Abstract
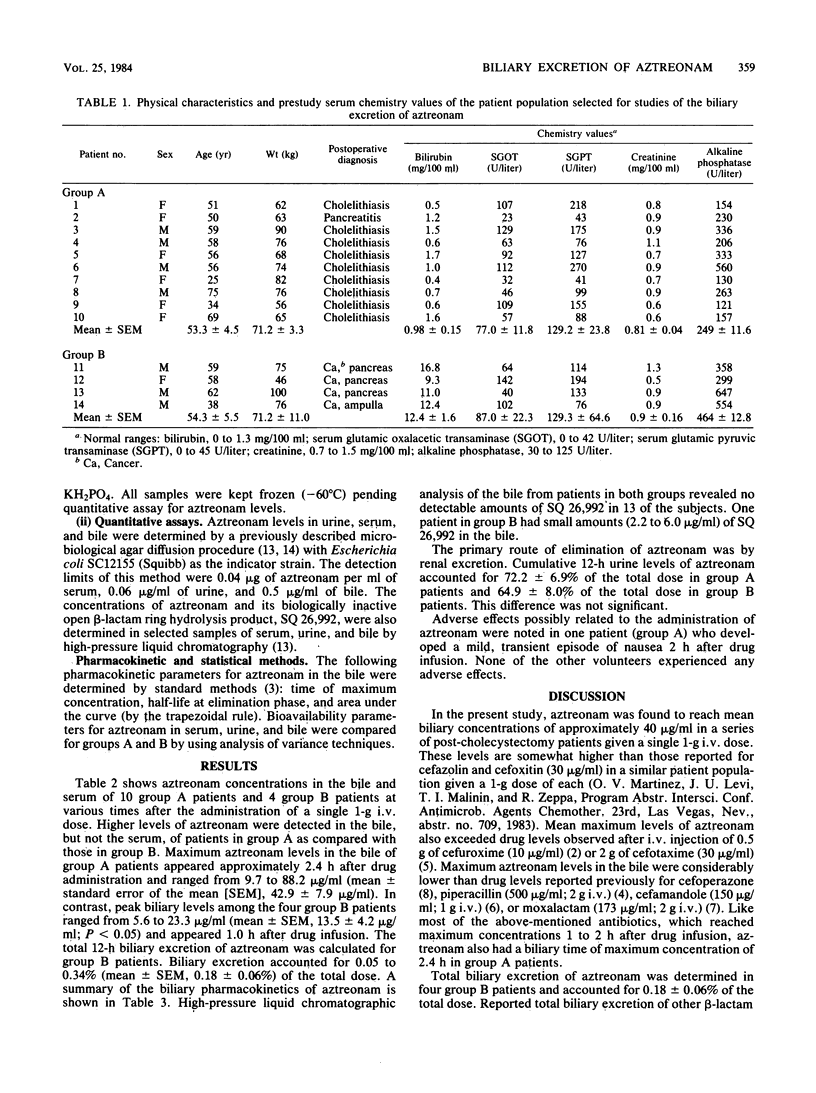
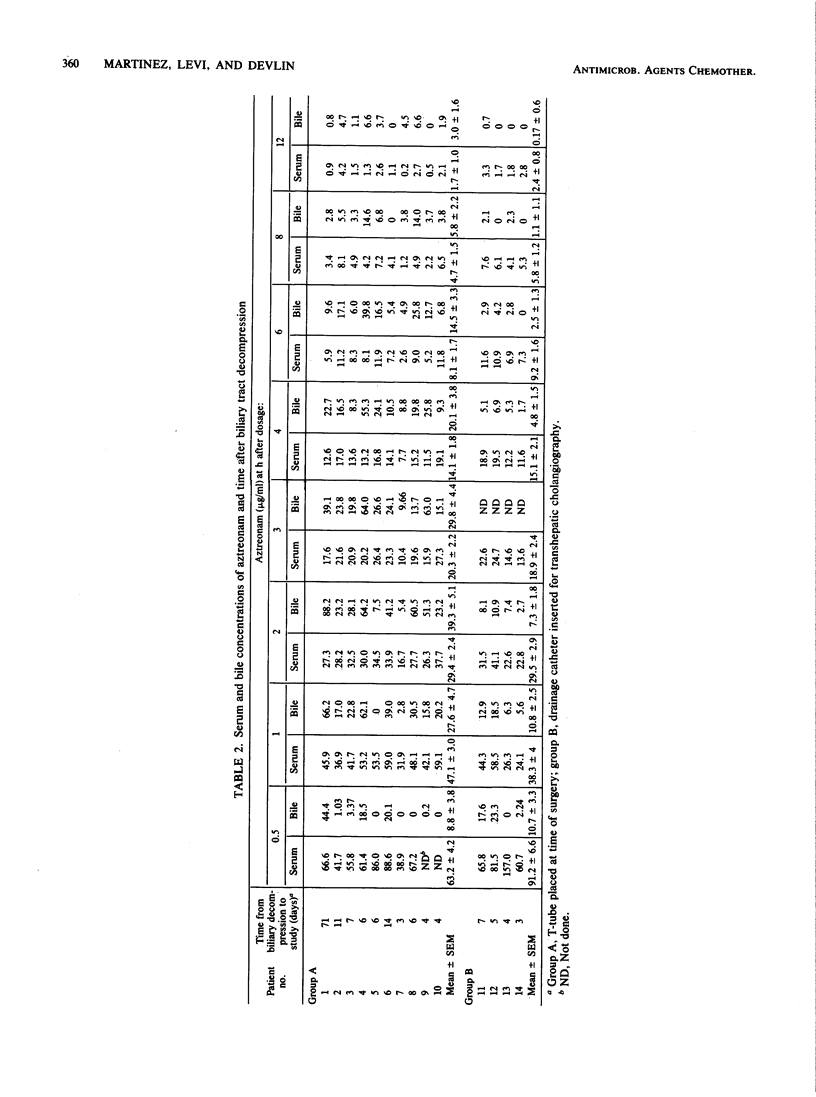
The biliary excretion of aztreonam was studied in 10 post-cholecystectomy patients with T-tube biliary drainage (group A) and four other subjects with obstructive biliary tract disease who had recent placement of external biliary drainage (group B). Maximum biliary levels ranged from 9.7 to 88.2 micrograms/ml (mean, 42.9 +/- 7.9 micrograms/ml) and occurred 2.4 h after injection of a single 1-g dose intravenously. Peak biliary levels observed in group B patients were approximately one-third those in group A. Cumulative 12-h biliary excretion (group B) accounted for 0.18 +/- 0.06% of the total dose. In the same period, urinary excretion accounted for 65 to 72% of the total dose. The lower biliary levels of aztreonam observed in group B patients relative to those in group A suggest that in patients with total biliary tract obstruction the liver may not recover full secretory capacity, at least within 3 to 7 days after biliary decompression.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brogard J. M., Haegele P., Dorner M., Lavillaureix J. Biliary excretion of a new semisynthetic cephalosporin, cephacetrile. Antimicrob Agents Chemother. 1973 Jan;3(1):19–23. doi: 10.1128/aac.3.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogard J. M., Kopferschmitt J., Pinget M., Arnaud J. P., Lavillaureix J. Cefuroxime concentrations in serum, urine and bile: pharmacokinetic profile. Proc R Soc Med. 1977;70(Suppl 9):42–50. doi: 10.1177/00359157770700S909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron J. A., Meyers B. R., Hirschman S. Z. Biliary concentrations of piperacillin in patients undergoing cholecystectomy. Antimicrob Agents Chemother. 1981 Feb;19(2):309–311. doi: 10.1128/aac.19.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kees F., Strehl E., Dominiak P., Grobecker H., Seeger K., Seidel G., Neuhaus B., Safrany L. Cefotaxime and desacetyl cefotaxime in human bile. Infection. 1983 Mar-Apr;11(2):118–120. doi: 10.1007/BF01641077. [DOI] [PubMed] [Google Scholar]
