Abstract
X-linked Mental Retardation (XLMR) occurs in 1 in 600 males and is highly genetically heterogeneous. We used a novel human X chromosome cDNA microarray (XCA) to survey the expression profile of X-linked genes in lymphoblasts of XLMR males. Genes with altered expression verified by Northern blot and/or quantitative PCR were considered candidates. To validate this approach, we documented the expected changes of expression in samples from a patient with a known X chromosome microdeletion and from patients with multiple copies of the X chromosome. We used our XCA to survey lymphoblast RNA samples from 43 unrelated XLMR males and found 15 genes with significant (≥1.5-fold) reduction in expression in at least one proband. Of these, subsequent analysis confirmed altered expression in 12. We followed up one, PLP2, at Xp11.23, which exhibits approximately fourfold decreased expression in two patients. Sequencing analysis in both patients revealed a promoter variant, −113C>A, that alters the core-binding site of the transcription factor ELK1. We showed that PLP2-(−113C>A) is sufficient to cause reduced expression using a luciferase reporter system and is enriched in a cohort of males with probable XLMR (14 of 239, 5.85%) as compared to normal males (9 of 577, 1.56%) (χ2 = 11.07, P < 0.001). PLP2 is expressed abundantly in the pyramidal cells of hippocampus and granular cells of the cerebellum in the brain. We conclude that our XCA screening is an efficient strategy to identify genes that show significant changes in transcript abundance as candidate genes for XLMR.
X-linked mental retardation (XLMR) is a genetically heterogeneous group of disorders caused by defects of genes on the X chromosome (Ropers and Hamel 2005). Collectively, XLMR disorders are more common than fragile X syndrome, occurring in 1.66/1000 males in the general population (0.22/1000 males) (Turner et al. 1996; Stevenson 2000). Numerous studies have established a 25%–30% male excess in the mentally retarded population and a substantial fraction of the male excess is thought to be due to defects of genes on the X chromosome (Wing 1971; Herbst and Miller 1980; Hane et al. 1996). Additionally, X-linked risk factors for mental retardation, i.e., allelic variants that are not sufficient in and of themselves but in combination with other genetic variables and/or environmental factors result in intellectual impairment, may also contribute to the strong male excess, particularly in patients with borderline to mild mental retardation (Ropers and Hamel 2005). Stevenson and colleagues estimated that ∼150–200 genes on the X chromosome are responsible for XLMR (Stevenson et al. 2000). Understanding the molecular basis of the various XLMR disorders will enable accurate diagnosis and counseling of patients and families with these disorders and should also provide valuable insight into aspects of neuronal function that are required for the normal development of human cognition.
Steady progress has been made over the last 15 years in the study of the molecular basis and pathological mechanisms of XLMR. A total of 59 genes responsible for XLMR have been characterized using mainly classical genetic approaches including characterization of chromosomal fragile sites, X:autosome translocations, X chromosome microdeletions/duplications, and linkage mapping using informative pedigrees followed by candidate gene studies (Fu et al. 1991; Gu et al. 1996; Billuart et al. 1998; Carrie et al. 1999; Zemni et al. 2000). More recently, large-scale sequencing of candidate genes in XLMR families identified several novel XLMR genes (Kalscheuer et al. 2003; Tarpey et al. 2005). Despite these achievements, our understanding of the molecular basis and mechanisms for many XLMR disorders remains limited (Chelly and Mandel 2001; Ropers and Hamel 2005). With the genes for the more common and severe XLMR disorders now identified, the majority of the remaining XLMR genes (∼100) are likely to be found in fewer individuals with smaller families and less severe mental retardation. The rarity of individual XLMR phenotypes, the vast genetic heterogeneity, and the paucity of large and informative pedigrees pose challenges for utilization of classical genetic strategies to identify the remaining XLMR genes.
The complete sequence of human X chromosome (Ross et al. 2005) and the large collection of X-linked expressed sequence tags (ESTs) provide molecular resources for the development of new approaches to tackle the XLMR problem. A cDNA microarray technology has been used successfully to monitor the relative abundance of mRNA transcripts for thousands of genes simultaneously (DeRisi et al. 1997; Duggan et al. 1999; Iyer et al. 1999). Reasoning that about a third of all disease-associated mutations reduce mRNA abundance (Mendell and Dietz 2001) and that this fraction may be even higher for X-linked genes (Read et al. 1988; Hernandez-Martin et al. 1999), we developed a custom-built, human X chromosome cDNA microarray (XCA) to identify genes that show a significant alternation in the steady-state level of their transcripts. These candidate genes can then be evaluated for mutations by sequencing in the affected individuals and in individuals with similar phenotypic features and/or mapping information. We report here data substantiating this approach. Additionally, we identified two unrelated males with XLMR who exhibited a substantial reduction (greater than fourfold) of PLP2 mRNA in their lymphoblasts.
Results
Microarray
We made a human XCA with 1777 human EST clones representing genes from 1653 independent Unigene loci on the human X chromosome (Fig. 1A,B). The EST clone set was initially selected based on the human Unigene Build 139 (http://www.ncbi.nlm.nih.gov/UniGene/build.html) and manually updated based on information from UCSC (genome. ucsc.edu) and Ensembl (www.ensembl.orgwww.ensembl.org) databases and from the recently completed sequence of the human X chromosome (Ross et al. 2005). Approximately two thirds of the EST clones are from genes with known or implied function. We obtained the EST clones from commercial sources (ATCC, OpenBiosys). Among the 59 XLMR genes listed in the XLMR database complied at Greenwood Genetic Center (http://www.ggc.org/xlmr/html, updated 4/2006), 57 have representative EST clones on the current XCA. The two that are not included are recently reported XLMR genes, SIZN2 and ZFP674 (Srivastava et al. 2005; Lugtenberg et al. 2006).
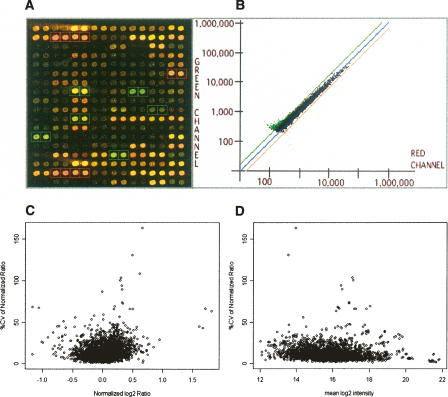
Figure 1.
A custom-built human X chromosome cDNA microarray (XCA). (A,B) A scanned microarray image and an example scatter-gram of data from the XCA, respectively. (C,D) The coefficients of variation (CV) of the normalized ratios across the microarray replicates of six normal controls (C) The percent CVs of normalized ratios plotted against the inter-microarray means of the base 2 logarithms of the normalized ratios across the biological replicates. (D) The percent CVs of normalized ratios plotted against the inter-microarray means of the log intensity on base 2 averages between the two channels of the normal and reference samples.
We performed standard dual fluorescent color cDNA microarray analysis using total cellular RNA from lymphoblasts of males with probable XLMR and normal males. To assemble a common reference RNA, we pooled equal amounts of total RNA from lymphoblast cell lines of six normal males. We labeled the reference RNA with Cy5 and the test RNA (isolated from either normal or patients) with Cy3. Fluorescent probes of either the control or XLMR patients were mixed with the fluorescent probe of the reference RNA and then hybridized to the cDNA targets on the microarray. The signal intensity ratios represent the relative abundance of individual transcripts from the test samples as compared to the reference RNA samples. As the result, the ratios for test samples from control males and affected males are comparable. Approximately 60% of EST clones generated reliable hybridization signals above the background as determined using the DeArray-IPLab Program (http://www.nhgri.nih.gov) and 40 of the 59 XLMR genes can be assayed for transcript levels in lymphoblasts.
