Abstract
Evidence suggests that anxiety is associated with a shift of visual attention toward threatening stimuli in the environment, such as facial expressions (Mogg & Bradley, 1999). More recent evidence, however, indicates that anxiety may be better characterized by a failure to rapidly disengage the visual attention system away from threat-related facial expressions (Fox, Russo, Bowles, & Dutton, 2001). The present study further investigates this delayed disengagement hypothesis. Results show that high trait-anxious individuals, in contrast to low trait-anxious individuals, take longer to classify peripheral target letters when fearful facial expressions were presented at fixation relative to sad, happy, or neutral expressions. These findings demonstrate a specific tendency to dwell on fear-relevant stimuli, as opposed to negative information in general. These findings are considered from an evolutionary perspective and the possible role of delayed disengagement from threat in the maintenance of anxiety states is also discussed.
It has been widely reported that threat-related stimuli have a special propensity to attract visual attentive processing (e.g., Williams, Watts, MacLeod, & Mathews, 1988, 1997). For instance, Öhman, Flykt, and Esteves (2001a) have pointed out that mammals have evolved in environments in which both resources and dangers were unpredictably distributed in space and time. Therefore, the reproductive success of individuals was dependent to a large extent on the ability to rapidly locate potentially dangerous events in the environment. In support of this notion, enhanced detection of threat-relevant stimuli such as angry facial expressions (Eastwood, Smilek, & Merikle, 2001; Fox, Lester, Russo, Bowles, Pichler, & Dutton, 2000; Öhman, Lundqvist, & Esteves, 2001b) and snakes and spiders (Öhman et al., 2001a) has been found in visual search tasks. These results demonstrate that fear-relevant stimuli are indeed detected rapidly and may result in a shift of visual attention to their location. Individual differences have also been found in that the faster detection of stimuli such as snakes and spiders is further enhanced in people who are phobic of these stimuli (Öhman et al., 2001a). In contrast, anxiety-related differences for the faster detection of angry facial expressions seem inconsistent (Byrne & Eysenck, 1995; Fox et al., 2000).
However, people with high levels of self-reported anxiety do seem to be more likely to allocate their visual attention towards the location of angry or fearful faces in tasks where two faces are presented side by side (e.g., Fox, 2002; Mogg & Bradley, 1999). Thus, there is a general tendency to allocate selective attention towards potentially threatening stimuli, and this may be further enhanced by high levels of anxiety and/or phobic fear. These results are supportive of an evolutionary hypothesis that a specialized neural and behavioural module has evolved for responding to threatening stimuli (e.g., emotional facial expressions; Öhman & Mineka, 2001). It is of particular interest that this evolved module may be hypersensitive in anxiety.
In addition to the above mechanism, however, there is also evidence for a second mechanism that may also be involved in anxiety (Fox, Russo, Bowles, & Dutton, 2001). To illustrate, Fox et al. (2001) presented facial expressions (angry, happy, neutral) as cues to a target location. On invalid trials (i.e., cue and target appear in different locations) it was found that high state-anxious people took longer to respond to a target when the (invalid) cue had been an angry face relative to when the cue had been a happy or a neutral expression. This differential pattern was not observed for those reporting low levels of state-anxiety (Fox et al., 2001). Thus, anxiety was associated with a tendency to dwell on threat-relevant stimuli such as angry expressions. It has also been shown that high trait-anxious people take longer to disengage their attention from threat-related pictures relative to positive or neutral pictures (Yiend & Mathews, 2001). Thus, the presence of fear-relevant stimuli in the environment may result in an anxiety-related failure to rapidly disengage from threatening stimuli.
Derryberry and Reed (1994) also employed a target detection task to investigate attentional biases in more general personality traits (e.g., extraversion and introversion). Cues oriented attention either towards a positive or a negative location. A positive location was one where points were gained if response times were fast enough and the participant received positive feedback. In contrast, a negative location was one where points were lost if response times were too slow and this resulted in negative feedback. Extraverts were slower to shift their attention away from positive locations while introverts were slower to shift their attention away from negative locations.