- Levi J. U., Livingstone A. S., Martinez O. V., Zeppa R., Malinin T., Hutson D. G., Einhorn N. C. Biliary concentrations of cefamandole and its use in biliary tract surgery. Scand J Infect Dis Suppl. 1980;Suppl 25:55–57. [PubMed] [Google Scholar]
- Martinez O. V., Levi J. U., Livingstone A., Malinin T. I., Zeppa R., Hutson D., Einhorn N. Biliary excretion of moxalactam. Antimicrob Agents Chemother. 1981 Aug;20(2):231–234. doi: 10.1128/aac.20.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. A review and summary of the pharmacokinetics of cefoperazone: a new, extended-spectrum beta-lactam antibiotic. Ther Drug Monit. 1981;3(2):121–128. doi: 10.1097/00007691-198102000-00002. [DOI] [PubMed] [Google Scholar]
- Pinget M., Brogard J. M., Dauchel J., Lavillaureix J. Biliary excretion of ampicillin, metampicillin and carbenicillin. J Antimicrob Chemother. 1976 Jun;2(2):195–201. doi: 10.1093/jac/2.2.195. [DOI] [PubMed] [Google Scholar]
- Ratzan K. R., Baker H. B., Lauredo I. Excretion of cefamandole, cefazolin, and cephalothin into T-tube bile. Antimicrob Agents Chemother. 1978 Jun;13(6):985–987. doi: 10.1128/aac.13.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzan K. R., Ruiz C., Irvin G. L., 3rd Biliary tract excretion of cefazolin, cephalothin, and cephaloridine in the presence of biliary tract disease. Antimicrob Agents Chemother. 1974 Oct;6(4):426–431. doi: 10.1128/aac.6.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swabb E. A., Leitz M. A., Pilkiewicz F. G., Sugerman A. A. Pharmacokinetics of the monobactam SQ 26,776 after single intravenous doses in healthy subjects. J Antimicrob Chemother. 1981 Dec;8 (Suppl E):131–140. doi: 10.1093/jac/8.suppl_e.131. [DOI] [PubMed] [Google Scholar]
- Swabb E. A., Singhvi S. M., Leitz M. A., Frantz M., Sugerman A. Metabolism and pharmacokinetics of aztreonam in healthy subjects. Antimicrob Agents Chemother. 1983 Sep;24(3):394–400. doi: 10.1128/aac.24.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]


