AAA-1 domain of one subunit highlighted red and its corresponding M-domain blue. The bound ATP molecules are shown as CPK models and are colored yellow.
(B) Enlarged view of the M-domain position in the Trap-ATP hexamer model as seen in (A). The figure shows that motif 2 of the M-domain is positioned between the D1-large domains of neighboring subunits. The position of motif 2 is supported by an independent sulfhydryl crosslinking study using E. coli ClpB (Haslberger et al., 2007). The position of G167 (G175 in E. coli ClpB) and A490 (S499 in E. coli ClpB), which were mutated to form a disulfide cross-link are depicted as green spheres.
(C) Enlarged view of the M-domain position as seen in the crystal structure of a TClpBAMPPNP monomer (Lee et al., 2003),. For clarity, only the AAA-1 domain of the TClpB-AMPPNP monomer is shown, which was superimposed via the D1-large domain onto the atomic model of the Trap-ATP hexamer. The AAA-1 domain is colored orange with the M-domain highlighted blue. Motif 1 and motif 2 of the M-domain are labeled accordingly. The figure shows the position of G167 and R475 (green spheres), which were mutated to form a disulfide crosslink (Lee et al., 2003).
In the ATP- and AMPPNP-bound states, motif 1 of the M-domain points away from the hexamer, while motif 2 is positioned between the D1-large domains of neighboring subunits and, thereby, could facilitate cooperative interactions within the AAA-1 ring (Figures 6A, 6B, and data not shown). Despite the limited resolution of our cryo-EM reconstructions, the position of motif 2 in our hexamer model is supported by an independent study using E. coli ClpB (Haslberger et al., 2007). Most remarkably, G167C and D170C (G175C and D178C in E. coli ClpB) can be cross-linked to A490C (S499C in E. coli ClpB) of the same ClpB subunit (Haslberger et al., 2007). While the cross-linking data strongly support our hexamer models, it is of interest to note that R475C and A490C are located on opposite surfaces of motif 2 (Figures 6A and 6B versus 6C), suggesting that the M-domain undergoes large motions.
Mechanistic Model of ClpB Mediated Protein Disaggregation
Our results are consistent with our previous notion that ClpB assists in the resolubilization of aggregates by substrate translocation through the central pore of the ClpB hexamer (Weibezahn et al., 2004). While it has been established that the DnaK chaperone system plays a crucial role both upstream and downstream of the ClpB-mediated threading activity (Weibezahn et al., 2004; Zietkiewicz et al., 2004; Zietkiewicz et al., 2006), our biochemical data confirm that ClpB can also bind substrates directly and independently of the DnaK chaperone system. The high-affinity substrate binding state of ClpB is consistent with a recent report suggesting that the solubilization of aggregated proteins relies on the continuous extraction of unfolded polypeptides by ClpB/DnaK (Schlieker et al., 2004a), an activity which presumably requires stable interaction between substrates and the ClpB hexamer.
We also show that the TClpB-AMPPNP and Trap-ATP hexamer structures are different (Figure 4), allowing us to identify the structural determinants for stable substrate binding (Figure 5A). While it is possible that the structure of the TClpB-AMPPNP hexamer represents an “off-pathway” state and, therefore, may not be comparable to the ATP-activated state, the structural determinants for high-affinity substrate binding are also not observed in the ADP and apo states (Figure 2 and Supplementary Figure 2). Moreover, it has been shown that AMPPNP can support some of Clp/Hsp100 functions that are of physiological relevance (Huang and Goldberg, 1997; Lee et al., 2003; Maurizi, 1991; Shorter and Lindquist, 2004; Shorter and Lindquist, 2006; Thompson and Maurizi, 1994), supporting the notion that the TClpB-AMPPNP hexamer represents a functionally relevant state. Since AMPPNP is not hydrolyzed by ClpB, AMPPNP cannot support all of ClpB’s activities. Taken together, we propose that our structure of the TClpB-AMPPNP hexamer could represent an early ATP-hydrolysis state, distinct from the ATP-activated state (represented by the Trap-ATP hexamer) and the ATP-hydrolyzed state (represented by the TClpB-ADP hexamer).
