Abstract
Gastric electrical stimulation (GES) has been suggested as a potential therapy for patients with obesity or gastric motility disorders. The aim of this study was to investigate the spinal mechanism of GES effects on gastric functions. Extracellular potentials of single spinal (T9–T10) neurons were recorded in pentobarbital anesthetized, paralyzed, ventilated male rats (n=19). Gastric distension (GD) was produced by air inflation of a balloon. One pair of platinum electrodes (1.0–1.5 cm apart) was sutured onto the serosal surface of the lesser curvature of the stomach. GES with four sets of parameters was applied for one minute: GES-A (6 mA, 0.3 ms, 40 Hz, 2s on, 3s off), GES-B (6 mA, 0.3 ms, 14 Hz, 0.1s on, 5s off), GES-C (6 mA, 3 ms, 40 Hz, 2s on, 3s off), GES-D (6 mA, 200 ms, 12 pulses/min). 62/158 (39%) spinal neurons responded to GD (20, 40, 60 mmHg, 20s. Most GD-responsive neurons (n=43) had excitatory responses; the remainder had inhibitory (n=12) or biphasic responses (n=7). GES-A, -B, -C and –D affected activity of 12/33 (36%), 4/31 (13%), 22/29 (76%) and 13/30 (43%) GD-responsive neurons, respectively. Bilateral cervical vagotomy did not significantly alter mean excitatory neuronal responses to GD (n=5) or GES (n=6). Resiniferatoxin (2.0 μg/kg, i.v.), an ultrapotent agonist of vanilloid receptor-1, abolished excitatory responses to GD and GES in 4/4 neurons recorded in vagotomized rats. The results suggested that GES mainly had an excitatory effect on T9–T10 spinal neurons with gastric inputs; neuronal responses to GES were strengthened with stimulation at an increased pulse width and/or number of pulses. The modulatory effect of GES involved thoracic spinal (sympathetic) afferent fibers containing vanilloid receptor-1.
Keywords: Gastric afferents, sympathetic afferents, vagal afferents, vanilloid receptor-1, spinal cord
Introduction
Basic and clinical investigations show that gastric electrical stimulation (GES) may be a suitable treatment for some gastric motility disorders and morbid obesity (Abell and Minocha 2002; D'Argent et al. 2002; Greenstein and Belachew 2002; Lin and Chen 2002; Liu et al. 2006). However, the neural and hormonal mechanisms underlying the effects of GES on gastric sensory and motor functions are little known. The stomach receives dual innervations by vagal and splanchnic (sympathetic) nerves in both humans and animals, which play an important role in the regulation of gastric function. Previous studies using atropine and vagotomy in dogs have indicated that vagal afferent and/or efferent pathways are involved in the regulation of GES on gastric motility (Chen et al. 2003; Grundfest-Bronaltowski et al. 1990; Liu et al. 2004; Ouyang et al. 2003). In rats, GES can activate vagal afferent fibers innervating the stomach (Peles et al. 2003) and modulate neuronal activity in the nucleus tractus solitarii, where vagal afferent fibers terminate (Qin et al. 2005). A few recent studies suggest that the effects of GES with different parameters on gastric motility also involve the sympathetic nerve system. For example, GES significantly inhibits postprandial antral contractions. Guanethidine, an adrenergic blocker, can prevent this effect of GES, suggesting involvement of sympathetic pathways (Zhu and Chen 2005). Intravenous injections of propranolol or phentolamine is reported to abolish long-pulse GES-induced tachygastria and antral hypomotility via the alpha- and beta-adrenergic sympathetic pathways (Ouyang et al. 2005). Thus, these studies provide evidence for a role of the gastric sympathetic efferent pathway in effects of GES on gastric motility. However, the effect of GES on spinal visceroreceptive processing for gastric primary afferent information has not been examined.
Many anatomic and neurochemical studies show that splanchnic (sympathetic) afferent fibers from the stomach mainly project to the caudal thoracic spinal cord (T8–T13) in rats and also some neurotransmitters and c-fos expressing neurons in the spinal cord are related to innocuous and/or noxious gastric manipulations (Green and Dockray 1988; Holzer et al. 2005; Neurhuber and Niedrle 1979; Ozaki and Gebhart 2001; Schicho et al. 2005; Sharkey et al. 1984; Traub et al 1996). However, to our knowledge, no study has been performed electrophysiologically to examine activity of thoracic spinal neurons receiving primary gastric afferent inputs, although we previously showed that gastric mechanical stimulation activated upper cervical spinal neurons in rats (Qin et al. 2003). The purposes of this study in rats were: 1) to characterize low thoracic (T9–T10) spinal neurons responding to gastric distension (GD); 2) to examine the effect of GES with different parameters on activity of these neurons; 3) to determine afferent pathways of gastric input to and GES effect on spinal neurons; 4) to investigate the dependence of GD responses and GES effects upon gastric afferent fibers expressing vanilloid receptor-1 (VR-1). Preliminary results of this study have been presented previously in an abstract (Qin et al. 2006).
