Abstract
To understand the role of glutathione (GSH) in the protection of cells from arsenite toxicity, we studied the mechanism of apoptotic cell death in cells genetically unable to synthesize GSH (GCS-2 cells). Arsenite stimulated an increase in protein ubiquitination in GCS-2 cells while the wild type cells were unaffected. Arsenite treatment increased lipid peroxidation and induced ubiquitination of molecular chaperone Hsp90 and impaired its ability to bind cochaperone p50Cdc-37 and client proteins Plk-1 and Cdk-4 in GCS-2 cells. Treatment with arsenite also partially inhibited proteasome activity in GCS-2 cells. In these cells stably transfected with GFPu (a reporter consisting of a short degron fused to the COOH-terminus of GFP), intracellular fluorescence increased, suggesting the accumulation of GFP aggregates. GCS-2 cells underwent apoptosis accompanied by release of cytochrome C into the cytoplasm. Taken together, these data suggest that a possible mechanism of arsenite-induced apoptosis is the accumulation of ubiquitinated proteins and impairment of the protein degradative pathway. Further, protection from arsenite-induced ubiquitination is mediated by GSH and to a lesser extent by available reducing equivalents in the cells.
Keywords: Glutathione, arsenite, proteasome, ubiquitination, Hsp90
Introduction
Environmental exposure to arsenite is linked to increased risk of cardiovascular disorders and cancer of the bladder, lung, and skin [1, 2]. Arsenic is also an important cytotoxin, exposure to which results in cell death and apoptosis [3]. However, little is known about the molecular mechanisms of arsenite-induced cellular toxicity; furthermore, a better understanding of the modulation of arsenic toxicity would contribute to our knowledge of the basic principles of cellular toxicity and might lead to better therapeutic strategies.
Thiol-containing compounds are a central component of many biologically significant reactions. The most abundant intracellular non-protein thiol, GSH, is found in millimolar concentrations in most cells [4, 5]. GSH has been implicated in the protection of cells against cytotoxins and in the metabolism of xenobiotics, including arsenite, through the formation of GSH conjugates [6–9]. A number of proteins with regulatory functions such as NFκB, AP-1, JAK/STATs, EGF receptor, and Hsps are sensitive to the cellular redox environment [10, 11]. Antioxidants such as GSH regulate these proteins by modulating the redox state of specific thiol residues of target proteins including transcription factors, stress kinases, and caspases [10, 11].
Oxidative stress contributes to cellular structural changes resulting in the misfolding of proteins. Chaperones and cochaperones are pivotal for stability and function of a large group of client proteins including protein kinases, mutated p53, cyclin D-associated Cdk-4, and Cdk-6 [12, 13]. Hsp90 is one of the most abundant and important chaperones that assists in protein folding and protects newly synthesized proteins from catabolism [14, 15]. Recruitment of protein kinase clients to the Hsp90 chaperone involves the cochaperone p50cdc37 acting as a scaffold, binding protein kinases via its N-terminal domain and Hsp90 via its C-terminal region [16, 17].
Protein ubiquitination, the covalent modification of proteins by the addition of ubiquitin, is a mechanism by which damaged proteins are marked for subsequent degradation by the 26S proteasome [18, 19]. In the ubiquitin-proteasome pathway, the sequential actions of E1, the ubiquitin-activating enzyme, E2, the ubiquitin conjugating enzyme (UBC), and E3, the ubiquitin-protein ligase, serve to attach the ubiquitin moiety (most often polyubiquitin chains) to acceptor lysine residues of protein substrates, which are in turn targeted for degradation by the 26S proteasome [20, 21]. The ability to degrade and regulate levels of intracellular proteins has made the proteasome an important molecule in the regulation of diverse biological processes including cell cycle progression, cell signaling, and apoptosis [18, 22].
Programmed cell death eliminates harmful or damaged cells in a highly regulated manner. Various extracellular and intracellular stimuli can trigger this process, and once initiated the signal is tranduced through a series of protein-protein interactions. There is accumulating evidence that supports a close relationship between the ubiquitin-proteasome pathway and the apoptotic machinery [18, 22]. Protein degradation mediated by polyubiquitination regulates the steady-state expression levels of many of the apoptosis regulators [22]. In most cell lines, proteasome inhibitors trigger apoptosis [23].
To investigate the role of GSH in arsenite-induced cytotoxicity, we have developed cell lines from 3.5 day post-coitus (dpc) embryos from both wild-type and γ-glutamate cysteine ligase catalytic subunit (Gclc)-deficient mice [24]. GCS-2 (Gclc-deficient cells) grown in 2.5 mM GSH for 48 h have ~2.4% GSH levels of wild type (BDC-1) cells. This value drops to the limit of detection after GSH has been withdrawn for 24 h. We have used GCS-2 cells as a model system to elucidate the molecular mechanisms underlying arsenite toxicity under low GSH conditions. Results presented here demonstrate that exposure of GSH-depleted GCS-2 cells to low concentrations of arsenite lead to polyubiquitination of molecular chaperone Hsp90, degradation of Hsp90 and its client proteins, impaired proteasomal function leading to accumulation of protein-ubiquitin conjugates and apoptosis. These results support the concept that GSH serves to protect cells against arsenite-induced toxicity.
Materials and Methods
Reagents
Polyclonal antibodies to Hsp90, Hsp70, Hsp40, Hsp105, and Hsp27, cytochrome C, p70 S6 kinase, p90 S6 kinases 1, 2, and 3 were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against ubiquitin and ubiquitin activating enzyme-E1 were from StressGen Biotechnologies (Victoria, British Columbia, Canada). Antibodies against phospho ERK, phospho p38 MAPK, and phospho JNK were purchased from Cell Signal Technologies (Beverley, MA). Secondary horseradish peroxidase-conjugated antibodies were from Bio-Rad Laboratories (Richmond, CA). α- 32P dATP was purchased from Perkin-Elmer Life Sciences (Boston, MA).
Cell Lines and Culture Conditions
All cell culture studies used M15 complete medium and cultures were done essentially as described earlier [24]. All GSH-dependent GCS-2 cells were maintained in medium containing 2.5 mM GSH and NAC-dependent GCS-2 cell lines were cultured in medium supplemented with 2 mM NAC. BDC-1 cells (wild type controls) were maintained in medium without NAC or GSH.
Measurement of Thiols
Thiols were measured using the high-performance liquid chromatography-electrochemical detection method of Kleinman and Richie [25] with minor modifications.
Treatment of Cells
Sodium arsenite was purchased from Sigma (St. Louis, MO). A 100 μM stock solution was prepared in PBS, pH 7.4 and was used at final concentrations ranging from 0–2 μM. Briefly, the GCS-2 cells were seeded at 1.5 x 106 cells per 10 cm dish, allowed to grow for 48 h either in the presence of 2.5 mM GSH or 2 mM NAC, depleted off GSH or NAC for 24 h, and treated with various concentrations of arsenite for up to 21 h. BDC-1 cells were treated under similar conditions as GCS-2 cells except that they were grown in the absence of NAC or GSH.
Cytotoxicity Assay
Cell viability, as an indicator of cytotoxicity, was determined by determining the capacity of BDC-1 and GCS-2 cells to reduce MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to formazan [26]. Briefly, a 5mg/ml solution of MTT (Sigma Chemical Co., St. Louis, MO) was prepared, filtered through a 0.2 μm filter, and used in the assays. Cell viability was determined on cells grown in 24-well plates. After arsenite treatment under appropriate conditions, MTT solution (40 μl) was added to each well, followed by incubation at 37°C for 4 h in a 5% CO2:95% air atmosphere. The cells were washed twice in phosphate-buffered saline and added acidic propanol (0.1 N HCl in absolute propanol) to each well. The absorbance was determined at 570 nm using a plate reader with acidic propanol as the blank. The MTT reduction was linear with time over a 4 h incubation period.