What is the structural basis for stable substrate binding? Our results show that ClpB binds substrates with high-affinity in a hexamer and tri-phosphate dependent manner. Our model suggests that all six subunits contribute toward a single, high-affinity substrate binding site. However, this does not exclude the possibility that ClpB may have additional, low-affinity binding sites. For instance, our structural models show that motif 1 of the M-domain points away from the hexamer (Figure 4), and could potentially interact with substrates perhaps in a transient manner.
As proposed for ClpA (Ishikawa et al., 2001), our results suggest that a substrate (or substrates) binds to the upper surface of the AAA-1 ring, bringing it into close contact with Tyr243 of the D1 loop, a residue critical for high-affinity substrate binding. Furthermore, in light of our Trap-ATP hexamer reconstruction, we propose that the N-terminal domains stabilize a ClpB-substrate interaction and could provide additional anchoring points, thereby facilitating the extraction of unfolded polypeptides.
ATP hydrolysis induces a conformational change in the top ring, which switches ClpB from a high-affinity to a low-affinity substrate binding state, concomitant with substrate threading through the central pore of the ClpB hexamer. In analogy with ClpA (Bukau et al., 2006; Hinnerwisch et al., 2005; Piszczek et al., 2005), we propose that substrate proteins are translocated through the central pore by a downward motion of the D1 loop, causing substrate unfolding. Contacts between the translocated substrate and the AAA-2 domains may, in turn, trigger a second conformational change, resulting in the opening of the bottom ring and release of the unfolded substrate at the distal end.
Conclusion
We have presented cryo-EM reconstructions of TClpB in four different nucleotide-bound and nucleotide-free states, which together reveal the structural transitions of the ClpB hexamer during its ATPase cycle. Our structural models show that motif 2 of the ClpB M-domain is positioned between the D1-large domains of neighboring subunits and could facilitate a concerted, ATP-driven conformational change in the AAA-1 ring. Moreover, we have shown that stable substrate binding requires tri-phosphates, which cannot be substituted with AMPPNP. While our results suggest that substrates bind with high affinity to the upper surface of the top ring, where they are in contact with the D1 loops from all six subunits, the structure of a substrate-bound complex remains to be determined.
Experimental Procedures
Protein Preparation
TClpB was overexpressed and purified as described (Lee et al., 2003). The TClpB Trap mutant (E271A/E668A) was generated from a C-terminal His6-tagged TClpB construct in pET24a by site-directed mutagenesis using QuikChange. The overexpression and purification of the Trap mutant is essentially the same as described for TClpB, except that the initial DEAE-Sepharose column step was replaced with Ni-NTA Agarose. TrfA-33 in pAT30 from the broad host range plasmid RK2 was a kind gift from A. Toukdarian and D.R. Helinski. TrfA was cloned by PCR into pProEX HTb to generate a cleavable N-terminal His6-tagged TrfA construct (pHisTrfA). His6TrfA was overexpressed in E. coli BL21 (DE3) RIL by growing the cells at 37 °C to mid-log phase in LB medium and inducing with 0.25 mM IPTG for 4 hr at 18 °C. His6TrfA was purified from the soluble lysate by affinity chromatography on a Ni-NTA Agarose column followed by a SP-Sepharose column. Purified His6TrfA was treated with TEV protease and applied to Ni-NTA agarose to remove the liberated His-tag, the TEV protease, and any uncleaved His6TrfA.
Fluorescent Polarization Measurements
FITC-Casein was purchased from Sigma-Aldrich. FAM-TrfA was prepared by mixing purified TrfA with 5-carboxyfluorescein succinimidyl ester at 1:10 molar ratio in 100 mM potassium phosphate pH 7.0. The reaction was stopped after 1 hr by adding 1 M Tris-HCl pH 8.0. The free dye was removed using a Sephadex G-25 column.