Using a similar spatial orienting task, Derryberry and Reed (2002) found that high trait-anxious individuals took longer to detect an uncued target when preceded by a threatening cue. This bias transpired when the stimulus onset asynchrony (SOA) between the cue and the target was 250 ms and not 500 ms. This finding is consistent with the literature showing that the attentional bias in anxiety operates early within the stream of information processing. More importantly, Derryberry and Reed's (2002) study provides further evidence that highly anxious people experience difficulties in disengaging their attention away from threat. They also suggested that another personality dimension (i.e., the ability to bring attention under voluntary control) might play a key role in the processing of threatening information. Attentional control was considered as a general capacity to control attention with regard to both positive as well as negative reactions. It was shown that highly anxious individuals with good attentional control were far better at disengaging from threatening cues than highly anxious people who had poor attentional control. This was only evident when the SOA between the cue and the target was 500 ms, suggesting that at the longer SOA voluntary factors may play a key role in reducing the bias.
Taken together, this recent research suggests that the ability to rapidly disengage from negative stimuli might be fairly generally impaired in anxiety. As discussed by Fox et al. (2001), this tendency maintains cognitive resources on the source of stress and may serve to maintain and enhance anxiety states (see also Fox, Russo, & Dutton, 2002). In contrast, the ability to rapidly disengage from threat-related material once identified may be a useful mechanism to keep anxiety levels under control. However, all of the previous research (Derryberry &Reed, 2002; Fox et al., 2001, 2002; Yiend & Mathews, 2001) has used threat-relevant stimuli such as negative feedback, angry faces, or negative pictures. It remains possible, therefore, that all of these results might have been due to a general tendency to dwell on negative stimuli and may not be specifically related to the fear-relevance or the threat-relevance of the stimuli. The aim of the present study was to address this issue by (1) examining whether attentional disengagement was delayed to fearful facial expressions in relation to happy and neutral facial expressions, and (2) whether delayed disengagement occurs with sad facial expressions. This is a first test of the evolutionary hypothesis that delayed disengagement may be specific to fear-relevant stimuli. If the role of the fearful expression as a social cue to warn of potential danger in the environment is critical, as we suspect, then an anxiety-related deficit in disengaging from facial expressions should only be found for the fearful expression, but not for the sad expression.
This prediction is in line with that proposed by Öhman et al. (2001b), who reported that threatening schematic faces were located faster than sad schematic facial expressions in a visual search task. They argued, that it was the threatening nature of the stimuli, and not the negative valence, that was responsible for the observed detection advantage. If previous results using disengagement tasks (e.g., Fox et al., 2001) are due to a general inability to disengage rapidly from negative stimuli then we should find equivalent results for sad and fearful expressions since they are both rated as “negative”. However, if the fear-relevance is critical then delayed disengagement should only occur for the fearful expression.
EXPERIMENT 1
The aim of this experiment was to determine whether high trait-anxious individuals would take longer to disengage their visual attention from faces expressing fear, relative to happy or neutral facial expressions. The design is similar to that of Fox et al. (2001, Exp. 5), in which it was found that high state-anxious people took longer to disengage their visual attention from threat-related words, relative to either positive or neutral words. However, the novelty of the current investigation resides in the use of fearful, as opposed to angry, facial expressions using a direct measure of disengagement. We decided to use fearful facial expressions as the threat-related stimuli because recent evidence has shown that the amygdala responds more strongly to fearful compared to angry faces (Whalen, Shin, McInerney, Fischer, Wright, & Rauch, 2001). An explanation for this increased amygdala activation may be due to the ambiguity in identifying the possible source of threat associated when encountering a fearful facial expression (Whalen, 1998). Nevertheless, fearful faces should represent a good threat-related stimulus for present purposes. The hypothesis is that high trait-anxious people will take longer to disengage from a fearful facial expression relative to a happy or a neutral expression.