Lipid Peroxidation Assay
Lipid peroxidation, as an indicator of oxidative stress, was measured in BDC-1 and GCS-2 cells using an assay kit supplied by Oxford Biomedical Research, Oxford, MI according to the manufacturer’s directions using 1, 1, 3, 3-tetramethoxypropane (TMOP) as the standard. The assay is based on the reaction of the chromogenic reagent, N-methyl-2-phenylindole with malondialdehyde (MDA) at 45°C. The resulting chromophore has maximal absorbance at 586 nm. Data are expressed as pmol MDA formed/mg protein.
RNA Isolation and Northern Blot Analysis
Total RNAs were isolated from cultured cells using TRIRzol reagent (InvitrogenLife Technologies, Carlsbad, CA) according to manufacturer’s instructions. Fifteen μg of total RNA from cells after various treatments were electrophoresed on 1% agarose and 2.2 M formaldehyde gels, transferred onto a Zeta-Probe nylon membrane (Bio-Rad), and hybridized to a 410-bp 32P-labeled cDNA probe corresponding to Hsp90 or with a 456-bp probe corresponding to Hsp70 cDNA. The blot was then stripped and re-probed with β-actin cDNA.
Proteasome Assay
The proteasomal activities of total cellular homogenates were measured using the proteasome specific substrate LLVY-AMC (Biomol, Plymouth Meeting, PA). This substrate is specifically used to assay the chymotryptic activity of the proteasome. Homogenates were prepared in the proteasome assay buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5 mM EDTA, and 0.5% NP-40) from the cells treated with and without arsenite. Reactions (100 μl) containing homogenate and the proteasome specific substrate were prepared in quadruplicate. Assays were done in 96-well plates and the fluorescence was measured with excitation λ360/emission λ460 using SpectraMax Gemini Fluorometer (Molecular Devices Corp. Sunnyvale, CA).
DNA Laddering Assay
For nonradioactive DNA laddering detection, DNA from treated and untreated cells was extracted in DNA isolation buffer (50 mM Tris-HC, 0.05% SDS, 0.1% Triton-X-100, 50 μg/ml RNase, 50 μg/ml proteinase K, and 25 mM EDTA), phenol/chloroform extracted, and ethanol precipitated. DNA (10 μg) was then analyzed on 1% agarose gels containing ethidium bromide (0.1 μg/ml) and visualized by UV transillumination.
Immunoprecipitation and Western Blot Analysis
Cells were washed twice with ice-cold PBS, and harvested in cell lysis buffer ( 50 mM Tris-HCL, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM disodium EDTA, 1% sodium deoxycholate) containing the protease inhibitors (Roche Biochemicals, Indianapolis, IN). Cells were lysed by sonication and the protein supernatants were clarified by centrifugation at 25,000 X g for 10 min at 4 ºC. Protein concentration was determined by the BCA protein assay reagent kit (Pierce, Rockford, IL). Hsp90 and p50cdc-37 from clarified lysates were immunoprecipitated with 1μg of specific polyclonal antibody against Hsp90 or p50 cdc-37 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and 20 μl of protein A/G plus agarose (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 2h at 4°C. Immunoprecipitated proteins were washed under stringent conditions, resolved on 7.5% SDS-polyacrylamide gels, transferred on to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ), and analyzed by western blotting. For immunoblotting of whole cell lysates, clarified cellular lysates were resolved on 10% SDS-polyacrylamide gels. Proteins were transferred to nitrocellulose membranes and analyzed by immunoblotting using various antibodies. The ECL blots were developed according to the manufacturer’s instructions.
Transfection
BDC-1 and GCS-2 cells were plated at a density of 5 X 104 cells per well in 6-well plates 24 h prior to transfection. The cells were transfected using the FuGENE transfection reagent (Roche) with 1 mg of either control plasmid containing neomycin or pGFPu containing neomycin. After 24 h of transfection, the cells were selected with 800μg/ml G-418 until single clones were recovered. Clones were further expanded and GFP fluorescence was monitored with and without arsenite treatment by confocal microscopy.
Results
Arsenite-Induced Cytotoxicity in GCS-2 Cells
It has been well documented that GSH is the major non-protein thiol that regulates the intracellular redox balance [4, 5] and that arsenite promotes apoptosis [3]. Availability of embryonic cell lines derived from Gclc−/− mice with little/no GSH (24) allowed us to examine the role of GSH depletion in arsenite-induced cytotoxicity. Arsenite dose response survival rates were compared between BDC-1 cells and GSH-deprived GCS-2 cells after 21 h of treatment (Fig. 1). GCS-2 cells exhibited decreased viability as the arsenite dose increased to 2 μM whereas BDC-1 cells were unaffected. The ED50 for GCS-2 cells was 1 μM whereas for BDC-1 cells (data not shown) it was 24 μM. To determine if other antioxidants could substitute for GSH, we rescued GCS-2 cells in the presence of thiol antioxidant NAC and examined arsenite-induced cytotoxicity. Because NAC is known not to complex with arsenite [27], we cultured GCS-2 cells in the presence of NAC for 48 h and divided them into two groups: one group was grown for an additional 24 h in NAC and treated with arsenite for 21 h. In a parallel experiment, NAC was withdrawn from GCS-2 cells for 24 h and treated with arsenite in the absence of NAC for 21 h. GCS-2 cells treated with arsenite in the absence of NAC showed decreased viability rates similar to GCS-2 cells deprived of GSH, while cells exposed to arsenite in the presence of NAC exhibited intermediate survival rates (Fig. 1). These data suggest GCS-2 cells are much more susceptible to arsenite toxicity and that it is a function of the levels of intracellular GSH and NAC could only partially protect these cells from arsenite-induced cell death in the absence of GSH.
Figure 1.
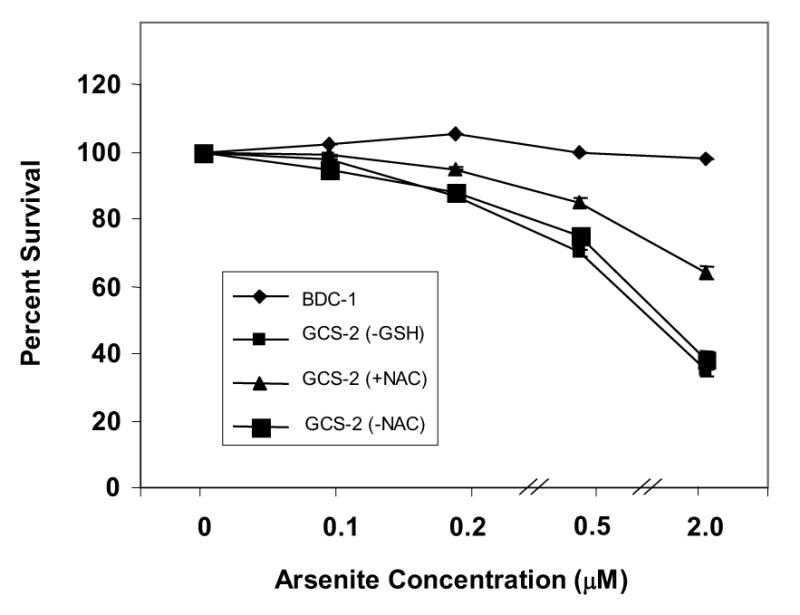
Determination of the cytotoxicity of arsenite in GCS-2 cells. Dose response to arsenite-induced cytotoxicity in GCS-2 and BDC-1 cells. Cytotoxicity was measured using the MTT assay as described in “Methods”. A fixed number of GCS-2 cells were allowed to grow in 24-well plates in the presence of 2.5 mM GSH or 2 mM NAC for 48 h. GSH was withdrawn for 24 h and both BDC-1 and GCS-2 cells were treated with varying concentrations of arsenite for 21 h. NAC-dependent cells were divided into two groups: One group was grown for an additional 24 h in NAC and treated with different concentrations of arsenite in the presence of NAC while NAC was withdrawn from the other group for 24 h and the cells were treated with varying concentrations of arsenite for 21 h, followed by the addition of 40 μl of MTT reagent to each well. The plates were incubated for 4 h at 37°C and read on a plate reader at 570 nm. Results are presented as mean ± SEM.