Trap and TClpB hexamers were generated by pre-incubation in 50 mM MOPS pH 7.5, 5 mM MgCl2, 150 mM KCl at either 55 °C or 25 °C in the absence (TClpB-apo) or presence of 1 mM nucleotide (Trap-ATP, TClpB-ATPγS, TClpB-AMPPNP, TClpB-ATP, and TClpB-ADP). Fluorescent polarization measurements were performed using a Beacon 2000 Fluorescence Polarization System at excitation and emission wavelengths of 490 nm and 535 nm, respectively. To measure binding affinities, a final concentration of 0.332 μg/ml -Casein FITC or FAM-TrfA was added to a pre-assembled hexamer in a 0.5 ml reaction volume. Samples were incubated for 5 min at 55 °C (FITC-casein) and either 37 °C or 25 °C (FAM-TrfA), and fluorescence polarization was measured at the same temperature. KD values were calculated where possible by plotting the change in fluorescence polarization against TClpB concentration.
Electron Cryomicroscopy
Sample Preparation
To generate the TClpB hexamer, TClpB (0.01 mg/ml) or Trap (0.01 mg/ml) were incubated at 55 °C for 10 to 15 min in 50 mM MOPS pH 7.5, 150 mM KCl, 5 mM MgCl2 with or without 2 mM nucleotide. To stabilize the pre-formed TClpB hexamer, glutaraldehyde was added to a final concentration of 0.01%. The cross-linking reaction was allowed to proceed for 45 min at 55 °C and quenched with glycine. The cross-linked TClpB hexamer was isolated by size-exclusion chromatography on a Superdex 200 10/30 column. It is noteworthy that the peak fraction of the cross-linked TClpB hexamer eluted at the same position as that of the non-cross-linked hexamer (data not shown). Finally, 3.5 μl of sample (0.1 mg/ml) were applied to glow discharged copper grids, blotted, and frozen in liquid ethane.
Data Collection
Frozen hydrated grids were imaged at -180 °C using a JEOL JEM-2010F transmission electron microscope operated at 200 keV and equipped with a GATAN cryo-holder. Images were recorded on a 4K x 4K CCD camera in low dose mode (15 e-/Å2) using the JEOL FasTEM system at a nominal magnification of 69,000x. This resulted in an image with a final spacing of 2.17 Å/pixel. Typically, focal pairs were recorded with the first image at 1.5 to 3.0 μm underfocus and the next image at an additional 1.5 μm underfocus. Only particles from the close-to-focus images were used in the single particle reconstruction.
Image Processing and 3D reconstruction
All image processing, analysis, and 3D reconstruction were done using the EMAN software suite (Ludtke et al., 1999). Particles were selected semiautomatically from the individual digital micrographs using boxer. The contrast transfer function (CTF) of the microscope was determined using ctfit. Our 21 Å resolution cryo-EM structure of the TClpB-AMPPNP hexamer (Lee et al., 2003) served as a starting model for each reconstruction. The models were refined iteratively using projection-based particle classification. A new model was constructed in each cycle from the class averages of the CTF amplitude and phase corrected particle images with Wiener filtration.
The initial refinement was carried out over 8 cycles of 12 iterations of class averaging with an angular step size of 8°. Producing class-averages by this iterative self-alignment process has been shown to eliminate initial model bias (Ludtke et al., 2004). Once the model converged, the next round of refinement cycles was carried out with a smaller angular step size until the model converged again. This procedure was repeated for several rounds. The final resolution for each reconstruction was calculated by dividing the starting data in half, performing two independent reconstructions, and then assessing their FSC at a threshold of 0.5. The final resolution is 11.2 Å for Trap-ATP, 12.1 Å for TClpB-AMPPNP, 16.7 Å for TClpB-ADP, and 17.7 Å for TClpB-apo.