Methods
Experiments were performed on 19 male Sprague-Dawley rats (Charles River Inc. 350–450 g) initially anesthetized with intraperitoneal injection of sodium pentobarbital (60 mg/kg). Experimental procedures followed the ethical guidelines of the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources Commission on Life Sciences and National Research Council (1996) and approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center. The right carotid artery and left jugular vein were cannulated for continuous blood pressure monitoring and for intravenous infusion of pentobarbital (10–15 mg/kg/hr) throughout experiments, respectively. During the experiment, the average blood pressure was kept between 80 to 120 mmHg. Rats also were unresponsive to noxious pinching of the feet or tip of the tail, and the pupils were constricted. The trachea was cannulated through a tracheotomy for artificial ventilation using a volume-control pump (55–60 strokes/min, 3.0–5.0 ml stroke volume). To provide and maintain muscle relaxation, pancuronium bromide (initial dose 0.4 mg/kg) was administered intravenously and animals were given supplemental doses (0.2 mg/kg) as needed during the experiment. Rectal temperature was kept between 36 and 38°C by a servo-controlled heating blanket and overhead infrared lamps
The procedure for performing gastric distension (GD) and gastric electrical stimulation (GES) was the same as in previous studies (Qin et al. 2003, 2005). Briefly, after midline laparotomy, gastric contents were removed through a small incision in the fundus wall. A latex balloon (3–4 cm in length) attached to polyethylene tubing (PE-240) with 3–5 small holes near the tip was inserted into the gastric cavity through the incision and fixed on the edge of the incision by a ligature. The air balloon had a greater volume than that of the stomach and provided no resistance to inflation when the stomach was distended. Intragastric pressure (60 mmHg, 20s) was used as a search stimulus and was monitored continuously via a pressure transducer and sphygmomanometer. In some neurons, graded GD (20, 40, 60 mmHg, 20s) was performed to determine stimulus-response relationships. For delivering GES, one pair of platinum electrodes with 5–8 circles of a spring (0.5 cm long, 1 cm apart, 32 GA, Fisher Scientific Inc.) was sutured onto the serosal surface of the lesser curvature of the stomach. Dental impression material was placed around the electrodes for isolation from other visceral organs and the abdominal wall. GES with four sets of parameters was applied for one minute: GES-A (pulse trains of standard parameters: 6 mA, 0.3 ms, 40 Hz, 2s-on and 3s-off), GES-B (pulse trains of decreased frequency: 6 mA, 0.3 ms, 14 Hz, 0.1s-on and 5s-off), GES-C (pulse trains of increased pulse width: 6 mA, 3 ms, 40 Hz, 2s-on and 3s-off), and GES-D (single long pulses: 6 mA, 200 ms, 12 pulses/min).
A laminectomy exposed the T9–T10 spinal segments for extracellular recording. Rats were mounted in a stereotaxic headholder and stabilized with clamps attached to L1–L2 and T5–T7 vertebral processes. Dura mater was carefully removed and the spinal cord was covered with warm agar (3–4% in saline) to improve recording stability. Carbon-filament glass microelectrodes were used for recording extracellular potentials of single T9–T10 spinal neurons within 0–1.2 mm from the dorsal surface and 0.5–1.5 mm lateral to midline in either the left or right side of the spinal cord. Extracellular potentials were fed into a window discriminator, displayed on an oscilloscope, and stored in a computer with Spike 3 data acquisition program (CED, Cambridge) for off-line analysis. After a spinal neuron responsive to GD and GES was studied, an electrolytic lesion (50 μA DC, 20 s) was made to mark the recording site. At the end of experiments, animals were euthanized with an intravenous euthanasia-5 solution or overdose of pentobarbital (200 mg/kg). The lower thoracic spinal cord was removed and placed in 10% buffered formalin solution. Frozen sections (55–60 μm) of spinal cord were viewed to find lesion sites where the neuronal recordings had been made. Locations were drawn on cross sections from the cytoarchitectonic scheme of Molander et al. (1984).
Somatic receptive fields of spinal neurons were examined for responses to innocuous brushing with a camel-hair brush, pressure with a blunt stick, and noxious pinching of skin with a blunt forceps. Neurons were classified as follows: low-threshold (LT) neurons responded to hair movement and/or pressure; high-threshold (HT) neurons responded only to noxious pinching of the somatic field; wide dynamic range (WDR) neurons responded to innocuous stimuli and also had greater responses to noxious pinch of somatic fields. Outlines and descriptions of receptive fields were recorded manually for all neurons examined.