Method
Participants
Participants were 66 students from the University of Essex campus community ranging in age from 18 to 50 years of age, with a modal age in the 20s. Participants were preselected from an undergraduate laboratory class. Thirty-three scoring 45 or higher on the trait anxiety scale of the Spielberger Trait–State Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) were assigned to the high-anxious group, while thirty-three scoring 35 or less on the trait anxiety scale were assigned to the low-anxious group. Scores on the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) were also available from a prior laboratory class. Each person had normal or corrected to normal vision and participated in one experimental session lasting about 25 min, for which they received payment of £2.00.
Materials and apparatus
Three different photographs were selected from the Ekman and Friesen (1975) database of facial expressions. All the photographs were of the same individual (PE) but each expressed a different emotion (fear, happy, and neutral). Each of the black and white photographs measured 6.8 cm × 10.3 cm in size and they were matched for brightness. In an initial pilot study, 12 students categorized the emotion of each of the faces as “happy”, “sad”, “fearful”, “angry”, “surprised”, “disgusted”, or “neutral”. It was found that 100% categorized the fearful expression as “fearful”, 100% categorized the happy expression as “happy”, and 80% rated the neutral face as “neutral”, while 20% categorized this face as “sad”. The target stimuli were the capital letters X and P and they were presented in Geneva font 24. The target letters were presented in one of four locations: 8 cm above, below, to the left, or to the right of the centrally located face. These locations were about 9° degrees of visual angle from the centre of fixation when viewed from 50 cm.
All stimuli were presented on a Power Mackintosh 7200/90 computer with a 29 cm × 21 cm Sony Trinitron Multiscan screen. Data presentation and collection were controlled by the PsyScope program (Cohen, MacWhinney, Flatt, & Provost, 1993) and responses were collected by means of a dedicated PsyScope button box, which ensures accuracy to within 1 ms.
Procedure
On arrival at the laboratory, participants were seated in a small cubicle containing a computer and the nature of the task was explained to them. They were told that they would see an asterisk at the centre of the screen and that they should keep their eyes fixated on this location. It was explained that the asterisk would be replaced by a face and that shortly afterwards a letter (X or P) would appear either above, below, to the left, or to the right of the face. They were instructed to categorize the letter as quickly and accurately as possible by pressing the red button for an X or the green button for a P, while keeping their eyes focused on the central face. Each trial began with an asterisk presented in the centre of the computer screen for 1000 ms. One of the facial expressions was then presented at the centre of the screen and after 600 ms a target letter (either X or P) was presented at one of the four locations for 50 ms. The face remained on the computer screen until the participant responded or until 2000 ms had elapsed. There was an intertrial interval of 500 ms.
Each participant completed a practice block of 24 trials, followed by 288 experimental trials. These trials were divided equally into trials with targets appearing above (72), below (72), to the left (72), and to the right (72) of the centrally presented face. For each target location, the centrally fixated face was equally often fearful (96), happy (96), and neutral (96). Likewise, the actual target letter (X or P) appeared equally often with each type of facial expression and in each target location. All conditions were presented in a different random order for each participant.
Design
We initially carried out a 2 (anxiety: High and low trait anxiety) × 2 (facial expression: Happy and neutral) mixed ANOVA . If this analysis was nonsignificant then happy and neutral facial expressions would be collapsed into a category labelled nonfear. We then carried out a 2 (anxiety: High and low trait anxiety) × 2 (facial expression: Fear and nonfear) mixed ANOVA. Anxiety was a between-subjects factor, while facial expression was a within-subject factor. The main dependent variable used was the reaction time (RT) to respond to target items.
Results
As shown in Table 1, the two groups of participants differed in terms of trait anxiety and in their BDI scores.
TABLE 1.
Mean scores on the STAI Trait Anxiety Scale and the Beck Depression Inventory (BDI) for the high and low trait-anxious groups in Experiments 1 and 2
|
Group |
|||
|---|---|---|---|
| Measure | High trait-anxious | Low trait-anxious | t(64) |
| Experiment 1 | |||
| n | 33 | 33 | |
| Trait anxiety | 54.2 (6.2) | 29.3 (4.0) | 19.4** |
| BDI | 10.4 (4.8) | 2.9 (2.8) | 7.8** |
| Experiment 2 | |||
| n | 33 | 33 | |
| Trait anxiety | 53.8 (6.7) | 29.4 (3.4) | 18.7** |
| BDI | 11.8 (7.8) | 2.7 (3.0) | 6.3** |
p < .001.