Fitting
To generate an atomic model for the Trap-ATP and TClpB-AMPPNP hexamer, respectively, we manually fitted an N-terminal truncated TClpB monomer into the corresponding cryo-EM maps using the program O (Jones et al., 1991). First, the AAA-1 domain was fitted as a rigid body via the D1-large domain. Next, we refitted the D1-small domain including the M-domain (residues 332-533) into the cryo-EM map. Finally, the long coiled-coil (residues 398-515) was moved into the mass density to further improve the fit. No mass density was observed that could account for a globular and stably folded N-terminal domain (data not shown).
Unlike the AAA-1 domain, the AAA-2 domain was fitted as a rigid body without breaking it into the D2-large and D2-small domains. Next, the helix-loop-helix motif (residues 710-739) was moved as a rigid body to fit the mass density. Finally, the flexible linker connecting the AAA-1 and AAA-2 domains (residues 530 to 545) was repositioned into the observed mass density to connect the two rings.
After manual fitting, the program RSRef2000 (Korostelev et al., 2002) was used to refine residues 329-339, 392-524, 528-554, and 709-739 against the cryo-EM map using local realspace simulated annealing refinement. The final coordinates were refined using conjugate gradient minimization in CNS (Brünger et al., 1998) with 6-fold rotational symmetry and no experimental energy terms to release minor steric clashes between symmetry related subunits. The final cross-correlation coefficient between the fitted Trap-ATP and TClpB-AMPPNP hexamer and the corresponding cryo-EM map is 0.82 and 0.79, respectively, as calculated using the program Foldhunter (Jiang et al., 2001).
Accession Numbers
The cryo-EM maps have been deposited in the EM data bank (http://www.ebi.ac.uk/msd/) under accession codes EMD-1241 (TClpB-apo), EMD-1242 (TClpB-ADP), EMD-1243 (TClpB-AMPPNP), and EMD-1244 (Trap-ATP).
Supplementary Material
Acknowledgments
We thank M.E. Sowa for initiating this project, A.B. Biter, B. Sielaff, and S.J. Ludtke for critical reading of this manuscript, and S.J. Ludtke for help with EMAN. We are grateful to W. Chiu for access to the cryo-EM facility, which is supported by the National Institute of Health (P41RR02250). This work was supported by grants from the Department of Defense (W81XWH-04-1-0033) and the American Heart Association Texas Affiliate (0665082Y) to S.L, and the National Institute of Health (R01GM67672) and the Welch Foundation (Q-1530) to F.T.F.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akoev V, Gogol EP, Barnett ME, Zolkiewski M. Nucleotide-induced switch in oligomerization of the AAA+ ATPase ClpB. Protein Sci. 2004;13:567–574. doi: 10.1110/ps.03422604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger AT, Adams PD, Clore GM, DeLano WI, Gros P, Grosse-Kunstleve RW, Jiang J-S, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. 1998;D54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Buhrman G, de Serrano V, Mattos C. Organic solvents order the dynamic switch II in Ras crystals. Structure. 2003;11:747–751. doi: 10.1016/s0969-2126(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Burton RE, Baker TA, Sauer RT. Nucleotide-dependent substrate recognition by the AAA+ HslUV protease. Nat. Struct. Mol. Biol. 2005;12:245–251. doi: 10.1038/nsmb898. [DOI] [PubMed] [Google Scholar]
- Cashikar AG, Schirmer EC, Hattendorf DA, Glover JR, Ramakrishnan MS, Ware DM, Lindquist SL. Defining a pathway of communication from the C-terminal peptide binding domain to the N-terminal ATPase domain in a AAA protein. Mol. Cell. 2002;9:751–760. doi: 10.1016/s1097-2765(02)00499-9. [DOI] [PubMed] [Google Scholar]