The majority (86%) of the coefficients of variation (CV) of the normalized signal intensity ratios across the six biological microarray replicates of the normal control samples were <20% (Fig. 1C,D), the mean CV was 12.4%, and the median CV was 10.2%. Figure 1C shows low CVs in the normalized ratios throughout the entire range. As expected, the means of normalized ratios are small, ranging primarily from −0.5 to +0.5 on the base log2 scale (equivalent to ratios of 0.7 to 1.4 on the straight scale). Significantly, the variance of the normalized ratios observed at the low signal intensities was not substantially increased compared with that seen at the high signal intensities (Fig. 1D).
Proof of principal studies
To determine if the XCA can detect a mutation reducing expression to negligible levels, we isolated RNA from a lymphoblast cell line (GM07691, CCR) from a patient with chronic granulomatous disease (CGD) due to a microdeletion of CYBB, which encodes the cytochrome b-245, β-peptide. We found a substantial reduction (7.5-fold) in the CYBB transcript in this cell line. To determine if the XCA can identify genes with increased expression, we determined the transcript levels of XIST and genes that reside in the pseudoautosomal region (PAR) in fibroblasts from an individual with 49,XXXXY (Supplemental Table 1). Since XIST expresses only from the inactivated X chromosomes and PAR genes are known to escape X inactivation, we expected to see an increase in the transcript levels of these genes in fibroblasts with 49,XXXXY as compared to fibroblasts with normal karyotype. We found that the XIST transcript in the 49,XXXXY fibroblast RNA was 2.76-fold higher than that in the normal 46,XY controls, and eight of the nine PAR genes on which we have informative data showed an increase in transcript levels of >1.5-fold (Supplemental Table 1). Taken together, these results indicate that when the quality of the microarray data is reliable, as defined using the DeArray-IPLab Program (www.nhgri.nih.gov), our XCA analysis is sufficiently sensitive to detect genes whose expression is altered (either decreased or increased) in a major way (≥1.5-fold).
Analysis of X-linked gene expression in lymphoblast RNA from XLMR males
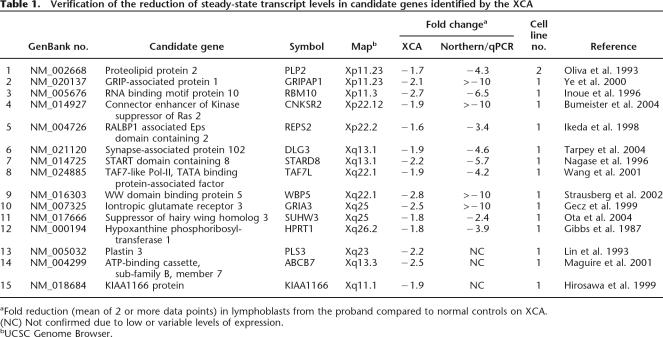
In an initial search for genes responsible for XLMR, we used our XCA to survey gene expression in lymphoblast RNA from 43 unrelated MR males with a pedigree consistent with X-linked inheritance. We focused our attention first on genes that showed significant reduction in expression in one or more of the samples. We prioritized potential candidate genes based on their tissue expression profile, the known or suggested function of their protein product, and association with pathways known to be essential to neuronal function. We identified 15 candidate genes using our XCA and confirmed transcript reduction in 12 in the proband by Northern blot and/or real-time quantitative PCR (Table 1). Two of the 12 confirmed candidate genes, HPRT1 and DLG3, are known XLMR genes (Gibbs and Caskey 1987; Tarpey et al. 2004). In GRIA3, which encodes inontropic glutamate receptor 3, we identified three missense variants that altered the conserved residues in known functional domains in a screening of ∼150 males with XLMR (Wu et al. 2005). We found that these missense variants cosegregate with the MR phenotype in the respective proband families and result in functional defects in the glutamate receptors (Y. Wu and T. Wang, unpubl.). The three unconfirmed genes were found to have either low or variable levels of expression in lymphoblasts. In addition, we identified 30 genes that showed an increase (≥1.5-fold) in the transcript abundance in one or more lymphoblast cell lines using the XCA (Supplemental Table 2).
Table 1.
Verification of the reduction of steady-state transcript levels in candidate genes identified by the XCA
aFold reduction (mean of 2 or more data points) in lymphoblasts from the proband compared to normal controls on XCA.
(NC) Not confirmed due to low or variable levels of expression.
bUCSC Genome Browser.
Identification of a functional promoter variant in PLP2
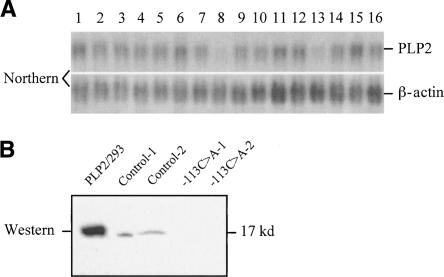
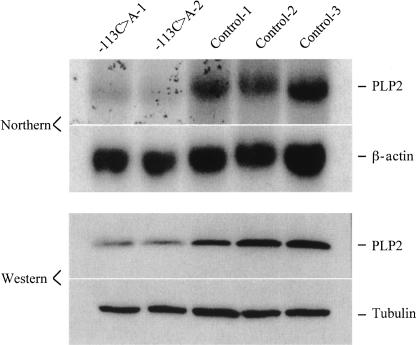
We identified an approximately fourfold reduction in expression of the proteolipid protein 2 (PLP2) gene at Xp11.23 in samples from two unrelated males with XLMR. We confirmed the XCA result in these patients using both Northern blot (Fig. 2A) and real-time RT-PCR (4.3-fold reduction). We generated a polyclonal antibody against the C terminus of human PLP2 protein and showed a corresponding reduction of PLP2 protein in cell lysate of lymphoblasts by immunoblot analysis (Fig. 2B).
Figure 2.
Confirmation of reduced PLP2 transcript and protein in lymphoblasts from unrelated males with XLMR. (A) Northern blot of total RNA from lymphoblast cell lines probed with a human PLP2 cDNA probe that detects an ∼1 kb mRNA. A cDNA probe for human beta-actin (Clontech) hybridized to the same blot provides a loading control. (Lane 1) reference RNA; (lane 2) normal male; (lanes 3–16) unrelated XLMR males. Note reduction of PLP2 mRNA in lanes 8 and 13. Both patients had a substantial reduction of PLP2 mRNA (greater than fourfold) by XCA analysis. (B) Immunoblot of PLP2 in protein lysate (20 μg/lane) from lymphoblasts of the two XLMR probands with reduced levels of PLP2 transcript and two control males. Protein lysate from HEK293 cells transfected with a PLP2–pcDNA3 expression construct was used as positive control (PLP2/293). The 17-kD PLP2 protein is indicated.
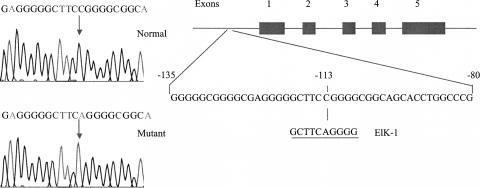
To determine the basis for the reduction of PLP2 expression, we sequenced all five exons, intron–exon boundaries, and 500 bp of the proximal promoter region. In both patients, we identified the same single nucleotide substitution (−113C>A) in the proximal promoter region (Fig. 3). This substitution alters the core, 3-bp consensus (TCC) binding sequence (YACTTCCGGT) for the transcription factor ELK1 that is predicted to abolish the binding completely (Shore and Sharrocks 1995).
Figure 3.
Identification of the −113C>A variant in the proximal promoter of PLP2 in a male proband with XLMR. The promoter variant (−113C>A) identified in the proband is predicted to alter the core-binding sequence of transcription factor ELK1. The predicted ELK1 binding site is repeated below.