Response times and error rate analyses
The mean percentage errors were subjected to a 2 (anxiety: High and low trait anxiety) × 2 (facial expression: Fear and nonfear) mixed ANOVA (see Table 2 for more details).1 This analysis did not reveal any significant main or interaction effects (all ps > .10). Incorrect trials and RTs less than 100 ms and greater than 1500 ms were eliminated from the RT analysis. A preliminary 2 (anxiety: High and low trait anxiety) × 2 (facial expression: Happy and neutral) mixed ANOVA was carried out to determine whether RT performance differed between the happy and neutral facial expressions. The main effects of facial expression, F(1, 64) = 2.32, MSE = 150.2, p > .10, anxiety group, F(1, 64) < 1, and Facial expression × Anxiety group interaction, F(1, 64) = 2.82, MSE = 150.2, p = .098, were not significant, thus happy and neutral trials were collapsed together (i.e., nonfear). A 2 (anxiety: High and low trait anxiety) × 2 (facial expression: Fear and nonfear) mixed ANOVA with participants as a random factor was then carried out (see footnote 1). This analysis revealed a significant main effect of facial expression, F(1, 64) = 4.89, MSE = 1351.1, p < .05 (fear = 488 ms, nonfear = 473 ms), and a significant Anxiety group × Facial expression interaction, F(1, 64) = 8.11, MSE = 1351.1, p < .01 (see Table 2).2 The main effect of anxiety group was not significant, F(1, 64) < 1 (high trait anxiety = 484 ms, low trait anxiety = 478 ms).
TABLE 2.
Mean correct response times in milliseconds (ms) and mean error percentages for the fear, nonfear, and sad, nonsad, facial expressions for high and low trait-anxious groups in Experiments 1 and 2
|
Group |
||
|---|---|---|
| Condition | High trait-anxious | Low trait-anxious |
| Experiment 1 | ||
| n | 33 | 33 |
| Fear | ||
| RT | 500.3 (96.3) | 474.7 (75.8) |
| Error | 3.6 (3.06) | 4.2 (4.52) |
| Nonfear | ||
| RT | 467.9 (73.0) | 478.8 (79.3) |
| Error | 3.8 (3.54) | 4.2 (2.89) |
| Experiment 2 | ||
| n | 33 | 33 |
| Sad | ||
| RT | 479.2 (88.4) | 470.8 (82.1) |
| Error | 5.4 (3.9) | 4.6 (3.11) |
| Nonsad | ||
| RT | 478.1 (88.2) | 470.5 (83.2) |
| Error | 5.4 (3.22) | 4.1 (2.62) |
Standard deviations are in parenthesis.
Planned contrasts revealed that RTs were slower following fearful expressions for the high trait-anxious group compared with nonfearful expressions, t(32) = 2.7, p < .025. For the low trait-anxious group, there was no difference in RTs following fearful expressions relative to nonfearful expressions, t(32) = −1.1, p > .10. Neither of the between group comparisons were significant.
Discussion
The results showed that high trait-anxious people took longer to categorize a target letter in peripheral vision when a fearful face was presented at fixation, relative to a nonfearful face. This pattern was not apparent in low trait-anxiety participants.
In our previous work we found an anxiety-related increase in dwell times on the emotional expression of anger relative to happy or neutral facial expressions in a cueing task (Fox et al., 2001, 2002). The present results are the first extension of these findings to fearful facial expressions. Thus, anxious individuals take longer to disengage their attention from the negative facial expressions of anger and fear, but not from the positive emotional expression of happiness or neutral expressions. The question arises as to whether this is a general negativity effect or is it specific to threat-related stimuli.