- DeLaBarre B, Brünger AT. Complete structure of p97/valosin-containing protein reveals communication between nucleotide domains. Nat. Struct. Biol. 2003;10:856–863. doi: 10.1038/nsb972. [DOI] [PubMed] [Google Scholar]
- DeLaBarre B, Brünger AT. Nucleotide dependent motion and mechanism of action of p97/VCP. J. Mol. Biol. 2005;347:437–452. doi: 10.1016/j.jmb.2005.01.060. [DOI] [PubMed] [Google Scholar]
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P, Mogk A, Zvi AP, Tomoyasu T, Bukau B. Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc. Natl. Acad. Sci. USA. 1999;96:13732–13737. doi: 10.1073/pnas.96.24.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maurizi MR, Esser L, Xia D. Crystal structure of ClpA, an HSP100 chaperone and regulator of ClpAP protease. J. Biol. Chem. 2002;277:46743–46752. doi: 10.1074/jbc.M207796200. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat. Rev. Mol. Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- Haslberger T, Weibezahn J, Zahn R, Lee S, Tsai FTF, Bukau B, Mogk A. M domains couple the ClpB threading motor with the DnaK chaperone activity. Mol. Cell. 2007 doi: 10.1016/j.molcel.2006.11.008. In press. [DOI] [PubMed] [Google Scholar]
- Hinnerwisch J, Fenton WA, Furtak KJ, Farr GW, Horwich AL. Loops in the central channel of ClpA chaperone mediate protein binding, unfolding, and translocation. Cell. 2005;121:1029–1041. doi: 10.1016/j.cell.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Huang H, Goldberg AL. Proteolytic activity of the ATP-dependent protease HslVU can be uncoupled from ATP hydrolysis. J. Biol. Chem. 1997;272:21364–21372. doi: 10.1074/jbc.272.34.21364. [DOI] [PubMed] [Google Scholar]
- Huyton T, Pye VE, Briggs LC, Flynn TC, Beuron F, Kondo H, Ma J, Zhang X, Freemont PS. The crystal structure of murine p97/VCP at 3.6Å. J. Struct. Biol. 2003;144:337–348. doi: 10.1016/j.jsb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Beuron F, Kessel M, Wickner S, Maurizi MR, Steven AC. Translocation pathway of protein substrates in ClpAP protease. Proc. Natl. Acad. Sci. USA. 2001;98:4328–4333. doi: 10.1073/pnas.081543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Maurizi MR, Steven AC. The N-terminal substrate-binding domain of ClpA unfoldase is highly mobile and extends axially from the distal surface of ClpAP protease. J. Struct. Biol. 2004;146:180–188. doi: 10.1016/j.jsb.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Jiang W, Baker ML, Ludtke SJ, Chiu W. Bridging the information gap: computational tools for intermediate resolution structure interpretation. J. Mol. Biol. 2001;308:1033–1044. doi: 10.1006/jmbi.2001.4633. [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr. 1991;A47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kedzierska S, Akoev V, Barnett ME, Zolkiewski M. Structure and function of the middle domain of ClpB from Escherichia coli. Biochem. 2003;42:14242–14248. doi: 10.1021/bi035573d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny I, Liberek K. Cooperative action of Escherichia coli ClpB protein and DnaK chaperone in the activation of a replication initiation protein. J. Biol. Chem. 2002;277:18483–18488. doi: 10.1074/jbc.M107580200. [DOI] [PubMed] [Google Scholar]
- Korostelev A, Bertram R, Chapman MS. Simulated-annealing real-space refinement as a tool in model building. Acta Crystallogr. 2002;D58:761–767. doi: 10.1107/s0907444902003402. [DOI] [PubMed] [Google Scholar]