Arsenite-Induced Ubiquitination of Cellular Proteins in GSH-depleted GCS-2 Cells
The role of GSH in arsenite-induced ubiquitination of proteins was investigated in GCS-2 cells that contain little or no GSH compared to BDC-1 cells. GCS-2 cells were grown for 48 hours in the presence of GSH, followed by depletion of GSH for 24 hours, and treatment with various concentrations of arsenite (0 to 2 μM) for up to 24 h. Exposure of GCS-2 cells to low concentrations of arsenite ubiquitinated the proteins in a dose-dependent manner, while BDC-1 cells remained unaffected (Fig. 2A). Untreated GCS-2 cells did not show ubiquitination indicating that the absence of GSH alone is not sufficient to increase protein damage by ubiquitination. The GCS-2 cells had 20-fold higher levels of ubiquitinated proteins compared to wild-type cells at low concentrations of arsenite (Fig. 2B). Ubiquitination was time-dependent and steadily increased over the course of the experiment (Fig. 2C).
Figure 2.
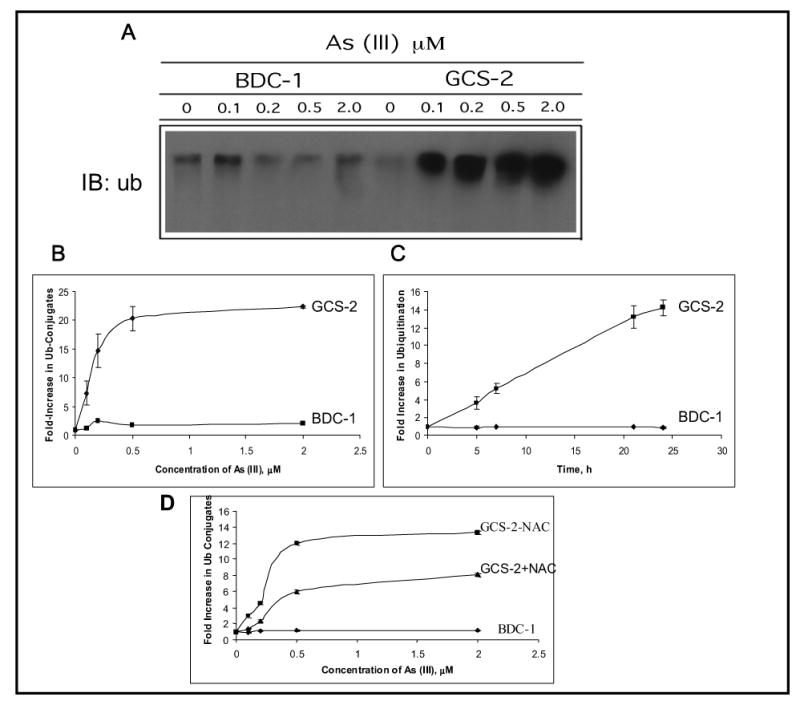
Effect of arsenite on protein ubiquitination in BDC-1 and GCS-2 cells. (A) Dose dependence of arsenite-induced ubiquitination in BDC-1 and GCS-2 cells. GCS-2 cells were plated at 1.5 x 106 cells/dish in the presence of GSH for 48 h. GSH was withdrawn from GCS-2 cells for 24 h and both cell types were treated with various concentrations of arsenite for 21 h. Cell extracts were prepared and 10 μg total protein was separated by SDS-PAGE and analyzed by immunoblot with polyclonal antibody against ubiquitin. (B) Quantification of the ubiquitin-protein conjugates by densitometry. Blots from (A) were then quantified to measure the high molecular weight ubiquitin-protein conjugates using a densitometer. Each point is an average of three independent determinations. (C) Time-dependent increase in the high molecular weight conjugates at 0.5 μM arsenite treatment. The experimental conditions used were as described in A, except cells were treated for various time points with 0.5μM arsenite. The blots were quantified as described in B. (D) Effect of NAC on arsenite-induced ubiquitination in BDC-1 and GCS-2 cells. GCS-2 cells were grown in the presence of 2 mM NAC for 48 h and divided into two groups: one group was grown in NAC for an additional 24 h in the presence of NAC and treated with 0.5 μM arsenite for 21 h. In a parallel experiment, NAC was withdrawn for 24 h from GCS-2 cells that were grown in the presence of NAC for 48 h and the cells were treated with various concentrations of arsenite for 21 h in the absence of NAC. Fold increase in ubiquitin conjugates were quantified as described in Fig. 2B.
We also treated GCS-2 cells grown in the presence of NAC for 48 h with arsenite for 21 h. In a parallel experiment, NAC was withdrawn for 24 h from GCS-2 cells that were grown in the presence of NAC for 48 h and treated with arsenite in the absence of NAC for 21 h. GCS-2 cells that were exposed to arsenite in the presence of NAC ubiquitinated proteins to half the extent as the cells in the absence of NAC (Fig. 2D). This finding indicates that NAC partially protects cells against arsenite-induced ubiquitination in the absence of GSH.
Arsenite Induces Changes in Gene Expression in GCS-2 Cells
To detect changes in gene expression, we analyzed protein levels and phosphorylation status by immunoblotting. We examined five representative categories of proteins: MAP kinases, transcription factors, heat shock proteins, ubiquitin pathway enzymes, and ribosomal S6 kinases (Table 1). Total protein levels of MAP kinases remained unchanged upon arsenite exposure. We found increased phosphorylation of ERK1/2, JNK1/2, and p38 MAP kinase in the GCS-2 cells, while BDC-1 cells did not show any obvious changes. We examined five heat shock proteins that function as molecular chaperones and found that only Hsp90 protein levels were decreased. This finding suggests that Hsp90 is involved in arsenite-induced toxicity (see below). Because we found increased ubiquitination of total proteins in GCS-2 cells (Fig. 2), we examined changes in ubiquitin pathway enzymes including ubiquitin activating enzyme (E1), some of the ubiquitin conjugating enzymes (E2), and representative members of the ubiquitin ligase family (E3) and found no detectable changes (Table 1). We also examined the phosphorylation status of ribosomal S6 kinases under our conditions and found no detectable differences between GSH-competent and GSH-deficient cells.
Table 1.
Arsenite-Induced Changes in Phosphorylation Status and Gene Expression in GCS-2 cells
| Proteins | Phosphorylation | Changes in protein levels |
|---|---|---|
| MAP Kinases | ||
| ERK1/2 | Increased | No change |
| JNK1/2 | Increased | No change |
| p38 MAPK | Increased | No change |
| Transcription Factors | ||
| ATF-2 | Increased | No change |
| C-Jun | Increased | No change |
| Heat Shock Proteins | ||
| Hsp105 | No change | No change |
| Hsp90 | No change | Decreased |
| Hsp70 | No change | No change |
| Hsp40 | No change | No change |
| Hsp27 | No change | No change |
| Ubiquitin Pathway Enzymes | ||
| Ubiquitin activating enzyme (E1) | ND* | No change |
| Ubiquitin conjugating enzymes (E2) | ||
| UBC-4 | ND | No change |
| UBC-8 | ND | No change |
| UBC-9 | ND | No change |
| Ubiquitin Ligase (E3) | ||
| E6-AP | ND | No change |
| Smurf-1 | ND | No change |
| Smurf-2 | ND | No change |
| NEDD-4 | ND | No change |
| Ribosomal S6 Kinases | ||
| p70 S6 kinase | No change | No change |
| p90 S6 kinases 1, 2, and 3 | No change | No change |
ND- not determined
Exposure to Arsenite Downregulates Hsp90 at Both mRNA and Protein Levels
We further examined changes in Hsp90 levels and found that exposure to 0.5 μM arsenite downregulated Hsp90 steady state total RNA levels by ~ 2-fold in GCS-2 cells while Hsp70 RNA levels were unaffected (Fig. 3A). Immunoblotting demonstrated that in GCS-2 cells, Hsp90 protein levels decreased to about 40% of the wild-type levels at 21–24 h (Fig. 3B). In Fig. 2B, it appears that Hsp90 protein levels are lower in the untreated GCS-2 cells than in untreated BDC-1 cells. Because only 2 out of 5 experiments appeared to show a decrease in Hsp90 expression in GCS-2 cells, we quantified signal intensities by scanning densitometry. We found that these changes were statistically not significant, with a p ≤ 0.19 (Student’s t test). Under the same conditions, Hsp70 protein levels did not change.