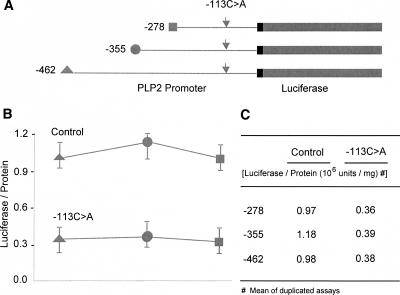
To determine if the PLP2-(−113C>A) variant was sufficient to explain the observed reduction in PLP2 expression, we assayed the transient expression of normal and mutant PLP2 promoter–luciferase constructs in HEK293 cells. We made a series of three PLP2 promoter constructs of different lengths in which −113C>A is the only sequence variant (Fig. 4A). After normalization for transfection efficiency by expression of a β-galactosidase vector cotransfected with our test constructs, we found that all three of the mutant PLP2 promoter–luciferase constructs showed an approximately three- to fourfold reduction of luciferase activity as compared to the corresponding control construct (Fig. 4B,C). We also measured PLP2 expression in fibroblasts from two additional male individuals who carry the −113C>A variant. Northern blots and immunoblots of RNA and protein, respectively, isolated from these cells showed a similar substantial reduction in PLP2 expression (Fig. 5).
Figure 4.
Luciferase reporter assay of the PLP2-(−113C>A) promoter variant. (A) PLP2-luciferase constructs. A nested set of proximal PLP2 promoter fragments of different lengths (−278, −355, −462 bps from the transcription start site) were cloned into a luciferase reporter vector (pGL3). The −113C>A variant was introduced by site-directed mutagenesis and confirmed by sequencing. (B) Expression of the PLP2-luciferase constructs transiently transfected into HEK293 cells. The mean of the luciferase activities from duplicate studies of each construct was calculated after normalization using the expression of a cotransfected beta-galactosidase expression vector. Note the reduced expression of luciferase activity in HEK293 cells transfected with the three constructs carrying the −113C>A SNP as compared to that with the corresponding control constructs.
Figure 5.
Northern and immunoblot studies of PLP2 expression in independent human fibroblast cell lines. Total RNA (10 μg) or protein lysate (20 μg) from fibroblast cell lines established from two males with the −113C>A SNP and three normal controls were studied by Northern and immunoblotting. β-actin and β-tubulin were used as loading controls, respectively.
To evaluate the frequency of the PLP2-(−113C>A) promoter variant in control and XLMR populations, we studied a cohort of 239 MR males with a pedigree that is consistent with X-link inheritance and 577 apparently normal males using allele-specific oligonucleotide hybridization followed by direct DNA sequencing of all positives. The ethnic backgrounds of the XLMR and control groups are listed in Table 2. Approximately one third of the control samples were from males with known college education and the remainder were from apparently normal males of unknown education background. We found a total of 14 chromosomes positive for the PLP2-(−113C>A) promoter variant in the males with probable XLMR (5.85%) and 9 chromosomes positive for the PLP2-(−113C>A) sequence variant in the controls (1.56%). In Caucasians, the allele frequency was 1.92% in the control cohort and 8.65% in the cohort of males with probable XLMR (χ2 = 10.21, P < 0.01). The overall difference in the PLP2-(−113C>A) allele frequency between the control and the XLMR cohorts was statistically significant (χ2 = 11.07, P < 0.001).
Table 2.
Allele frequency of PLP2-(−113C>A) promoter variant in males with probable XLMR and normal controls
aApparent normal males without frank mental retardation.
bAfrican Americans, Hispanics, Asians, Middle Easterns.
We performed segregation analysis in seven of the 14 proband families in which additional DNA samples were available, and no genetic causes have been identified (Supplemental Fig. 1). We confirmed cosegregation of the PLP2-(−113C>A) allele with the MR phenotype in six families (A–F). In the seventh family (G), we confirmed the PLP2-(−113C>A) variant in the proband and his mother who is a heterozygote for this allele. The proband has mild mental retardation, microcephaly, cerebral palsy, and spastic quadriparesis. His maternal half brother, who does not carry the PLP2-(−113C>A) variant, presents with only mild mental retardation.
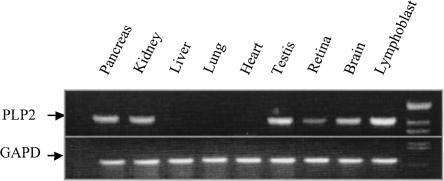
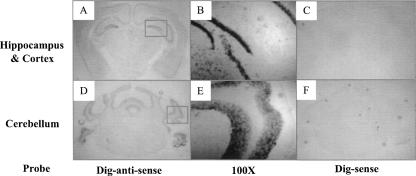
PLP2 cDNAs were cloned from a cDNA library made from a colon cancer cell line, and subsequent study suggested it functions as an ER channel (Oliva et al. 1993). As an initial step to determine if reduced PLP2 expression caused by the −113C>A variant could have an effect on the CNS, we determined the expression pattern of PLP2 using a human multitissue RT-PCR assay. PLP2 mRNA was detectable in multiple tissues, including brain, retina, testis, kidney, and pancreas (Fig. 6). Additionally, using in situ hybridization on the coronal sections of adult mouse brain using Digoxin-labeled antisense PLP2 probes, we detected low level expression of PLP2 transcript throughout the cortex with relatively abundant signal in the pyramidal cell layer of the hippocampus CA1-CA3 regions, the granular cell layer of the dentate gyrus, and granular cell layer of cerebellum (Fig. 7).
Figure 6.
Tissue expression profile of PLP2 by multi-tissue RT-PCR. Total cellular RNA (0.5 μg) from various human tissues (Clontech) was RT-PCR amplified using a One-step RT-PCR kit (Qiagen) and the products resolved on 2% agarose gel. GAPD was used as internal control for sample normalization. The products of the PLP2 and GAPD amplicons are indicated by arrows and were confirmed by sequencing.
Figure 7.
In situ hybridization study of PLP2 expression in adult mouse brain. DIG-labeled sense and antisense probes for murine PLP2 were generated using a DIG-RNA-labeling kit (Roche). In situ hybridization was performed on fresh frozen coronal sections (10 μm) of adult mouse brain. Note the positive hybridization signals in the hippocampus (A,B) and cerebellum (D,E) with the antisense but not the sense probes (C,F). (B,E) Enlarged images of the indicated areas in B and E, respectively.
Discussion
We developed and tested a strategy using a custom-built XCA to identify X-linked genes that exhibit significant alternations (≥1.5-fold) in steady-state mRNA levels. Control experiments showed that our array detected significant alternations in transcript levels reflecting an X-linked gene deletion in a hemizygous male and extra copies of active genes in a 49,XXXXY sample. Previously, a human XCA was used successfully to study X-linked genes that escape X chromosome inactivation (Sudbrak et al. 2001). Our rationale for using this array to screen for candidate genes for XLMR is based on the fact that a fraction of the mutations at any given locus result in a dramatic change in the abundance of the transcript. Genetic mechanisms that are known to result in changes in the steady-state transcript levels include promoter mutations, gene duplications, deletions, abnormal RNA splicing, frame-shift, and nonsense mutations that are associated with nonsense-mediated RNA decay (NMD). Previous studies have shown that ∼30% of mutations at any given gene loci result in a reduction of steady-state mRNA due to an NMD-related mechanism alone (Mendell and Dietz 2001). We focused our initial study on genes with reduced rather than increased transcript levels since the molecular defects that reduce steady-state transcript levels are easily recognized.