To determine the influence of vagal afferent pathways on lower thoracic spinal neurons with gastric input, the vagi were prepared for transection. The left and right cervical vagus nerves were separated from the carotid artery and silk suture was looped around each nerve trunk. Bilateral vagus nerves were cut with scissors after gently pulling the tie around the nerve so that spinal neuronal responses to GD or GES could be compared before and after vagotomy. To characterize phenotype of afferent nerve fibers from stomach to spinal cord, resiniferatoxin (RTX), a potent analogue of capsaicin, was used to inactivate capsaicin-sensitive afferent fibers containing vanilloid receptor-1 (VR1). Systemic administration of RTX desensitizes and finally induces permanent degeneration of VR1-expressing primary sensory neurons in adult rats (Szallasi and Blumberg 1989; Pan et al. 2003). Therefore, RTX was used as a pharmacological tool to determine the role of VR1-containing nerve fibers in gastric afferent pathways. A stock solution of RTX (1 mg, FW 628.7, Sigma) was dissolved in 0.5 ml ethanol and 0.5 ml Tween 80. The bottle was wrapped in foil and stored in a −80ºC freezer. On the experimental day, 2 μl RTX was removed from the stock solution and diluted with 1 ml normal saline. The intravenous dose of RTX was 2 μg/kg. Gastric and somatic stimuli were examined for spinal neuronal responses 20 min after RTX administration.
Off-line analysis of the neural signals was done by using the Spike 3 data-acquisition system (CED, Cambridge). Activity was measured using rate histograms (1 s/bin). Spontaneous activity of neurons was determined by counting activity for 10 s before gastric stimulation to obtain impulses per second (imp/s). Neuronal responses (imp/s) during gastric stimuli were defined as increases or decreases (≥ 20%) in maximal activity compared to spontaneous activity. Latency of responses was measured from the onset of GD to the onset of a response. The duration of spinal neuronal response to a stimulus was measured from the onset of a response to the time point of recovery to control activity. If evoked activity by GD or GES in some neurons, for some reason, was not recovery completely for a long period of time, the duration of responses was measured from the onset of responses to the time point at which a new basal line or control activity occurred. Raw tracings of neuronal responses to GES were processed by a Spike 3 digital filter to eliminate GES artifacts. Data are presented as means ± S.E. Statistical comparisons were made using ANOVA followed by Tukey’s test and Chi’s square. Comparison of data were considered statistically significant if P < 0.05.
Results
Gastric distension (GD, ≥40 mmHg) changed activity of 62/158 (39%) spinal neurons that were located in T9 (n=30) and T10 (n=32) segments. Thirty-eight GD-responsive neurons were recorded from left side and 24 neurons from right side of the spinal cord. Electrolytic lesions of recording sites for 31 GD-responsive neurons in the T9–T10 spinal cord were verified histologically. Neurons excited by GD were primarily located in laminae I-III, V and VII, whereas the majority of spinal neurons inhibited by GD were found in laminae V and VII (Fig. 1A-C).
Fig. 1.