Figure 3.
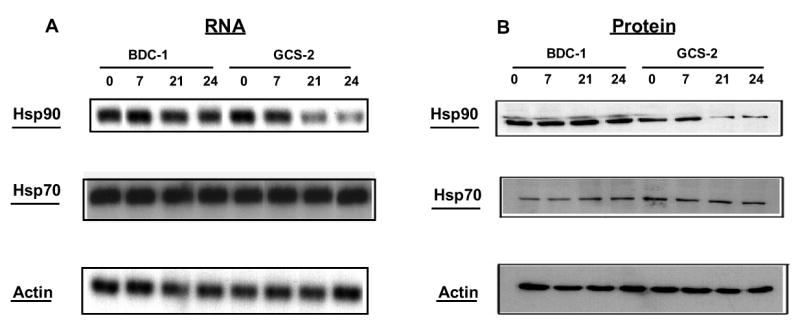
Determination of the effects of arsenite on Hsp90 and Hsp70 mRNA and protein levels. A. Northern blot analysis of Hsp90 and Hsp70. GCS-2 and BDC-1 cells were incubated with 0.5 μM arsenite for the indicated times and total RNA was isolated. 15 μg of RNA was analyzed by northern blot with Hsp90 (top) cDNA probe. The Hsp90 membrane was stripped and reprobed with Hsp70 (middle) cDNA probe. Hsp70 membrane was stripped and reprobed with actin (bottom) cDNA probe to verify equal loading. Incubation times in hours are indicated along the top. B. Analysis of Hsp90 and Hsp70 protein levels. Cells were treated as in A, and 50 μg of cytosolic extract was separated by SDS-PAGE and analyzed by immunoblot with Hsp90-specific (top) or Hsp70-specific (middle) antibody. Equivalent loading was verified with an anti-actin (bottom) antibody.
Arsenite Downregulates Hsp90 by Ubiquitination in GSH-Depleted GCS-2 Cells
In the GCS-2 cells, there was a marked diminution in the cellular content of Hsp90 protein upon treatment with low concentrations of arsenite (Fig. 4A). Hsp90 was immunoprecipitated from the cytosol with an Hsp90-specific polyclonal antibody and analyzed by immunoblot with an anti-ubiquitin antibody. In GCS-2 cells treated with arsenite, there are bands in the high molecular weight region (>90 kDa) ranging in size from 100 to 250 kDa. These data indicate that Hsp90 is polyubiquitinated in these cells after arsenite treatment (Fig 4B). There was no evidence of Hsp90 ubiquitination in BDC-1 cells under the same conditions (Fig. 4B). These findings suggest that low/absent levels of GSH and exposure to arsenite result in Hsp90 ubiquitination in GCS-2 cells.
Figure 4.
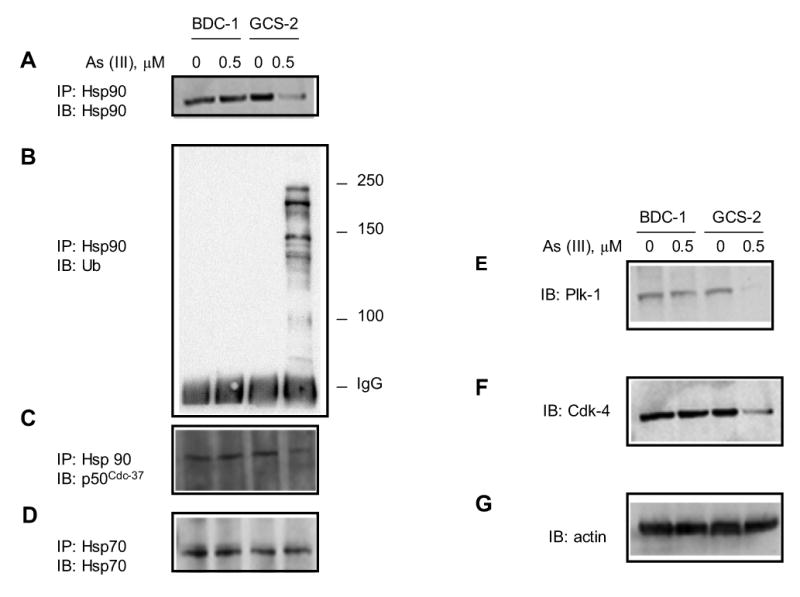
Effect of arsenite on Hsp90 expression, ubiquitination, and association with co-chaperone and client proteins. (A) Effect of arsenite on the steady-state levels of Hsp90. Hsp90 was immunoprecipitated from total cell lysates with anti-Hsp90 antibody and immunoblotted with the same antibody. (B) Effect of arsenite on Hsp90 ubiquitination in GCS-2 cells. Hsp90 was immunoprecipitated with anti-Hsp90, separated by SDS-PAGE and analyzed by immunoblot with anti-ubiquitin antibody. (C) Effect of arsenite on Hsp90 association with its cochaperone p50cdc37. Cell lysates were immunoprecipitated with anti-Hsp90 antibody and blotted with anti-p50cdc37 antibody. (D) Effect of arsenite on steady-state protein levels of Hsp70. Cell lysates were immunoprecipitated with anti-Hsp70 antibody and immunoblotted with the same antibody. This also serves as a loading control. (E) Effect of arsenite on Plk-1 expression in GCS-2 cells. Total cell lysates were immunoblotted with anti-Plk-1 antibody. (F) Effect of arsenite on cyclin D-associated cdk-4 expression in GCS-2 cells. Plk-1 membrane was stripped and immunoblotted using anti-cdk-4 antibody. (G) Cdk-4 membrane was stripped and reprobed with actin-specific antibody to control for protein loading.
Arsenite Affects Hsp90 Binding with its Cochaperone
We examined if ubiquitination of Hsp90 affects its ability to function as a chaperone, as analyzed by its ability to bind its major cochaperone p50cdc-37 [16, 17]. Cytosolic extracts from arsenite-treated and untreated BDC-1 and GCS-2 cells were immunoprecipitated with anti-Hsp90 antibody. Proteins were separated by SDS-PAGE and analyzed by immunoblot with p50cdc-37-specific antibody. We found that the treatment with arsenite diminished the interaction of Hsp90 with p50cdc-37 by ~ 50% in GCS-2 cells whereas it was unaffected in BDC-1 cells (Fig. 4C). We next asked if the ubiquitination of Hsp90 and its reduced levels also destabilized its client proteins, leading to deficiencies in multiple unrelated mechanisms. We studied polo-like kinase-1 (Plk-1), a prominent regulator of cellular events such as spindle formation, chromosome segregation, and cytokinesis [28]. Cdk4 is also assisted in folding and subsequently stabilized by Hsp90 [29]. Both Plk-1 and Cdk-4 diminished after treatment with 0.5 μM arsenite in GCS-2 cells but remained unaltered in BDC-1 cells (Figs. 4E and 4F). Thus arsenite causes ubiquitination of Hsp90 and downregulates Hsp90 client proteins in GCS-2 cells under low/absent GSH conditions.
Arsenite Impairs Proteasome Function in GCS-2 Cells
The ubiquitin-proteasome pathway is the primary route for degrading ubiquitin-tagged proteins and some non-ubiquitinated proteins [20]. Since ubiquitinated proteins accumulate in arsenite-treated GCS-2 cells, we assayed proteasome function in these cells in comparison with BDC-1 cells. Proteasome activity was measured using a fluorogenic substrate of the chymotrypsin-like peptidase of the proteosome. We found that in the GCS-2 cells treated with 0.5 μM arsenite, proteasome activity decreased 25–30% compared to untreated GCS-2 and BDC-1 cells, and BDC-1 cells treated with arsenite (Fig. 5A).