Utilizing our XCA approach to identify candidate genes for primary genetic defects on the X chromosome has several advantages. It allows rapid, quantitative study of multiple samples for the expression profile of all X-linked genes, and it is not limited by the requirement of multiple affected families or large pedigrees with multiple affected individuals. These advantages are particularly relevant to the identification of the genetic defects in rare and heterogeneous genetic disorders, such as XLMR. Our XCA approach is limited by the requirement for the relative abundance of transcript levels in accessible tissues (lymphoblasts, fibroblasts) and the inability to detect mutations that do not alter transcript levels. In addition, nonspecific variations or secondary changes in transcript abundance and artifacts introduced in cell culture or microarray may all contribute to the noises in the initial data. Confirming these changes in independent samples from the proband, identifying responsible genomic variants, and segregation analysis of these variants in the proband families should help to verify the XCA findings.
PLP2 is one of the 12 genes with confirmed reduction in transcript levels in one or more unrelated males with probable XLMR. We found a promoter variant (−113C>A) that was 3.75-fold more common in a cohort of males with probable XLMR as compared to normal controls. The fact that we also found this allele in “normal” males suggests this variant is not sufficient to cause frank mental retardation by itself but may contribute increased risk to impaired intellectual functioning and/or modification of clinical phenotype of the patients. While our results strongly suggest that the PLP2-(−113C>A) variant is sufficient to cause the observed reduction in PLP2 transcript levels, it remains possible that other SNPs in linkage disequilibrium with this variant may also affect PLP2 expression.
The PLP2-(−113C>A) variant disrupts the core-binding site for transcription factor ELK1 and is likely responsible for reduced PLP2 expression at mRNA and protein levels. ELK1 has a restricted, 9-bp binding site (YACTTCCGGT) (Shore and Sharrocks 1995) with three base pairs forming an invariant TCC core necessary for the binding of all members of the ETS family. ELK1 is expressed abundantly in neurons in various regions of the brain (Sgambato et al. 1998) and is one of the key targets of MAPK1/3 in the MAPK pathway (Hipskind et al. 1994; Yang et al. 1998). MAPK proteins are known to be involved in regulation of neuronal proliferation and differentiation and of hippocampal long-term potentiation (English and Sweatt 1996; Martin et al. 1997). Recently, ELK1-deficient mice have been characterized and showed mild impairment in Fos activation in amygdala, hippocampus, and cortex (Cesari et al. 2004). Additional studies will be needed to understand the roles of PLP2, a potential downstream target of ELK1 in the MAPK pathway, in the development of human cognitive function.
Methods
Patient samples
The majority of the samples (n = 209, 88%) in our XLMR cohort (n = 239) were recruited at the Greenwood Genetic Center, South Carolina, and the Johns Hopkins Hospital, Baltimore, Maryland, under human subject research protocols that were approved by the relevant Institutional Review Board. Each patient was evaluated by a clinical geneticist and underwent laboratory testing to rule out detectable chromosomal anomaly, fragile X syndrome, and known inborn errors of metabolism. After an informed consent, 5–10 mL of blood were collected from each patient to establish lymphoblast cell lines by EB transformation. Genetic evidence for X-linkage in these families was established based on the following criteria: (1) responsible locus mapped by linkage analysis or other methods to a region on the X, (2) affected males in two or more generations in a pedigree consistent with X-linked inheritance (no male-to-male transmission, females not affected or only mildly affected), (3) two or more affected males in the same generation in a pedigree consistent with X-linked inheritance, (4) evidence of skewed pattern of X-inactivation (≥80:20) in leukocytes of heterozygous females, and (5) clinical diagnosis of XLMR syndromes for which the causative genes are unknown. Among the 239 samples in our XLMR cohort, 78% of the proband families have two or more affected males and met either criteria 1, 2, or 3 while 22% of the proband families have single affected males and met either criteria 4 or 5. A fraction of the samples (n = 30, 12%) with established X-linked inheritance were obtained from the human genetics cell repositories at ECACC (Salisbury, UK) and Coriell Cell Repositories (CCR, Camden, NJ).
RNA preparation
Lymphoblasts were cultured in RPMI1640 with 15% fetal calf serum in 5% CO2 at 37°C and harvested during log phase growth. Fibroblasts were cultured in DMEM with 10% fetal calf serum in 5% CO2 at 37°C and harvested when 70% confluent. Total RNA was prepared using Trizol (Invitrogen) and followed by RNeasy Midi-RNA preparation kit (Qiagen) (http://oncwebl.onc.jhmi.edu/microarray).
Microarray fabrication and hybridization
The cDNA inserts from EST clones were amplified by PCR using universal M13 forward and M13 reverse primers, purified using Montage PCR96 purification kit (Millipore), and printed onto polylysine-coated glass slides using OmniGrid Microarray Printer (GeneMachine) at the Oncology Microarray Core of Johns Hopkins University (http://oncweb1.onc.jhmi.edu/microarray). Following a standard protocol, 10–20 μg of total RNA were labeled with either Cy5 (reference RNA) or Cy3-dUTP (sample RNA) using PowerScrip Fluorecent-labeling kit (Clontech) and hybridized to the XCA (http://oncweb1.onc.jhmi.edu/microarray). The Array image was obtained using an Axon GenePix 4000A scanner (Axon Instruments) and processed using DeArray-IPLab developed at NHGRI (Chen et al. 1997).
Data analysis
We computed the signal intensity ratios, (R/G or Cy5/Cy3), and log transformed them on the base 2. We used the print-tip LOESS method to make within microarray normalization on M = log2(R/G) by assessing the relationship of M = log2(R/G) vs. A = 1/2 log2(R/G) in which A is the average of the log intensities, i.e., A = 1/2 [log2(R) + log2(G)]. To achieve expression value consistency between microarrays, we normalized microarray data with limma using the “scale” method, which ensures the same medium-absolute-deviation (MAD) of the log ratios across microarrays.
To assess the variability of the microarray data, we analyzed the CVs of the normalized ratios across the six biological microarray replicates of the normal control samples in a gene-wise manner. For the microarray data, 87.9% of features have at least three non-flagged data points across the six arrays and were included in the computation. The variability of the normalized ratios was examined in detail by plotting their percent CVs against the inter-microarray means of the averaged intensities (on the scale of base 2 logarithm) and the inter-microarray means of normalized ratios (on the scale of base 2 logarithm), respectively.
We identified differentially expressed genes by comparing individual patients with the six normal controls by linear model analysis (Smyth 2004) using limma, in which an empirical Bayes method is used to moderate the standard errors of the estimated log-fold changes of gene expression. This approach results in more stable inference and improved power for experiments with small numbers of microarrays (Smyth 2004). The moderated t-statistic P-values were adjusted for multiple testing by Benjamini and Hochberg’s method to control false discovery rate (FDR). Differentially expressed genes were selected based on the FDRs. The Bioconductor package, limma, is available at http://www.bioconductor.org under R environment.
Northern blot
Ten micrograms of total RNA from individual lymphoblast cell lines were used for Northern blot analysis following a standard protocol (Sambrook et al. 1989).
Mutation analysis
Exons, exon–intron boundaries, and ∼500 bp of the proximal promoter of the human PLP2 gene were PCR-amplified and sequenced using an ABI 3100 sequencer. Allele-specific oligonucleotide hybridization (ASO) (Bender et al. 2005) was used to determine allele frequency in sample populations. PCR primers and oligonucleotide probes were listed in Table 3 of the Supplemental material.
Luciferase assay
Human PLP2 promoter fragments 278 bp, 355 bp, and 462 bp in length were subcloned into a luciferase reporter gene construct (pGL3, Promega). The −113C>A variant was introduced using a Quick-change Site-Directed Mutagenesis Kit (Stratagene) following the manufacturer’s instructions. HEK293 cells cultured in DMEM with 10% fetal calf serum in 5% CO2 at 37°C were harvested 48 h after transfection using Fugene 6 (Roche). Luciferase activity (units/mg protein) was determined with a luciferase assay kit (Promega) using a luminometer (Monolight 2010) according to the manufacturer’s instructions. A β-galactosidase control vector (Promega) was cotransfected for normalization of transfection efficiency.