- Lee S, Sowa ME, Watanabe Y, Sigler PB, Chiu W, Yoshida M, Tsai FTF. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell. 2003;115:229–240. doi: 10.1016/s0092-8674(03)00807-9. [DOI] [PubMed] [Google Scholar]
- Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- Ludtke SJ, Chen D-H, Song J-L, Chuang DT, Chiu W. Seeing GroEL at 6 Å resolution by single-particle electron cryomicroscopy. Structure. 2004;12:1129–1136. doi: 10.1016/j.str.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lum R, Tkach JM, Vierling E, Glover JR. Evidence for an unfolding/threading mechanism for protein disaggregation by Saccharomyces cerevisiae Hsp104. J. Biol. Chem. 2004;279:29139–29146. doi: 10.1074/jbc.M403777200. [DOI] [PubMed] [Google Scholar]
- Maurizi MR. ATP-promoted interaction between ClpA and ClpP in activation of Clp protease from Escherichia coli. Biochem. Soc. Trans. 1991;19:719–723. doi: 10.1042/bst0190719. [DOI] [PubMed] [Google Scholar]
- Maurizi MR, Xia D. Protein binding and disruption by Clp/Hsp100 chaperones. Structure. 2004;12:175–183. doi: 10.1016/j.str.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Mogk A, Schlieker C, Strub C, Rist W, Weibezahn J, Bukau B. Roles of individual domains and conserved motifs of the AAA+ chaperone ClpB in oligomerization, ATP hydrolysis, and chaperone activity. J. Biol. Chem. 2003;278:17615–17624. doi: 10.1074/jbc.M209686200. [DOI] [PubMed] [Google Scholar]
- Mogk A, Tomoyasu T, Goloubinoff P, Rüdiger S, Röder D, Langen H, Bukau B. Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 1999;18:6934–6949. doi: 10.1093/emboj/18.24.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi K, Watanabe Y, Yohda M, Yoshida M. Heat-inactivated proteins are rescued by the DnaK.J-GrpE set and ClpB chaperones. Proc. Natl. Acad. Sci. USA. 1999;96:7184–7189. doi: 10.1073/pnas.96.13.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- Ogura T, Wilkinson AJ. AAA+ superfamily ATPases: common structure-diverse function. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- Piszczek G, Rozycki J, Singh SK, Ginsburg A, Maurizi MR. The molecular chaperone, ClpA, has a single high affinity peptide binding site per hexamer. J. Biol. Chem. 2005;280:12221–12230. doi: 10.1074/jbc.M411733200. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Hong SW, Vierling E, Lindquist S. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell. 2000;12:479–492. doi: 10.1105/tpc.12.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y, Lindquist S. Hsp104 required for induced thermotolerance. Science. 1990;248:1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- Sauer RT, Bolon DN, Burton BM, Burton RE, Flynn JM, Grant RA, Hersch GL, Joshi SA, Kenniston JA, Levchenko I, et al. Sculpting the proteome with AAA(+) proteases and disassembly machines. Cell. 2004;119:9–18. doi: 10.1016/j.cell.2004.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlieker C, Tews I, Bukau B, Mogk A. Solubilization of aggregated proteins by ClpB/DnaK relies on the continuous extraction of unfolded polypeptides. FEBS Lett. 2004a;578:351–356. doi: 10.1016/j.febslet.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Schlieker C, Weibezahn J, Patzelt H, Tessarz P, Strub C, Zeth K, Erbse A, Schneider-Mergener J, Chin JW, Schultz PG, et al. Substrate recognition by the AAA+ chaperone ClpB. Nat. Struct. Mol. Biol. 2004b;11:607–615. doi: 10.1038/nsmb787. [DOI] [PubMed] [Google Scholar]