Figure 5.
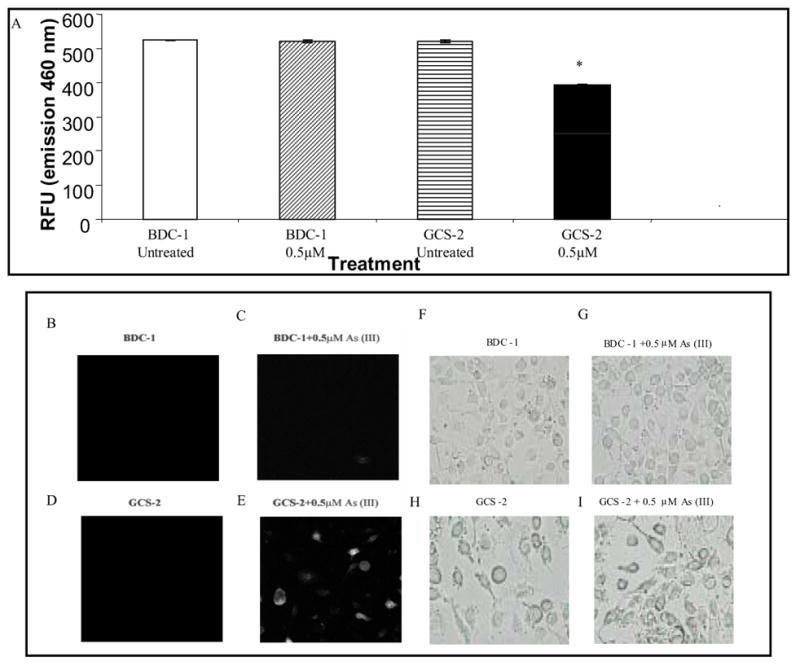
Effect of arsenite on proteasome function in GCS-2 cells. (A) In vitro functional assay for proteasome activity. The chymotrypsin-like peptidase activity of the proteasome was assayed with fluorogenic substrate succinyl-leu-leu-val-tyr-AMC. Cells were treated with arsenite, washed, lysed, and assayed for chymotrypsin activity. Values are presented as mean ± SEM for 5 determinations. p ≤ 0.001; RFU, relative fluorescence unit. (B) In vivo functional assay for the proteasome. BDC-1 and GCS-2 cells were transfected with pGFPu containing neomycin as described under “Materials and Methods”. Stably transfected cells were isolated, treated with and without arsenite and 21 h later the cells were assayed for intracellular fluorescence by confocal microscopy. B, D, F, and H are untreated cells; C, E, G, and I are cells treated with 0.5 μM arsenite. B-E is confocal micrographs of GFPu expression in cells. F-H is the corresponding light micrographs.
To investigate the specific relationship between aggregation of ubiquitin-protein conjugates and proteasome function, we transfected a reporter construct consisting of a short degron oligonucleotide sequence (corresponding peptide sequence ACKNWFSSLSHFVIHL) fused to GFP-C1 plasmid (GFPu) into BDC-1 and GCS-2 cells. GFPu is normally unstable [half-[30]. GCS-2 cells containing stably-time (t1/2) = 20–30 min] compared to GFP (t1/2 > 10 h) transfected GFPu were cultured for 48 h, GSH was withdrawn for 24 h, and the cells were treated with 0.5 μM arsenite for 21 h and monitored for fluorescence. GFPu fluorescence was not observed in BDC-1 cells or in GCS-2 cells without arsenite treatment (Fig. 5B, C, and D). In contrast, GCS-2 cells treated with 0.5 μM arsenite for 21 h showed increased GFPu fluorescence suggesting that GFPu accumulated intracellularly as aggregates (Fig. 5E).
Arsenite-Induced Lipid Peroxidation in GCS-2 Cells
Lipid peroxidation is a well-established mechanism of cellular injury and is used as an indicator of oxidative stress in cells and tissues [31]. MDA formation was determined in GCS-2 cells that were treated with arsenite for 21 h after GSH depletion for 24 h (Fig. 6A). There was ~ 10-fold increase in MDA formation in GCS-2 cells treated with arsenite compared to BDC-1 cells treated with arsenite.
Figure 6.
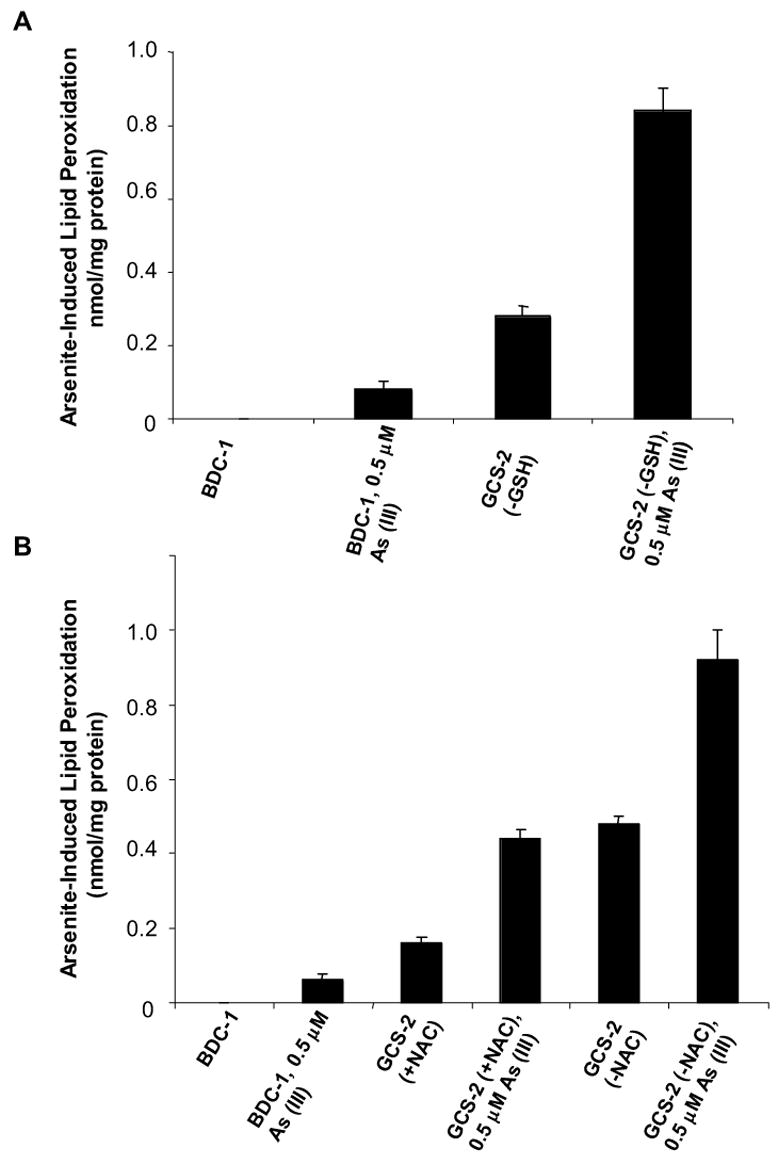
Effect of arsenite on lipid peroxidation in GCS-2 cells. (A) Arsenite-induced lipid peroxidation in BDC-1 and GSH-dependent GCS-2 cells. Experimental conditions are as described in Fig. 1 except that the cells were treated with 0.5 μM arsenite for 21 h. The amount of MDA formed in untreated BDC-1 cells was below the limits of detection. (B) Arsenite-induced lipid peroxidation in BDC-1 and NAC-dependent GCS-2 cells. The results in Fig. 6A and 6B are presented as mean ± SEM for 3 determinations.