In situ hybridization
A 600-bp murine PLP2 cDNA was cloned into pBluescript (Invitrogen). DIG-labeled sense and antisense probes were generated using a DIG-RNA-labeling kit (Roche). In situ hybridization was performed on fresh frozen coronal sections (10 μm) of adult mouse brain following a standard protocol (Roche).
Immunoblot
HEK293 cells were cultured in DMEM with 10% of FBS in 5% CO2 at 37°C. The cells were harvested using trypsin and lysed in 1% triton in phosphate buffered saline (PBS) with the cocktail protease inhibitor (Roche). Total protein concentration was measured using a BCA kit (Pierce). Twenty micrograms of total protein were loaded onto 12% SDS-PAGE gel in 2× Laemmli buffer containing 2-mercaptoethanol after denaturing for 8 min at 80°C. Proteins were transferred to Hybond-P membranes (Amersham), blocked with 5% nonfat milk in TBST (Tris-buffered saline with 0.1% triton), then incubated with rabbit anti-human PLP2 (raised against peptide TPVRQPRHTAAPTDPADGPV-COOH from the C terminus of PLP2) followed by horseradish peroxidase conjugated goat anti-rabbit immunoglobin G (Bio-Rad) and a chemiluminescence substrate (Pierce). The blotted membranes were then exposed to X-ray films (Kodak).
Acknowledgments
We thank Jeff Trent and Yidong Chen for help in the development of our array, Hai Xu of the Microarray Core at the Johns Hopkins Oncology Center for assistance with microarray printing, and Sandy Muscelli for assistance with manuscript preparation. This work was supported in part by K23HD044789 from NICHD (T.W.), Basil O’Connor Award from the March of Dimes Foundation (T.W.), Passano Physician Scientist Award (T.W.), Foundation Fighting Blindness HHMI (D.V.), HD26202 from NICHD (C.S.), MRRC Grant HD24061 from NICHD. D.V. is an Investigator with the Howard Hughes Medical Institute.
Footnotes
[Supplemental material is available online at www.genome.org. The microarray expression data from this study have been submitted to GEO under accession no. GPL4997.]
Article published online before print. Article and publication date are at http://www.genome.org/cgi/doi/10.1101/gr.5336307
References
- Bender H., Almashanu S., Steel G., Hu C., Lin W., Willis A., Pulver A., Valle D., Almashanu S., Steel G., Hu C., Lin W., Willis A., Pulver A., Valle D., Steel G., Hu C., Lin W., Willis A., Pulver A., Valle D., Hu C., Lin W., Willis A., Pulver A., Valle D., Lin W., Willis A., Pulver A., Valle D., Willis A., Pulver A., Valle D., Pulver A., Valle D., Valle D. Functional consequences of PRODH missense mutations. Am. J. Hum. Genet. 2005;76:409–420. doi: 10.1086/428142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billuart P., Bienvenu T., Ronce N., des Portes V., Vinet M., Zemni R., Roest Crollius H., Carrie A., Fauchereau F., Cherry M., Bienvenu T., Ronce N., des Portes V., Vinet M., Zemni R., Roest Crollius H., Carrie A., Fauchereau F., Cherry M., Ronce N., des Portes V., Vinet M., Zemni R., Roest Crollius H., Carrie A., Fauchereau F., Cherry M., des Portes V., Vinet M., Zemni R., Roest Crollius H., Carrie A., Fauchereau F., Cherry M., Vinet M., Zemni R., Roest Crollius H., Carrie A., Fauchereau F., Cherry M., Zemni R., Roest Crollius H., Carrie A., Fauchereau F., Cherry M., Roest Crollius H., Carrie A., Fauchereau F., Cherry M., Carrie A., Fauchereau F., Cherry M., Fauchereau F., Cherry M., Cherry M., et al. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature. 1998;392:923–926. doi: 10.1038/31940. [DOI] [PubMed] [Google Scholar]
- Bumeister R., Rosse C., Anselmo A., Camonis J., White M.A., Rosse C., Anselmo A., Camonis J., White M.A., Anselmo A., Camonis J., White M.A., Camonis J., White M.A., White M.A. CNK2 couples NGF signal propagation to multiple regulatory cascades driving cell differentiation. Curr. Biol. 2004;14:439–445. doi: 10.1016/j.cub.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Carrie A., Jun L., Bienvenu T., Vinet M., McDonell N., Couvert P., Zemni R., Cardona A., Van Buggenhout G., Frints S., Jun L., Bienvenu T., Vinet M., McDonell N., Couvert P., Zemni R., Cardona A., Van Buggenhout G., Frints S., Bienvenu T., Vinet M., McDonell N., Couvert P., Zemni R., Cardona A., Van Buggenhout G., Frints S., Vinet M., McDonell N., Couvert P., Zemni R., Cardona A., Van Buggenhout G., Frints S., McDonell N., Couvert P., Zemni R., Cardona A., Van Buggenhout G., Frints S., Couvert P., Zemni R., Cardona A., Van Buggenhout G., Frints S., Zemni R., Cardona A., Van Buggenhout G., Frints S., Cardona A., Van Buggenhout G., Frints S., Van Buggenhout G., Frints S., Frints S., et al. A new member of the IL-1 receptor family highly expressed in hippocampus and involved in X-linked mental retardation. Nat. Genet. 1999;23:25–31. doi: 10.1038/12623. [DOI] [PubMed] [Google Scholar]
- Cesari F., Brecht S., Vintersten K., Vuong L., Hofmann M., Klingel K., Schnorr J., Arsenian S., Schild H., Herdegen T., Brecht S., Vintersten K., Vuong L., Hofmann M., Klingel K., Schnorr J., Arsenian S., Schild H., Herdegen T., Vintersten K., Vuong L., Hofmann M., Klingel K., Schnorr J., Arsenian S., Schild H., Herdegen T., Vuong L., Hofmann M., Klingel K., Schnorr J., Arsenian S., Schild H., Herdegen T., Hofmann M., Klingel K., Schnorr J., Arsenian S., Schild H., Herdegen T., Klingel K., Schnorr J., Arsenian S., Schild H., Herdegen T., Schnorr J., Arsenian S., Schild H., Herdegen T., Arsenian S., Schild H., Herdegen T., Schild H., Herdegen T., Herdegen T., et al. Mice deficient for the Ets transcription factor Elk-1 show normal immune responses and mildly impaired neuronal gene activation. Mol. Cell. Biol. 2004;24:294–305. doi: 10.1128/MCB.24.1.294-305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Mandel J., Mandel J. Monogenic causes of X-linked mental retardation. Nat. Rev. Genet. 2001;2:669–680. doi: 10.1038/35088558. [DOI] [PubMed] [Google Scholar]
- Chen Y., Dougherty E., Bittner M., Dougherty E., Bittner M., Bittner M. Ratio-based decisions and the quantitative analysis of cDNA microarray images. J. Biomed.Opt. 1997;2:364–374. doi: 10.1117/12.281504. [DOI] [PubMed] [Google Scholar]