Experiment 2 was designed to test whether the anxiety-related increased dwell time on negative facial expressions (anger and fear) would also be found for the emotional expression of sadness. This comparison will allow us to determine whether difficulty in disengaging is related to negative emotional expressions generally, or whether the delay is specific to emotional expressions indicating potential danger. Facial expressions of anger and fear are potent social cues indicating potential danger, while a sad expression does not indicate potential danger.
EXPERIMENT 2
Experiment 2 examined whether high trait-anxious people would take longer to disengage from the emotional expression of sadness, relative to nonsad (neutral and happy) facial expressions. The design was identical to Experiment 1 except that a sad expression was used instead of a fearful expression. No increase in dwell time was expected in this experiment because the emotional expression of sadness is not related to the fear detection system. It is important to test this condition since our previous results with angry (Fox et al., 2001, 2002) and fearful (Experiment 1) expressions may be attributable to negative affect more generally and may have little to do with the expressions indicating potential danger.
Method
Participants
An additional 66 students were recruited from the University of Essex campus community ranging in age from 17 to 38 years of age, with a modal age in the 20s. Thirty-three participants scoring 45 or higher on the trait anxiety scale were assigned to the high-anxious group, while thirty-three scoring 35 or less on the trait anxiety scale were assigned to the low-anxious group. Each person had normal or corrected to normal vision and participated in one experimental session lasting about 25 min, for which they received payment of £2.00.
Materials and apparatus
Three different photographs were selected from the Ekman and Friesen (1975) database of facial expressions. All the photographs were of the same individual (JM) but each expressed a different emotion (sad, happy, and neutral). The stimulus dimensions were the same as in Experiment 1. In an initial pilot study, 40 students categorized the emotion of each of the faces as “happy”, “sad”, “fearful”, “angry”, “surprised”, “disgusted”, or “neutral”. It was found that 97.5% categorized the sad expression as “sad”, with the remaining 2.5% categorizing this face as “disgusted”; 97.5% categorized the happy expression as “happy”, while 2.5% categorized this face as “surprised”. Finally, 70% of students rated the neutral face as “neutral”, 20% as “surprised”, 5% as “happy”, and 2.5% categorized the neutral face as “fearful” and “disgusted”, respectively. The target stimuli were the capital letters X and P, as in Experiment 1, and they were presented in the same font and in the same locations. The computer hardware and software was the same as in Experiment 1.
Procedure
The procedure and number of trials were identical to that of Experiment 1, and the same questionnaires were administered.
Design
As in Experiment 1, we initially carried out a 2 (anxiety: High and low trait anxiety) × 2 (facial expression: Happy and neutral) mixed ANOVA . Again, this analysis was conducted to determine whether the happy and neutral trials could be collapsed into a category labelled as nonsad. This analysis was followed by a 2 (anxiety: High and low trait anxiety) × 2 (facial expression: Sad and nonsad) mixed ANOVA. Anxiety was a between-subjects factor, while facial expression was the within-subject factor. The main dependent variable was RT to target items.
Results
As shown in Table 1, the two groups of participants differed in terms of trait anxiety and BDI scores.
Response times and error rate analyses
As in Experiment 1, the mean percentage errors were subjected to a 2 (anxiety: High and low trait anxiety) × 2 (facial expression: Sad and nonsad) mixed ANOVA (see footnote 1). No significant main or interaction effects were found (all ps > .10). The mean percentage errors are presented in Table 2. Incorrect trials and RTs less than 100 ms and greater than 1500 ms were eliminated from the analysis. As in Experiment1, a preliminary 2 (anxiety: High and low trait anxiety) × 2 (facial expressions: Happy, neutral) mixed ANOVA was carried out. There were no significant main or interaction effects (all Fs < 1). Therefore, the data for these nonsad expressions were collapsed and the data (presented in Table 2) were analysed by using a 2 (anxiety: High and low trait anxiety) × 2 (facial expressions: Sad, nonsad) mixed ANOVA. None of the main effects, nor the Anxiety group × Facial expression interaction were significant, Fs (1, 64) < 1.