- Shorter J, Lindquist S. Hsp104 catalyzes formation and elimination of self-replicating Sup35 prion conformers. Science. 2004;304:1793–1797. doi: 10.1126/science.1098007. [DOI] [PubMed] [Google Scholar]
- Shorter J, Lindquist S. Destruction or potentiation of different prions catalyzed by similar Hsp104 remodeling activities. Mol. Cell. 2006;23:425–438. doi: 10.1016/j.molcel.2006.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires CL, Pedersen S, Ross BM, Squires C. ClpB is the Escherichia coli heat shock protein F84.1. J. Bacteriol. 1991;173:4254–4262. doi: 10.1128/jb.173.14.4254-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tek V, Zolkiewski M. Stability and interactions of the amino-terminal domain of ClpB from Escherichia coli. Protein Sci. 2002;11:1192–1198. doi: 10.1110/ps.4860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MW, Maurizi MR. Activity and specificity of Escherichia coli ClpAP protease in cleaving model peptide substrates. J. Biol. Chem. 1994;269:18201–18208. [PubMed] [Google Scholar]
- Wang J. Nucleotide-dependent domain motions within rings of the RecA/AAA(+) superfamily. J. Struct. Biol. 2004;148:259–267. doi: 10.1016/j.jsb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Takano M, Yoshida M. ATP binding to nucleotide binding domain (NBD)1 of the ClpB chaperone induces motion of the long coiled-coil, stabilizes the hexamer, and activates NBD2. J. Biol. Chem. 2005;280:24562–24567. doi: 10.1074/jbc.M414623200. [DOI] [PubMed] [Google Scholar]
- Watanabe Y-H, Motohashi K, Yoshida M. Roles of the two ATP binding sites of ClpB from Thermus thermophilus. J. Biol. Chem. 2002;277:5804–5809. doi: 10.1074/jbc.M109349200. [DOI] [PubMed] [Google Scholar]
- Weibezahn J, Schlieker C, Bukau B, Mogk A. Characterization of a trap mutant of the AAA+ chaperone ClpB. J. Biol. Chem. 2003;278:32608–32617. doi: 10.1074/jbc.M303653200. [DOI] [PubMed] [Google Scholar]
- Weibezahn J, Tessarz P, Schlieker C, Zahn R, Maglica Z, Lee S, Zentgraf H, Weber-Ban EU, Dougan DA, Tsai FTF, et al. Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell. 2004;119:653–665. doi: 10.1016/j.cell.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Ye J, Osborne AR, Groll M, Rapoport TA. RecA-like motor ATPases-lessons from structures. Biochim. Biophys. Acta. 2004;1659:1–18. doi: 10.1016/j.bbabio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Yonath A, Sielecki A, Moult J, Podjarny A, Traub W. Crystallographic studies of protein denaturation and renaturation. 1. Effects of denaturants on volume and X-ray pattern of cross-linked triclinic lysozyme crystals. Biochem. 1977;16:1413–1417. doi: 10.1021/bi00626a027. [DOI] [PubMed] [Google Scholar]
- Zietkiewicz S, Krzewska J, Liberek K. Successive and synergistic action of the Hsp70 and Hsp100 chaperones in protein disaggregation. J. Biol. Chem. 2004;279:44376–44383. doi: 10.1074/jbc.M402405200. [DOI] [PubMed] [Google Scholar]
- Zietkiewicz S, Lewandowska A, Stocki P, Liberek K. Hsp70 chaperone machine remodels protein aggregates at the initial step of Hsp70-Hsp100-dependent disaggregation. J. Biol. Chem. 2006;281:7022–7029. doi: 10.1074/jbc.M507893200. [DOI] [PubMed] [Google Scholar]
- Zolkiewski M. ClpB cooperates with DnaK, DnaJ, and GrpE in suppressing protein aggregation. J. Biol. Chem. 1999;274:28083–28086. doi: 10.1074/jbc.274.40.28083. [DOI] [PubMed] [Google Scholar]
- Zolkiewski M, Kessel M, Ginsburg A, Maurizi MR. Nucleotide-dependent oligomerization of ClpB from Escherichia coli. Protein Sci. 1999;8:1899–1903. doi: 10.1110/ps.8.9.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.