- DeRisi J., Iyer V., Brown P., Iyer V., Brown P., Brown P. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- Duggan D., Bittner M., Chen Y., Meltzer P., Trent J., Bittner M., Chen Y., Meltzer P., Trent J., Chen Y., Meltzer P., Trent J., Meltzer P., Trent J., Trent J. Expression profiling using cDNA microarrays. Nat. Genet. 1999;21:10–14. doi: 10.1038/4434. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- English J., Sweatt J., Sweatt J. Activation of p42 mitogen-activated protein kinase in hippocampal long-term potentiation. J. Biol. Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- Fu Y., Kuhl D., Pizzuti A., Pieretti M., Sutcliffe J., Richards S., Verkerk A., Holden J., Fenwick R.J., Warren S., Kuhl D., Pizzuti A., Pieretti M., Sutcliffe J., Richards S., Verkerk A., Holden J., Fenwick R.J., Warren S., Pizzuti A., Pieretti M., Sutcliffe J., Richards S., Verkerk A., Holden J., Fenwick R.J., Warren S., Pieretti M., Sutcliffe J., Richards S., Verkerk A., Holden J., Fenwick R.J., Warren S., Sutcliffe J., Richards S., Verkerk A., Holden J., Fenwick R.J., Warren S., Richards S., Verkerk A., Holden J., Fenwick R.J., Warren S., Verkerk A., Holden J., Fenwick R.J., Warren S., Holden J., Fenwick R.J., Warren S., Fenwick R.J., Warren S., Warren S., et al. Variation of the CGG repeat at the fragile X site results in genetic instability: Resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gecz J., Barnett S., Liu J., Hollway G., Donnelly A., Eyre H., Eshkevari H.S., Baltazar R., Grunn A., Nagaraja R., Barnett S., Liu J., Hollway G., Donnelly A., Eyre H., Eshkevari H.S., Baltazar R., Grunn A., Nagaraja R., Liu J., Hollway G., Donnelly A., Eyre H., Eshkevari H.S., Baltazar R., Grunn A., Nagaraja R., Hollway G., Donnelly A., Eyre H., Eshkevari H.S., Baltazar R., Grunn A., Nagaraja R., Donnelly A., Eyre H., Eshkevari H.S., Baltazar R., Grunn A., Nagaraja R., Eyre H., Eshkevari H.S., Baltazar R., Grunn A., Nagaraja R., Eshkevari H.S., Baltazar R., Grunn A., Nagaraja R., Baltazar R., Grunn A., Nagaraja R., Grunn A., Nagaraja R., Nagaraja R., et al. Characterization of the human glutamate receptor subunit 3 gene (GRIA3), a candidate for bipolar disorder and nonspecific X-linked mental retardation. Genomics. 1999;62:356–368. doi: 10.1006/geno.1999.6032. [DOI] [PubMed] [Google Scholar]
- Gibbs R., Caskey C., Caskey C. Identification and localization of mutations at the Lesch-Nyhan locus by ribonuclease A cleavage. Science. 1987;236:303–305. doi: 10.1126/science.3563511. [DOI] [PubMed] [Google Scholar]
- Gu Y., Shen Y., Gibbs R., Nelson D., Shen Y., Gibbs R., Nelson D., Gibbs R., Nelson D., Nelson D. Identification of FMR2, a novel gene associated with the FRAXE CCG repeat and CpG island. Nat. Genet. 1996;13:109–113. doi: 10.1038/ng0596-109. [DOI] [PubMed] [Google Scholar]
- Hane B., Schroer R., Arena J., Lubs H., Schwartz C., Stevenson R., Schroer R., Arena J., Lubs H., Schwartz C., Stevenson R., Arena J., Lubs H., Schwartz C., Stevenson R., Lubs H., Schwartz C., Stevenson R., Schwartz C., Stevenson R., Stevenson R. Nonsyndromic X-linked mental retardation: Review and mapping of MRX29 to Xp21. Clin. Genet. 1996;50:176–183. doi: 10.1111/j.1399-0004.1996.tb02622.x. [DOI] [PubMed] [Google Scholar]
- Herbst D., Miller J., Miller J. Nonspecific X-linked mental retardation II: The frequency in British Columbia. Am. J. Med. Genet. 1980;7:461–469. doi: 10.1002/ajmg.1320070407. [DOI] [PubMed] [Google Scholar]
- Hernandez-Martin A., Gonzalez-Sarmiento R., De Unamuno P., Gonzalez-Sarmiento R., De Unamuno P., De Unamuno P. X-linked ichthyosis: An update. Br. J. Dermatol. 1999;141:617–627. doi: 10.1046/j.1365-2133.1999.03098.x. [DOI] [PubMed] [Google Scholar]
- Hipskind R., Baccarini M., Nordheim A., Baccarini M., Nordheim A., Nordheim A. Transient activation of RAF-1, MEK, and ERK2 coincides kinetically with ternary complex factor phosphorylation and immediate-early gene promoter activity in vivo. Mol. Cell. Biol. 1994;14:6219–6231. doi: 10.1128/mcb.14.9.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosawa M., Nagase T., Ishikawa K., Kikuno R., Nomura N., Ohara O., Nagase T., Ishikawa K., Kikuno R., Nomura N., Ohara O., Ishikawa K., Kikuno R., Nomura N., Ohara O., Kikuno R., Nomura N., Ohara O., Nomura N., Ohara O., Ohara O. Characterization of cDNA clones selected by the GeneMark analysis from size-fractionated cDNA libraries from human brain. DNA Res. 1999;6:329–336. doi: 10.1093/dnares/6.5.329. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Ishida O., Hinoi T., Kishida S., Kikuchi A., Ishida O., Hinoi T., Kishida S., Kikuchi A., Hinoi T., Kishida S., Kikuchi A., Kishida S., Kikuchi A., Kikuchi A. Identification and characterization of a novel protein interacting with Ral-binding protein 1, a putative effector protein of Ral. J. Biol. Chem. 1998;273:814–821. doi: 10.1074/jbc.273.2.814. [DOI] [PubMed] [Google Scholar]
- Inoue A., Takahashi K.P., Kimura M., Watanabe T., Morisawa S., Takahashi K.P., Kimura M., Watanabe T., Morisawa S., Kimura M., Watanabe T., Morisawa S., Watanabe T., Morisawa S., Morisawa S. Molecular cloning of a RNA binding protein, S1-1. Nucleic Acids Res. 1996;24:2990–2997. doi: 10.1093/nar/24.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V., Eisen M., Ross D., Schuler G., Moore T., Lee J., Trent J., Staudt L., Hudson J.J., Boguski M., Eisen M., Ross D., Schuler G., Moore T., Lee J., Trent J., Staudt L., Hudson J.J., Boguski M., Ross D., Schuler G., Moore T., Lee J., Trent J., Staudt L., Hudson J.J., Boguski M., Schuler G., Moore T., Lee J., Trent J., Staudt L., Hudson J.J., Boguski M., Moore T., Lee J., Trent J., Staudt L., Hudson J.J., Boguski M., Lee J., Trent J., Staudt L., Hudson J.J., Boguski M., Trent J., Staudt L., Hudson J.J., Boguski M., Staudt L., Hudson J.J., Boguski M., Hudson J.J., Boguski M., Boguski M., et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- Kalscheuer V., Freude K., Musante L., Jensen L., Yntema H., Gecz J., Sefiani A., Hoffmann K., Moser B., Haas S., Freude K., Musante L., Jensen L., Yntema H., Gecz J., Sefiani A., Hoffmann K., Moser B., Haas S., Musante L., Jensen L., Yntema H., Gecz J., Sefiani A., Hoffmann K., Moser B., Haas S., Jensen L., Yntema H., Gecz J., Sefiani A., Hoffmann K., Moser B., Haas S., Yntema H., Gecz J., Sefiani A., Hoffmann K., Moser B., Haas S., Gecz J., Sefiani A., Hoffmann K., Moser B., Haas S., Sefiani A., Hoffmann K., Moser B., Haas S., Hoffmann K., Moser B., Haas S., Moser B., Haas S., Haas S., et al. Mutations in the polyglutamine binding protein 1 gene cause X-linked mental retardation. Nat. Genet. 2003;35:313–315. doi: 10.1038/ng1264. [DOI] [PubMed] [Google Scholar]
- Lin C., Park T., Chen Z.P., Leavitt J., Park T., Chen Z.P., Leavitt J., Chen Z.P., Leavitt J., Leavitt J. Human plastin genes: Comparative gene structure, chromosome location, and differential expression in normal and neoplastic cells. J. Biol. Chem. 1993;268:2781–2792. [PubMed] [Google Scholar]