Combined analysis
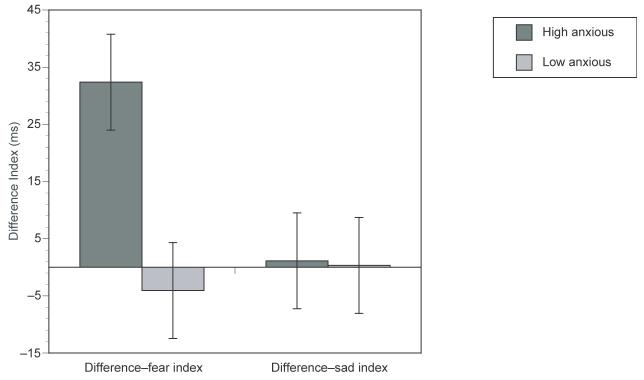
To assess whether RTs were increased for high trait-anxious people on fearful expression trials relative to sad expression trials, the data from Experiments 1 and 2 were combined. The dependent variable was the difference between RTs on fearful or sad expression trials relative to the nonfear (or nonsad) expression trials (i.e., the difference–fear index, and the difference–sad index). This resulted in a 2 (negative expression: Fearful, sad) × 2 (anxiety: High and low trait anxiety) between-subjects ANOVA with subjects as a random factor (see Figure 1). The critical interaction between negative expression and anxiety group was significant, F(1, 128) = 7.22, MSE = 724.8, p < .01.3 The interaction was mainly driven by a higher difference–fear index for the high, relative to the low trait-anxious group (+32.4 vs. −4.1, respectively), t(64) = 2.8, p < .01, while there was no significant between-group difference for the difference–sad index (+1.1 vs. +0.33), t(64) < 1. There was a significant difference between the difference–fear index and the difference–sad index for the high trait-anxious groups (+32.4 vs. +1.1), t(64) = 2.5, p < .025. Finally, there was no significant difference between the difference–fear index and the difference–sad index for low trait-anxious groups (−4.1 vs. +0.3), t(64) < 1.
Figure 1.
Difference index for fear (difference between RTs on fearful and nonfearful expression trials) and sad (difference between RTs on sad and nonsad expression trials) in milliseconds (ms) for high and low trait anxious participants. A positive score indicates slower responses on the fearful (sad) rather than nonfearful (nonsad) trials.
Discussion
The results of Experiment 2 demonstrate that there was no anxiety-related difference in dwell time for sad relative to nonsad facial expressions. In contrast to the results for fearful expressions (Experiment 1), high trait-anxious participants did not take longer to disengage from the negative facial expression of sadness. A combined analysis provided statistical evidence that anxiety groups differed on the fear index but not on the sad index. This indicates that fearful, but not sad, expressions impair attentional disengagement in anxious individuals. The effect size for the equivalent comparison between fear versus nonfear in Experiment 1, among high trait-anxiety participants, was d = .46. Assuming that this is the expected effect size for the sad versus nonsad manipulation, Experiment 2 had a power of .83 (one-tailed) to detect a significantly slower RT for sad than nonsad facial expressions in anxious participants. Thus, the results suggest that sad expressions do not hold the attention of anxious people to the same extent as fearful expressions.
GENERAL DISCUSSION
Across two experiments, it has been shown that extended dwell time was exhibited by high trait anxious individuals to fearful, but not to sad, facial expressions. These results, in conjunction with those of Fox et al. (2001, 2002), imply that it is fearful and angry faces that are particularly effective in disrupting the disengage component of visual attention in anxious people. We suggest that this is due to the increased salience of angry and fearful faces as sociobiological cues of potential danger, arguably hard-wired into our brains through the course of our evolutionary history. Such a contention is supported by the work of Öhman et al. (2001b), which demonstrates that fearful schematic faces are more rapidly located than sad schematic faces. They argued that it was the threat, not the negative, characteristic of the fearful face that was pivotal for the threat advantage found in the visual search task. Furthermore, Öhman et al. (2001a) have also found that stimuli posing a specific biological threat to our early survival (e.g., snakes, spiders) are detected more rapidly than nonthreatening stimuli. It is likely that the faster detection of angry and fearful faces, together with that of other biologically salient stimuli, conferred a significant evolutionary advantage on our early ancestors in a hostile, unpredictable environment.