To determine if NAC protects GCS-2 cells against arsenite-induced oxidative stress, we exposed cells grown in the presence of NAC and cells that were depleted of NAC for 24 h, to arsenite for 21 h. Cells that were treated with arsenite in the presence of NAC had ~ half the MDA levels compared to cells exposed to arsenite in the absence of NAC suggesting that NAC provides partial protection against arsenite-induced toxicity (Fig. 6B).
Release of Cytochrome C into Cytosol in GSH-depleted GCS-2 Cells
Release of cytochrome C into the cytosol is indicative of mitochondrial damage [32]. We evaluated cytochrome C release into the cytosol as a function of time and arsenite treatment in BDC-1 and GCS-2 cells. Cytosolic fractions of BDC-1 and GCS-2 cells were assayed for the presence of cytochrome C by immunoblot with anti-cytochrome C antibody. While there was no measurable cytochrome C present in the untreated or arsenite-treated BDC-1 cells, cytochrome C was readily detectable in the cytoplasm of untreated GSH-depleted GCS-2 cells (Fig. 7A) and the levels increased after arsenite treatment by approximately 2-fold at 21-24 h in the GCS-2 cells. This result suggests that these cells are poised to undergo apoptotic cell death and arsenite tips the balance toward apoptosis (Fig. 7).
Figure 7.
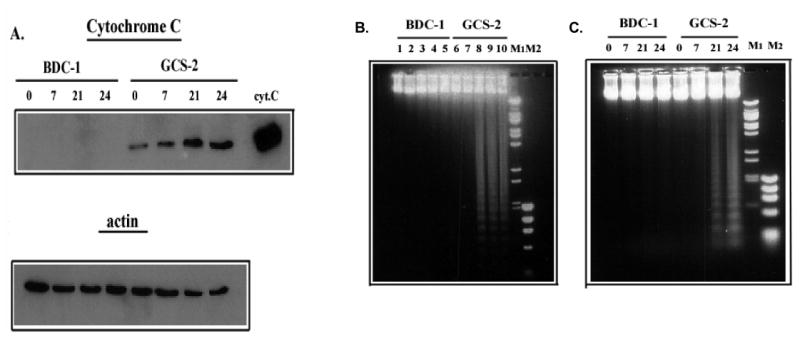
Effect of arsenite on cytochrome C release in BDC-1 and GCS-2 cells. (A) Time-course of the effect of arsenite on the release of cytochrome C in BDC-1 and GCS-2 cells. Cells were treated with 0.5 μM arsenite for the indicated times, and cytosolic extracts were separated by SDS-PAGE and immunoblotted with cytochrome C-specific antibody. Commercially available cytochrome C served as the positive control. The membrane was stripped and reprobed with an anti-actin antibody to ensure equal loading. (B) Dose response of arsenite-induced apoptosis in GCS-2 cells. Cells were treated with various concentrations of arsenite for 21 h, and DNA was isolated and analyzed on 1% agarose gels. Lanes 1 and 6, untreated; lanes 2 and 7, 0.2 μM; lanes 3 and 8, 0.5 μM; lanes 4 and 9, 1 μM; lanes 5 and 10, 2 μM arsenite treated. (C) Time-course of arsenite-induced apoptosis in GCS-2 cells. Cells were treated with 0.5 μM arsenite for various time points and DNA was isolated and analyzed as described in “Methods”.
Arsenite Induces Apoptosis in GCS-2 Cells
To determine if arsenite induces apoptosis in our system, we exposed GSH-deficient GCS-2 cells to varying concentrations of arsenite for 21 h, and analyzed extracted DNA. BDC-1 cells showed no signs of DNA fragmentation, whereas GCS-2 cells showed a dose-dependent increase in DNA laddering at concentrations ≥ 0.5 μM arsenite (Fig. 7B). While no DNA laddering was obvious up to 7 h of arsenite treatment, DNA fragmentation became evident at 21 h at 0.5 μM arsenite treatment (Fig. 7C). Thus, the absence of GSH and the accompanying exposure to arsenite induce internucleosomal DNA fragmentation in arsenite-treated GCS-2 cells.
Discussion
In this study, we have taken advantage of Gclc-deficient GCS-2 cells as an experimental system to assess the effects of very low/absent levels of GSH on arsenite-induced toxicity, protein ubiquitination and apoptosis. Our results are in general agreement with our previous results (24) and the recently reported arsenite toxicity studies performed on mouse embryo fibroblasts derived from glutamate-cysteine ligase modifier subunit-deficient (Gclm−/−) mice that have GSH levels ranging from 9–16% of the wild type levels [Fig. 1 and ref. 33]. The ED50 for GCS-2 cells was 1 μM (Fig. 1), whereas for BDC-1 cells, it was 24 μM (data not shown). These data suggest that GCS-2 cells are very sensitive to arsenite and the cytotoxicity of arsenite in these cells directly correlates with the intracellular levels of GSH.
Intracellular GSH also plays a vital role not only in the transport of arsenic and its methylated derivatives but also in arsenic metabolism especially in the detoxification of monomethylarsonic acid (MMA; refs. 8, 9). At present it is not clear if because of lack of GSH in GCS-2 cells, they can convert inorganic arsenite into methylated arsenicals. Without a ready source of methylated arsenicals, it is likely that all of the cytotoxic effects of arsenic in these cells are attributable to inorganic arsenic species.
Although accumulation of ubiquitin protein conjugates has been shown to occur with a number of stressors, a direct correlation between the availability of GSH in the cell and the accumulation of high molecular protein aggregates has not been established [34–37]. Bredfeldt et al observed that reduction in GSH levels could cause the accumulation of ubiquitinated proteins in UROtsa cells even though the actual level of GSH after GSH depletion by the Gcl inhibitor DL-buthionine-(R,S)-sulfoximine (BSO) in these cells was not documented [38]. Our findings demonstrate that GSH, and to a lesser extent NAC, can protect cells against arsenite-induced protein ubiquitination (Fig. 2). In this context, we also note that although endogenous cysteine concentrations in GSH and NAC-rescued cells are comparable [24], our data indicate that NAC could only partially reverse the ubiquitination of proteins in the GCS-2 cells (Fig. 2D). This result suggests that even though NAC can support cell growth as well as GSH does, it cannot perform all the functions of GSH in the cell.
In the absence of GSH as an anti-oxidant, vicinal dithiol-specific SH reagent such as arsenite could react directly with the highly conserved Cys589/590 pair in Hsp90 impairing its chaperone activity [39]. Withdrawal of GSH and treatment with arsenite downregulated molecular chaperone Hsp90 at the transcriptional level and by ubiquitination, diminished its ability to bind cochaperone p50Cdc37, and destabilized Hsp90 client proteins Plk-1 and Cdk-4 in GCS-2 cells (Fig. 4). How does arsenic-induced down-regulation of Hsp90 gene expression result in cytotoxicity and cell death? Molecular chaperone Hsp90 is a central molecule in the proper folding of proteins. Inhibition of Hsp90 and its ubiquitination by arsenite could lead to the formation misfolded polypeptides which not only lose their function but they may also form toxic species, including oligomers or larger aggregates. These aggregates may be prevented from reaching their cellular destination due to retention and/or degradation. This increased retention and accumulation of insoluble aggregates may be further exacerbated by arsenite-induced partial inhibition of the proteasome.
Enhanced ubiquitination of Hsp90 and diminution of the cellular content of its client proteins have also been noted in cancer cells after exposure to the signal transduction inhibitor hypericin [12]. Whereas arsenite mainly induces apoptosis, hypericin causes mitotic cell death [12]. However, in both cases, Hsp90 inactvation by ubiquitination is a potential mechanism culminating in cell death.
Hsp90, p50cdc37, and Plk-1 are all known to be over-expressed in certain types of cancers [28, 40]. In fact, treatment of tumors with chemotherapeutic agents such as geldanamycin and radicicol, inhibitors of Hsp90, is known to induce significant tumor cytotoxicity [14, 41]. Unlike geldanamycin and radicicol, which inactivate Hsp90 through interaction with the ADP/ATP binding pocket, arsenite exerts its cytotoxic effect through Hsp90 ubiquitination [12, 41].