- Lugtenberg D., Yntema H., Banning M., Oudakker A., Firth H., Willatt L., Raynaud M., Kleefstra T., Fryns J., Ropers H., Yntema H., Banning M., Oudakker A., Firth H., Willatt L., Raynaud M., Kleefstra T., Fryns J., Ropers H., Banning M., Oudakker A., Firth H., Willatt L., Raynaud M., Kleefstra T., Fryns J., Ropers H., Oudakker A., Firth H., Willatt L., Raynaud M., Kleefstra T., Fryns J., Ropers H., Firth H., Willatt L., Raynaud M., Kleefstra T., Fryns J., Ropers H., Willatt L., Raynaud M., Kleefstra T., Fryns J., Ropers H., Raynaud M., Kleefstra T., Fryns J., Ropers H., Kleefstra T., Fryns J., Ropers H., Fryns J., Ropers H., Ropers H., et al. ZFF674: A new kruppel-associated box-containing zinc-finger gene involved in nonsyndromic X-linked mental retardation. Am. J. Hum. Genet. 2006;78:256–278. doi: 10.1086/500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A., Hellier K., Hammans S., May A., Hellier K., Hammans S., May A., Hammans S., May A., May A. X-linked cerebellar ataxia and sideroblastic anaemia associated with a missense mutation in the ABC7 gene predicting V411L. Br. J. Haematol. 2001;115:910–917. doi: 10.1046/j.1365-2141.2001.03015.x. [DOI] [PubMed] [Google Scholar]
- Martin K., Michael D., Rose J., Barad M., Casadio A., Zhu H., Kandel E., Michael D., Rose J., Barad M., Casadio A., Zhu H., Kandel E., Rose J., Barad M., Casadio A., Zhu H., Kandel E., Barad M., Casadio A., Zhu H., Kandel E., Casadio A., Zhu H., Kandel E., Zhu H., Kandel E., Kandel E. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- Mendell J., Dietz H., Dietz H. When the message goes awry: Disease-producing mutations that influence mRNA content and performance. Cell. 2001;107:411–414. doi: 10.1016/s0092-8674(01)00583-9. [DOI] [PubMed] [Google Scholar]
- Nagase T., Seki N., Ishikawa K., Tanaka A., Nomura N., Seki N., Ishikawa K., Tanaka A., Nomura N., Ishikawa K., Tanaka A., Nomura N., Tanaka A., Nomura N., Nomura N. Prediction of the coding sequences of unidentified human genes. V. The coding sequences of 40 new genes (KIAA0161–KIAA0200) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res. 1996;3:17–24. doi: 10.1093/dnares/3.1.17. [DOI] [PubMed] [Google Scholar]
- Oliva M., Wu T., Yang V., Wu T., Yang V., Yang V. Isolation and characterization of a differentiation-dependent gene in the human colonic cell line HT29-18. Arch. Biochem. Biophys. 1993;302:183–192. doi: 10.1006/abbi.1993.1197. [DOI] [PubMed] [Google Scholar]
- Ota T., Suzuki Y., Nishikawa T., Otsuki T., Sugiyama T., Irie R., Wakamatsu A., Hayashi K., Sato H., Nagai K., Suzuki Y., Nishikawa T., Otsuki T., Sugiyama T., Irie R., Wakamatsu A., Hayashi K., Sato H., Nagai K., Nishikawa T., Otsuki T., Sugiyama T., Irie R., Wakamatsu A., Hayashi K., Sato H., Nagai K., Otsuki T., Sugiyama T., Irie R., Wakamatsu A., Hayashi K., Sato H., Nagai K., Sugiyama T., Irie R., Wakamatsu A., Hayashi K., Sato H., Nagai K., Irie R., Wakamatsu A., Hayashi K., Sato H., Nagai K., Wakamatsu A., Hayashi K., Sato H., Nagai K., Hayashi K., Sato H., Nagai K., Sato H., Nagai K., Nagai K., et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat. Genet. 2004;36:40–45. doi: 10.1038/ng1285. [DOI] [PubMed] [Google Scholar]
- Read A., Mountford R., Forrest S., Kenwrick S., Davies K., Harris R., Mountford R., Forrest S., Kenwrick S., Davies K., Harris R., Forrest S., Kenwrick S., Davies K., Harris R., Kenwrick S., Davies K., Harris R., Davies K., Harris R., Harris R. Patterns of exon deletions in Duchenne and Becker muscular dystrophy. Hum. Genet. 1988;80:152–156. doi: 10.1007/BF00702859. [DOI] [PubMed] [Google Scholar]
- Ropers H., Hamel B., Hamel B. X-linked mental retardation. Nat. Rev. Genet. 2005;6:46–57. doi: 10.1038/nrg1501. [DOI] [PubMed] [Google Scholar]
- Ross M., Grafham D., Coffey A., Scherer S., McLay K., Muzny D., Platzer M., Howell G., Burrows C., Bird C., Grafham D., Coffey A., Scherer S., McLay K., Muzny D., Platzer M., Howell G., Burrows C., Bird C., Coffey A., Scherer S., McLay K., Muzny D., Platzer M., Howell G., Burrows C., Bird C., Scherer S., McLay K., Muzny D., Platzer M., Howell G., Burrows C., Bird C., McLay K., Muzny D., Platzer M., Howell G., Burrows C., Bird C., Muzny D., Platzer M., Howell G., Burrows C., Bird C., Platzer M., Howell G., Burrows C., Bird C., Howell G., Burrows C., Bird C., Burrows C., Bird C., Bird C., et al. The DNA sequence of the human X chromosome. Nature. 2005;434:325–337. doi: 10.1038/nature03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T., Fritsch E.F., Maniatis T., Maniatis T. Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Sgambato V., Vanhoutte P., Pages C., Rogard M., Hipskind R., Besson M., Caboche J., Vanhoutte P., Pages C., Rogard M., Hipskind R., Besson M., Caboche J., Pages C., Rogard M., Hipskind R., Besson M., Caboche J., Rogard M., Hipskind R., Besson M., Caboche J., Hipskind R., Besson M., Caboche J., Besson M., Caboche J., Caboche J. In vivo expression and regulation of Elk-1, a target of the extracellular-regulated kinase signaling pathway, in the adult rat brain. J. Neurosci. 1998;18:214–226. doi: 10.1523/JNEUROSCI.18-01-00214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore P., Sharrocks A., Sharrocks A. The ETS-domain transcription factors Elk-1 and SAP-1 exhibit differential DNA binding specificities. Nucleic Acids Res. 1995;23:4698–4706. doi: 10.1093/nar/23.22.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth G. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Cho G., Bhat S., Lim Y., Zand D., Gao J., Roger R., Simensen R., Collins J., Golden J., Cho G., Bhat S., Lim Y., Zand D., Gao J., Roger R., Simensen R., Collins J., Golden J., Bhat S., Lim Y., Zand D., Gao J., Roger R., Simensen R., Collins J., Golden J., Lim Y., Zand D., Gao J., Roger R., Simensen R., Collins J., Golden J., Zand D., Gao J., Roger R., Simensen R., Collins J., Golden J., Gao J., Roger R., Simensen R., Collins J., Golden J., Roger R., Simensen R., Collins J., Golden J., Simensen R., Collins J., Golden J., Collins J., Golden J., Golden J., et al. SIZN1. 12th International Workshop on Fragile X and X-linked Mental Retardation. Williamsburg, VA: 2005. —A new gene implicated in non-syndromic X-linked mental retardation; p. 43. [Google Scholar]