It is important, however, not to lose sight of the fact that detection of stimuli in the environment comprises only one component of the attentional system. Posner and Petersen (1990), for instance, contend that there are three distinct neural subtraits, which underpin visual-spatial attention: Shift, engagement, and disengagement. It seems likely, that all three of these neural subtraits may have conferred evolutionary advantages on our early ancestors. Within this framework, an impaired ability to rapidly shift attention towards, or to rapidly disengage attention from threatening stimuli in the environment may be disadvantageous. The present results indicate that it is indeed threat-related facial expressions that delay attentional disengagement rather than negative expressions more generally. This compliments the findings of Öhman et al. (2001b), who found that angry, and not sad faces, were detected more quickly.
An obvious question concerns the implications of delayed disengagement from threat. While this issue has not been directly addressed in the present study, there are, we feel, strong grounds for speculation. One possibility is that rapid disengagement from threatening information may serve to keep anxiety at low levels (see Fox et al., 2001). Likewise, failing to rapidly disengage from threat may serve to maintain anxiety due to the visual-spatial attention system remaining fixated on the source of stress. In turn, this extended dwell time may manifest itself in the long term as rumination and worry, both of which are considered to be central to clinical anxiety disorders (Mathews, 1990).
From an evolutionary perspective, it might be expected that all individuals should disengage very quickly from threatening stimuli. This raises the question of why slower disengagement from threat only seems to occur in the high anxious. One possibility has to do with the intensity of the threat presented. For example, recent evidence has shown that high-trait anxious individuals were more vigilant towards morphed angry facial expressions of an intermediate (not mild or high) threat intensity in a variant of the dot-probe task (Wilson & MacLeod, 2003). However, when encountering an angry facial expression classified as extremely threatening, all participants displayed greater orientation toward such stimuli. Likewise, all participants directed their attention away from angry facial expressions that were classified as mildly threatening. Therefore, studies that employ threatening stimuli that are placed somewhere in the middle of a threat intensity continuum, such as our own, are more likely to find anxiety-related differences (see Mogg & Bradley, 1998).
There are several issues that have not been directly addressed in the current study but which merit further investigation. The first relates to whether the delay in attentional disengagement is either location or object based. Since all facial expressions were presented centrally in the same location in the present study, we cannot investigate this question. However, whether anxiety-related disengagement occurs from threatening objects or from the locations recently occupied by threat is an interesting question for future research.
A second issue concerns the possibility that highly anxious individuals may dwell longer on any arousing stimuli (e.g., surprised facial expressions) and not just stimuli of a threatening or fearful nature. Most threatening stimuli are, of course, also likely to be highly arousing as indeed are surprised facial expressions. Future research, utilizing both threat-related and surprised facial expressions, may determine whether it is the threatening or the arousing aspect of a stimulus that is primarily responsible for delays in disengagement.
To sum up, it is suggested that as well as visual-spatial attention system enabling rapid detection of threat-related stimuli, attentional processes may also act to delay disengagement from fear-relevant stimuli in highly anxious people. A useful focus for future research would be to determine which of these mechanisms is the more potent in the maintenance of anxiety. The present two experiments have shown that this anxiety-related delay in disengagement is specific to fear-relevant stimuli rather than being a more general problem in disengaging from negative information. This provides support for the evolutionary view that stimuli become salient, not because they are negative, but because they indicate potential danger in the environment (e.g., Öhman & Mineka, 2001).
Footnotes
The research reported here was supported by grant number 064290/Z/01/Z from the Wellcome Trust awarded to Elaine Fox and Riccardo Russo.