Arsenite not only enhanced the accumulation of protein-ubiquitin conjugates, but also inhibited proteasome function by about 25% in GSH-deficient GCS-2 cells, thereby providing a mechanism for reduced degradation of the ubiquitinated proteins (Fig. 5). Blocking proteasome function by proteasome-specific inhibitors leads to intracellular accumulation of ubiquitin-conjugated proteins [42].
Oxidative stress has been attributed as an important aspect of arsenite toxicity [31]. In the present study, exposure to arsenite induced cellular lipid peroxidation in both wild type and GSH-deficient cells (Fig. 6). However, more substantial increases in MDA were observed after arsenite treatment in GCS-2 cells that were depleted of GSH or NAC suggesting an increased oxidative stress in the GCS-2 cells. This increased oxidative stress in GCS-2 cells could enhance protein ubiquitination and apoptosis.
Our data indicate that untreated GCS-2 cells had detectable levels of cytochrome C in the cytosol, which increased upon exposure to arsenite (Fig. 7A). However we did not detect DNA laddering at the same time point (Fig. 7C). These findings concur with our earlier observations that GSH depletion is sufficient to cause cytochrome C release into the cytosol [32]. Cytochrome C release increased with the cell’s commitment to irreversible apoptosis since we observed DNA fragmentation in the GCS-2 cells only at 21 h after arsenite treatment (Fig. 7C). Most previous studies on the induction of apoptosis in normal cells involved acute exposure to concentrations of arsenite ranging from 40-400 μM [3]. However, others have observed that the mechanism of resistance of tumor cells to the chemotherapeutic effects of arsenic is the up-regulation of GSH, and GSH depletion by (BSO) restored arsenic sensitivity [43, 44]. JNK activation along with down-regulation of GSH may be an important mechanism by which arsenite induces apoptosis in tumor cells [44]. This is consistent with our finding of JNK activation in GSH-deficient GCS-2 cells (Table 1) may tip the balance toward apoptosis. Recently, Fernandez et al. have observed that As2O3 (6 μM) caused apoptosis preferentially in U-937 human promonocytic cells and this effect was potentiated by depletion of GSH induced by BSO [45]. In addition, there are reports that suggest that mitochondrial function is affected by oxidative stress [46–48]. In a GSH compromised state, arsenite could alter mitochondrial membrane permeability via a direct effect on the adenine nucleotide translocator [47] and perturbation of mitochondrial permeability transition pore [46, 48] resulting in apoptosis.
In summary, under conditions of reduced GSH availability, the cytotoxicity of arsenic may be viewed in terms of protein ubiquitination and Hsp90 inactivation, leading to destabilization of Hsp90 client proteins. These changes, along with partial inhibition of the proteasome, lead to intracellular aggregation of high molecular weight ubiquitinated protein conjugates. Ultimately damaged proteins could accumulate and induce cell death (Fig. 8). Further exploration into the role of cellular redox status might provide clues to the underlying mechanism of Hsp90-mediated signal transduction and posttranslational regulation of its client proteins.
Figure 8.
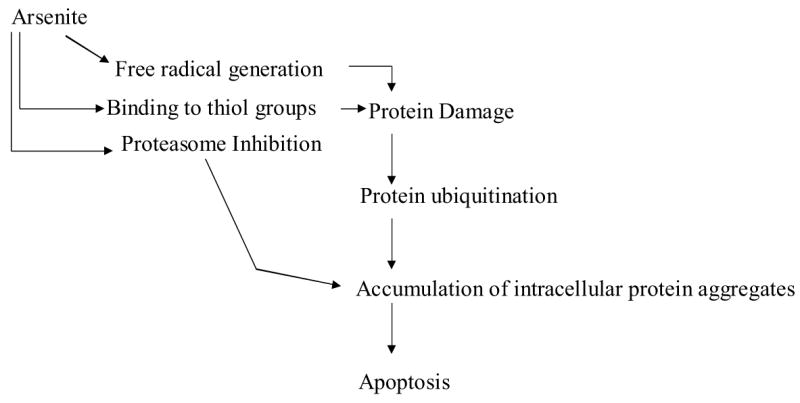
Schematic representation of events induced by arsenite in the absence of GSH culminating in apoptotic cell death.
Acknowledgments
This work was supported by NIH grant ES-08668. We thank Ms. Weili Liu for excellent technical assistance and Dr. Richard Sifers for the GFPu plasmid.
Abbreviations
- Cdk-4
cyclin dependent kinase-4
- Gcl
glutamate cysteine ligase
- GSH
glutathione
- Hsp90
heat shock protein 90
- MDA
malondialdehyde
- NAC
N-acetyl cysteine
- Plk-1
polo-like kinase-1
- SDS-PAGE
sodium dodecylsulfate polyacrylamide gel electrophoresis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kitchin KT. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol. 2001;172:249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- 2.Simeonova PP, Wang S, Toriuma W, Kommineni V, Matheson J, Unimye N, Kayama F, Harki D, Ding M, Vallyathan V, Luster MI. Arsenic mediates cell proliferation and gene expression in the bladder epithelium: association with activating protein-1 transactivation. Cancer Res. 2000;60:3445–3453. [PubMed] [Google Scholar]
- 3.Lau ATY, He Q-Y, Chiu J-F. A proteome analysis of the arsenite response in cultured lung cell: evidence for in vitro oxidative stress-induced apoptosis. Biochem J. 2004;382:641–650. doi: 10.1042/BJ20040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosower NS, Kosower EM. The glutathione status of cells. Int Rev Cytol. 1978;54:109–160. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- 5.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 6.Lieberman MW, Barrios R, Carter BZ, Habib GM, Lebovitz RM, Rajagopalan S, Sepulveda AR, Shi ZZ, Wan DF. Gamma-glutamyl transpeptidase. What does the organization and expression tell us about its function? Am J Path. 1995;147:1175–1185. [PMC free article] [PubMed] [Google Scholar]
- 7.Ballatori N, Wang W, Lieberman MW. Accelerated methyl mercury elimination in gamma-glutamyl transpeptidase-deficient mice. Am J Pathol. 1998;152:1049–1055. [PMC free article] [PubMed] [Google Scholar]
- 8.Kala SV, Kala G, Prater CI, Sartorelli AC, Lieberman MW. Formation and urinary excretion of arsenic triglutathione and methylarsenic diglutathione. Chem Res Toxicol. 2004;17:243–249. doi: 10.1021/tx0342060. [DOI] [PubMed] [Google Scholar]
- 9.Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, Lieberman MW. The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem. 2000;275:33404–33408. doi: 10.1074/jbc.M007030200. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann NY Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 11.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Rad Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 12.Blank M, Mandel M, Keisari Y, Meruelo D, Lavie G. Enhanced ubiquitinylation of heat shock protein 90 as a potential mechanism for mitotic cell death in cancer cells induced with hypericin. Cancer Res. 2003;63:8241–8247. [PubMed] [Google Scholar]
- 13.Zhao Q, Boschelli F, Caplan A, Arndt KT. Identification of a conserved sequence motif that promotes Cdc37 and cyclin D1 binding to Cdk4. J Biol Chem. 2004;279:12560–12564. doi: 10.1074/jbc.M308242200. [DOI] [PubMed] [Google Scholar]
- 14.Pratt WB, Toft DO. (2003) Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 15.Young JC, Barral JM, Hartl FU. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28:541–547. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Roe SM, Ali MM, Meyer P, Vaughan CK, Panaretou B, Piper PW, Prodromou C, Pearl LH. The mechanism of Hsp90 regulation by the protein kinase-specific cochaperone p50 (cdc 37) . Cell. 2004;116:87–98. doi: 10.1016/s0092-8674(03)01027-4. [DOI] [PubMed] [Google Scholar]
- 17.Tatebe H, Shiozaki K. Identification of Cdc37 as a novel regulator of the stress-responsive mitogen-activated protein kinase. Mol Cell Biol. 2003;23:5132–5142. doi: 10.1128/MCB.23.15.5132-5142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman J, Xue D. To live or die by the sword: the regulation of apoptosis by the proteasome. Dev Cell. 2004;6:460–461. doi: 10.1016/s1534-5807(04)00104-2. [DOI] [PubMed] [Google Scholar]