- Stevenson R. Splitting and lumping in the nosology of XLMR. Am. J. Med. Genet. 2000;97:174–182. doi: 10.1002/1096-8628(200023)97:3<174::AID-AJMG1034>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Stevenson R., Schwartz C., Schroer R., Schwartz C., Schroer R., Schroer R. X-linked mental retardation. Oxford University Press; New York: 2000. [Google Scholar]
- Strausberg R.L., Feingold E.A., Grouse L.H., Derge J.G., Klausner R.D., Collins F.S., Wagner L., Shenmen C.M., Schuler G.D., Altschul S.F., Feingold E.A., Grouse L.H., Derge J.G., Klausner R.D., Collins F.S., Wagner L., Shenmen C.M., Schuler G.D., Altschul S.F., Grouse L.H., Derge J.G., Klausner R.D., Collins F.S., Wagner L., Shenmen C.M., Schuler G.D., Altschul S.F., Derge J.G., Klausner R.D., Collins F.S., Wagner L., Shenmen C.M., Schuler G.D., Altschul S.F., Klausner R.D., Collins F.S., Wagner L., Shenmen C.M., Schuler G.D., Altschul S.F., Collins F.S., Wagner L., Shenmen C.M., Schuler G.D., Altschul S.F., Wagner L., Shenmen C.M., Schuler G.D., Altschul S.F., Shenmen C.M., Schuler G.D., Altschul S.F., Schuler G.D., Altschul S.F., Altschul S.F., et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc. Natl. Acad. Sci. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbrak R., Wieczorek G., Nuber U., Mann W., Kirchner R., Erdogan F., Brown C., Wohrle D., Sterk P., Kalscheuer V., Wieczorek G., Nuber U., Mann W., Kirchner R., Erdogan F., Brown C., Wohrle D., Sterk P., Kalscheuer V., Nuber U., Mann W., Kirchner R., Erdogan F., Brown C., Wohrle D., Sterk P., Kalscheuer V., Mann W., Kirchner R., Erdogan F., Brown C., Wohrle D., Sterk P., Kalscheuer V., Kirchner R., Erdogan F., Brown C., Wohrle D., Sterk P., Kalscheuer V., Erdogan F., Brown C., Wohrle D., Sterk P., Kalscheuer V., Brown C., Wohrle D., Sterk P., Kalscheuer V., Wohrle D., Sterk P., Kalscheuer V., Sterk P., Kalscheuer V., Kalscheuer V., et al. X chromosome-specific cDNA arrays: Identification of genes that escape from X-inactivation and other applications. Hum. Mol. Genet. 2001;10:77–83. doi: 10.1093/hmg/10.1.77. [DOI] [PubMed] [Google Scholar]
- Tarpey P., Parnau J., Blow M., Woffendin H., Bignell G., Cox C., Cox J., Davies H., Edkins S., Holden S., Parnau J., Blow M., Woffendin H., Bignell G., Cox C., Cox J., Davies H., Edkins S., Holden S., Blow M., Woffendin H., Bignell G., Cox C., Cox J., Davies H., Edkins S., Holden S., Woffendin H., Bignell G., Cox C., Cox J., Davies H., Edkins S., Holden S., Bignell G., Cox C., Cox J., Davies H., Edkins S., Holden S., Cox C., Cox J., Davies H., Edkins S., Holden S., Cox J., Davies H., Edkins S., Holden S., Davies H., Edkins S., Holden S., Edkins S., Holden S., Holden S., et al. Mutations in the DLG3 gene cause nonsyndromic X-linked mental retardation. Am. J. Hum. Genet. 2004;75:318–324. doi: 10.1086/422703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey P., Gryer A., Gecz J., Goodship J., Partington M., Price S., Schwartz C., Stevenson R., Tolmie J., Turner G., Gryer A., Gecz J., Goodship J., Partington M., Price S., Schwartz C., Stevenson R., Tolmie J., Turner G., Gecz J., Goodship J., Partington M., Price S., Schwartz C., Stevenson R., Tolmie J., Turner G., Goodship J., Partington M., Price S., Schwartz C., Stevenson R., Tolmie J., Turner G., Partington M., Price S., Schwartz C., Stevenson R., Tolmie J., Turner G., Price S., Schwartz C., Stevenson R., Tolmie J., Turner G., Schwartz C., Stevenson R., Tolmie J., Turner G., Stevenson R., Tolmie J., Turner G., Tolmie J., Turner G., Turner G., et al. 12th International Workshop on Fragile X and X-linked Mental Retardation. Williamsburg, VA: 2005. Prevalence of mutations in X-linked mental retardation genes in familial mental retardation; p. 43. [Google Scholar]
- Turner G., Webb T., Wake S., Robinson H., Webb T., Wake S., Robinson H., Wake S., Robinson H., Robinson H. Prevalence of fragile X syndrome. Am. J. Med. Genet. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wang P.J., McCarrey J.R., Yang F., Page D.C., McCarrey J.R., Yang F., Page D.C., Yang F., Page D.C., Page D.C. An abundance of X-linked genes expressed in spermatogonia. Nat. Genet. 2001;27:422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- Wing L. Severely retarded children in a London area: Prevalence and provision of services. Psychol. Med. 1971;1:405–415. doi: 10.1017/s0033291700044792. [DOI] [PubMed] [Google Scholar]
- Wu Y., Jiang Y., Zhang L., Splaine M., Huganir R., Schwartz C., Stevenson R., Valle D., Wang T., Jiang Y., Zhang L., Splaine M., Huganir R., Schwartz C., Stevenson R., Valle D., Wang T., Zhang L., Splaine M., Huganir R., Schwartz C., Stevenson R., Valle D., Wang T., Splaine M., Huganir R., Schwartz C., Stevenson R., Valle D., Wang T., Huganir R., Schwartz C., Stevenson R., Valle D., Wang T., Schwartz C., Stevenson R., Valle D., Wang T., Stevenson R., Valle D., Wang T., Valle D., Wang T., Wang T. 12th International Workshop on Fragile X and X-linked Mental Retardation. Williamsburg, VA: 2005. Mutations in ionotropic AMPA receptor 3 (GluR3) in males with X-linked mental retardation; p. 84. (Abstract) [Google Scholar]
- Yang Y., Speed T., Speed T. Design and analysis of comparative microarray experiments. In: Speed T., editor. Statistical analysis of gene expression microarray data. Chapman & Hall/CRC; New York: 2003. pp. 35–91. [Google Scholar]
- Ye B., Liao D., Zhang X., Zhang P., Dong H., Huganir R.L., Liao D., Zhang X., Zhang P., Dong H., Huganir R.L., Zhang X., Zhang P., Dong H., Huganir R.L., Zhang P., Dong H., Huganir R.L., Dong H., Huganir R.L., Huganir R.L. GRASP-1: A neuronal RasGEF associated with the AMPA receptor/GRIP complex. Neuron. 2000;26:603–617. doi: 10.1016/s0896-6273(00)81198-8. [DOI] [PubMed] [Google Scholar]
- Zemni R., Bienvenu T., Vinet M., Sefiani A., Carrie A., Billuart P., McDonell N., Couvert P., Francis F., Chafey P., Bienvenu T., Vinet M., Sefiani A., Carrie A., Billuart P., McDonell N., Couvert P., Francis F., Chafey P., Vinet M., Sefiani A., Carrie A., Billuart P., McDonell N., Couvert P., Francis F., Chafey P., Sefiani A., Carrie A., Billuart P., McDonell N., Couvert P., Francis F., Chafey P., Carrie A., Billuart P., McDonell N., Couvert P., Francis F., Chafey P., Billuart P., McDonell N., Couvert P., Francis F., Chafey P., McDonell N., Couvert P., Francis F., Chafey P., Couvert P., Francis F., Chafey P., Francis F., Chafey P., Chafey P., et al. A new gene involved in X-linked mental retardation identified by analysis of an X;2 balanced translocation. Nat. Genet. 2000;24:167–170. doi: 10.1038/72829. [DOI] [PubMed] [Google Scholar]