An ANOVA was carried out on the mean RTs (in ms) that had one between-subject factor (anxiety: High and low trait anxiety) and one within-subject factor (facial expression: Happy, neutral, and fear). This analysis revealed a significant main effect of facial expression, F(2, 128) = 4.69, MSE = 975.8, p < .05 (fear = 488 ms, happy = 472 ms, neutral = 475) and a significant Anxiety group × Facial expression interaction, F(2, 128) = 7.67, MSE = 975.8, p < .01. In Experiments 1 and 2 the mean percentage errors were subjected to a 2 (anxiety: High and low trait anxiety) × 3 (facial expression: Happy, neutral, and fear/sad) mixed ANOVA. These analyses did not reveal any significant main or interaction effects (all ps > .10).
A 2 (anxiety: High and low trait anxiety) × 2 (facial expression: Fear and nonfear) ANCOVA (BDI entered as a covariate) was also carried out. This analysis still yielded a significant Anxiety group × Facial expression interaction, F(1, 63) = 6.40, MSE = 1361.6, p < .025, suggesting that the observed bias is an anxiety-related effect. However, we would like to point out that although ANCOVA is often used in the cognition and emotion literature to partial out the effect of depression on the dependent variable, this technique may not be appropriate because the average level of the covariate is not expected to be comparable between high and low anxious individuals.
A 2 (negative expression: Fearful, sad) × 2 (anxiety: High and low trait anxiety) ANCOVA (BDI entered as a covariate) was carried out and revealed a significant Anxiety group × Facial expression interaction, F(1, 127) = 7.06, MSE = 730.4, p < .025.
REFERENCES
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychology. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Byrne A, Eysenck MW. Trait anxiety, anxious mood, threat detection. Cognition and Emotion. 1995;9:549–562. [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Derryberry D, Reed MA. Temperament and attention: Orienting toward and away from positive and negative signals. Journal of Personality and Social Psychology. 1994;66:1128–1139. doi: 10.1037//0022-3514.66.6.1128. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Eastwood JD, Smilek D, Merikle PM. Differential attentional guidance by unattended faces expressing positive and negative emotions. Perception and Psychophysics. 2001;63:1004–1013. doi: 10.3758/bf03194519. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1975. [Google Scholar]
- Fox E. Processing emotional facial expressions: The role of anxiety and awareness. Cognitive, Affective, and Behavioral Neuroscience. 2002;2:52–63. doi: 10.3758/cabn.2.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K. Facial expressions of emotion: Are angry faces detected more efficiently? Cognition and Emotion. 2000;14:61–92. doi: 10.1080/026999300378996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles RJ, Dutton K. Do threatening stimuli draw or hold visual attention in sub-clinical anxiety? Journal of Experimental Psychology: General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Dutton K. Attentional bias for threat: Evidence for delayed disengagement from emotional faces. Cognition and Emotion. 2002;16:355–379. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A. Why worry? The cognitive function of anxiety. Behavioural Research and Therapy. 1990;28:455–468. doi: 10.1016/0005-7967(90)90132-3. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational view analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley B. Orienting of attention to threatening facial expressions presented under conditions of restricted awareness. Cognition and Emotion. 1999;13:713–740. [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001a;130:466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Öhman A, Lundqvist D, Esteves F. The face in the crowd revisited: A threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001b;80:381–396. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka SM. Fear, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consultant Psychologists Press; 1983. [Google Scholar]
- Whalen PJ. Fear, vigilance and ambiguity: Initial neuroimaging studies of the human amgydala. Current Directions in Psychological Science. 1998;7:177–188. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. Chichester, UK: Wiley; 1988. [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. 2nd ed. Chichester, UK: Wiley; 1997. [Google Scholar]
- Wilson E, MacLeod C. Contrasting two accounts of anxiety-linked attentional bias: Selective attention to varying levels of stimulus threat intensity. Journal of Abnormal Psychology. 2003;112:212–218. doi: 10.1037/0021-843x.112.2.212. [DOI] [PubMed] [Google Scholar]
- Yiend J, Mathews A. Anxiety and attention to threatening pictures. Quarterly Journal of Experimental Psychology. 2001;54A:665–681. doi: 10.1080/713755991. [DOI] [PubMed] [Google Scholar]