- 19.Jesenberger V, Jentsch S. Deadly encounter: ubiquitin meets apoptosis. Nat Rev Mol Cell Biol. 2002;3:112–121. doi: 10.1038/nrm731. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz DC, Hochstrasser M. A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem Sci. 2003;28:321–328. doi: 10.1016/S0968-0004(03)00113-0. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Yu X. Regulation of apoptosis: the ubiquitous way. FASEB J. 2003;17:790–799. doi: 10.1096/fj.02-0654rev. [DOI] [PubMed] [Google Scholar]
- 22.Lee JC, Peter ME. Regulation of apoptosis by ubiquitination. Immunol Rev. 2003;193:39–47. doi: 10.1034/j.1600-065x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 23.Drexler HC. The role of p27Kip1 in proteasome inhibitor induced apoptosis. Cell Cycle. 2003;2:438–44. [PubMed] [Google Scholar]
- 24.Shi ZZ, Osei-Frimpong J, Kala G, Kala SV, Barrios RJ, Habib GM, Lukin DJ, Danney CM, Matzuk MM, Lieberman MW. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci USA. 2000;97:5101–5106. doi: 10.1073/pnas.97.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinman WA, Richie JP., Jr Determination of thiols and disulfides using high- performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1995;672:73–80. doi: 10.1016/0378-4347(94)00194-a. [DOI] [PubMed] [Google Scholar]
- 26.Rossi mR, Somji S, Garrett SH, Sens MA, Nath J, Sens DA. Expression of hsp27, hsp60, hsc70, and hsp70 stress response genes in cultured human urothelial cells (UROtsa) exposed to lethal and sublethal concentrations of sodium arsenite. Environ Health Perspect. 2002;110:1225–1232. doi: 10.1289/ehp.021101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirano S, Cui X, Li S, Kanno S, Kobayashi Y, Hayakawa T, Shraim A. Difference in uptake and toxicity of trivalent and pentavalent inorganic arsenic in rat heart microvessel endothelial cells. Arch Toxicol. 2003;77:305–312. doi: 10.1007/s00204-003-0447-x. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Erikson RL. Polo-like kinase (Plk) 1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci USA. 2003;100:5789–5794. doi: 10.1073/pnas.1031523100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Hirshberg M, McLaughlin SH, Lazar GA, Grossman JG, Nielsen PR, Sobott F, Robinson CV, Jackson SE, Laue ED. Biochemical and structural studies of the interaction of Cdc37 with Hsp90. J Mol Biol. 2004;16:891–907. doi: 10.1016/j.jmb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 31.Qu W, Kasprzak KS, Kadiiska M, Liu J, Chen H, Maciag A, Mason RP, Waalkes MP. Mechanisms of arsenic-induced cross-tolerance to nickel cytotoxicity, genotoxicity, and apoptosis in rat liver epithelial cells. Toxicol Sci. 2001;63:189–195. doi: 10.1093/toxsci/63.2.189. [DOI] [PubMed] [Google Scholar]
- 32.Valverde M, Rojas E, Kala SV, Kala G, Lieberman MW. Survival and cell death in cells constitutively unable to synthesize glutathione. Mutat Res. 2006;594:172–180. doi: 10.1016/j.mrfmmm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Kann S, Estes C, Reichard JF, Huang M-Y, Sartor MA, Schwemberger S, Chen Y, Dalton TP, Shertzer HG, Xia Y, Puga A. Butylhydroquinone protects cells genetically deficient in glutathione biosynthesis from arsenite-induced apotosis without significantly ahanging their prooxidant status. Toxicol Sci. 2005;87:365–384. doi: 10.1093/toxsci/kfi253. [DOI] [PubMed] [Google Scholar]
- 34.Shang F, Taylor A. Oxidative stress and recovery from oxidative stress are associated with altered ubiquitin conjugating and proteolytic activities in bovine lens epithelial cells. Biochem J. 1995;307:297–303. doi: 10.1042/bj3070297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsirigotis M, Zhang M, Chiu RK, Wouters BG, Gray DA. Sensitivity of mammalian cells expressing mutant ubiquitin to protein-damaging agents. J Biol Chem. 2001;276:46073–46078. doi: 10.1074/jbc.M109023200. [DOI] [PubMed] [Google Scholar]
- 36.Fujimuro M, Sawada H, Yokosawa H. Dynamics of ubiquitin conjugation during heat-shock response revealed by using a monoclonal antibody specific to multi-ubiquitin chains. Eur J Biochem. 1997;249:427–433. doi: 10.1111/j.1432-1033.1997.00427.x. [DOI] [PubMed] [Google Scholar]
- 37.Kirkpatrick DS, Dale KV, Catania JM, Gandolfi AJ. Low-level arsenite causes accumulation of ubiquitinated proteins in rabbit renal cortical slices and HEK293 cells. Toxicol Appl Pharmacol. 2003;186:101–109. doi: 10.1016/s0041-008x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 38.Bredfeldt TG, Kopplin MJ, Gandolfi AJ. Effect of arsenite on UROtsa cells: low-level arsenite causes accumulation of ubiquitinated proteins that is enhanced by reduction in cellular glutathione levels. Toxicol Appl Pharmacol. 2004;198:412–418. doi: 10.1016/j.taap.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Nardai G, Sass B, Eber J, Orosz G, Csermely P. Reactive cysteines of the 90-kDa heat shock protein, Hsp90. Arch Biochem Biophys. 2000;384:59–67. doi: 10.1006/abbi.2000.2075. [DOI] [PubMed] [Google Scholar]
- 40.Schwarze SR, Fu VX, Jarrard DF. Cdc37 enhances proliferation and is necessary for normal human prostate epithelial cell survival. Cancer Res. 2003;63:4614–4619. [PubMed] [Google Scholar]
- 41.Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, Ortiz KL, Sieck LK, Isaccs JS, Brechbiel MW, Mitchell JB, Neckers LM, Gius D. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res. 2003;63:8984–8995. [PubMed] [Google Scholar]
- 42.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 43.Kito M, Akao Y, Ohishi N, Yagi K, Nozawa Y. Arsenic trioxide-induced apoptosis and its enhancement by buthionine sulfoximine in hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2002;291:861–867. doi: 10.1006/bbrc.2002.6525. [DOI] [PubMed] [Google Scholar]
- 44.Davison K, Mann KK, Waxman S, Miller WH., Jr JNK activation is a mediator of arsenic trioxide-induced apoptosis in acute promyelocytic leukemia cells. Blood. 2004;103:3496–3502. doi: 10.1182/blood-2003-05-1412. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez C, Ramos AM, Sancho P, Amran D, de Blas E, Aller P. 12-O-tetradecanoylphorbol-13-acetate may both potentiate and decrease the generation of apoptosis by the antileukemic agent arsenic trioxide in human promonocytic cells. Regulation by extracellular signal-regulated protein kinases and glutathione. J Biol Chem. 2004;279:3877–3884. doi: 10.1074/jbc.M310665200. [DOI] [PubMed] [Google Scholar]
- 46.Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J Biol Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 47.Belzacq A-S, El Hamel C, Viera HLA, Cohen I, Haouzi D, Métivier D, Marchetti P, Brenner C, Kroemer G. Adenine nucleotide translocator mediates the mitochondrial membrane permeabilization induced by ionidamine, arsenite and CD437. Oncogene. 2001;20:7579–7587. doi: 10.1038/sj.onc.1204953. [DOI] [PubMed] [Google Scholar]
- 48.Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin SA, Zamazami N, Xie Z, Reed J, Kroemer G. Arsenite induces apoptosis via a direct effect on the mitochondrial permeability transition pore. Exp Cell Res. 1999;249:413–421. doi: 10.1006/excr.1999.4519. [DOI] [PubMed] [Google Scholar]