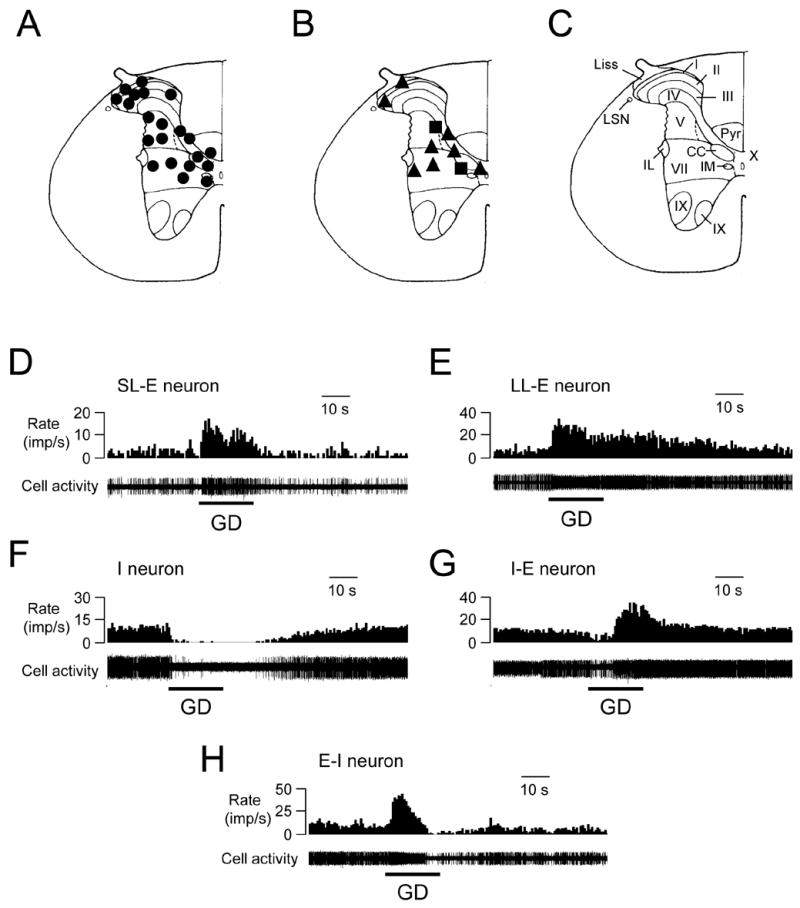
Recording sites and response patterns of lower thoracic (T9–T10) spinal neurons to gastric distension (GD, 60 mmHg). A-B: Locations of spinal neurons responding to GD. The black circles represent neurons excited by GD. The black triangles represent neurons inhibited by GD. The black squares represent neurons excited/inhibited by GD. C: Schematic drawing of the T10 spinal segment (Molander et al. 1984). I-X indicates laminae; Liss, Liss’s tract; LSN, lateral spinal nucleus; Pyr, pyramidal tract; IL, intermediolateral nucleus. IM, intermedial nucleus. CC, column of Clarke. D: Short-lasting excitatory (SL-E) response to GD. E: Long-lasting excitatory (LL-E) response to GD. F: Inhibitory (I) response to GD. G: Inhibitory-excitatory (I-E) response to GD. H: Excitatory-inhibitory (E-I) response to GD.
Response patterns to GD
Of the GD-responsive neurons, 43 (69%) neurons had excitatory (E) responses, 12 neurons had inhibitory (I) responses and 7 neurons had a biphasic pattern of responses (6 E-I, 1 I-E). Examples of these neuronal responses are shown in Fig. 1D-H. Quantitative analyses of spontaneous activity and responses of spinal neurons to noxious GD (60 mmHg, 20s) are shown in Table 1. A majority (81%, 35/43) of excitatory responses exhibited an adaptation-like patterns to GD, i.e. an initial greater increased activity followed by slowly a decreasing discharge rate during maintained GD. The reminder of the neurons exhibited sustained response pattern during GD. No neuron with an on-off response patterns (rapidly adapting type) to GD was found. Based on the intragastric pressure that produced a response, neurons excited or inhibited by GD were divided into the following two subgroups: low threshold (LT) neurons responded to intragastric pressure 20 mmHg; high threshold (HT) neurons responded to 40 mmHg pressure. Thus, 30/43 (70%) E neurons, 7/12 (58%) I neurons, all E-I (n=6) neurons and one I-E neuron were classified as LT neurons, whereas the remainder were HT neurons. Examples of these neurons are shown in Fig.2A-D. Responses of spinal neurons to graded GD (20, 40, 60 mmHg, 20 s) also were examined in neurons excited (n=6) or inhibited (n=4) by GD, which indicated that these neurons encode intensity of GD in a linear manner (Fig. 2E, F). In addition, a comparison between GD responses of spinal neurons recorded from left or right side of spinal cord was performed. The average excitatory response of left spinal neurons (22.1±2.7 imp/s, n=26) to GD (60 mmHg, 20s) was significant greater than the average response of right spinal neurons (12.9±2.0, n=17, P<0.01), although no statistical differences were found in other neuronal groups with different response patterns.
Table 1.
Response patterns and characteristics of T9–T10 spinal neurons to noxious gastric distension (GD, 60 mmHg, 20s).
| Responses to GD | n | Spontaneous activity (imp/s) | Latency (s) | E-Response (imp/s) | I-Responses (imp/s) | Duration (s) |
|---|---|---|---|---|---|---|
| Excitation (E) | 43 | 8.6±1.2 | 1.9±0.2 | 18.4±1.9 | / | 37.0±3.1 |
| Inhibition (I) | 12 | 13.2±2.0 | 2.1±0.4 | / | 9.4±2.8 | 33.1±8.6 |
| E-I | 6 | 9.6±2.6 | 4.2±2.8 | 11.9±2.7 | 8.4±2.0 | 53.3±11.6 |
| I-E | 1 | 12.1 | 4.7 | 28.1 | 5.3 | 61.4 |
Fig. 2.
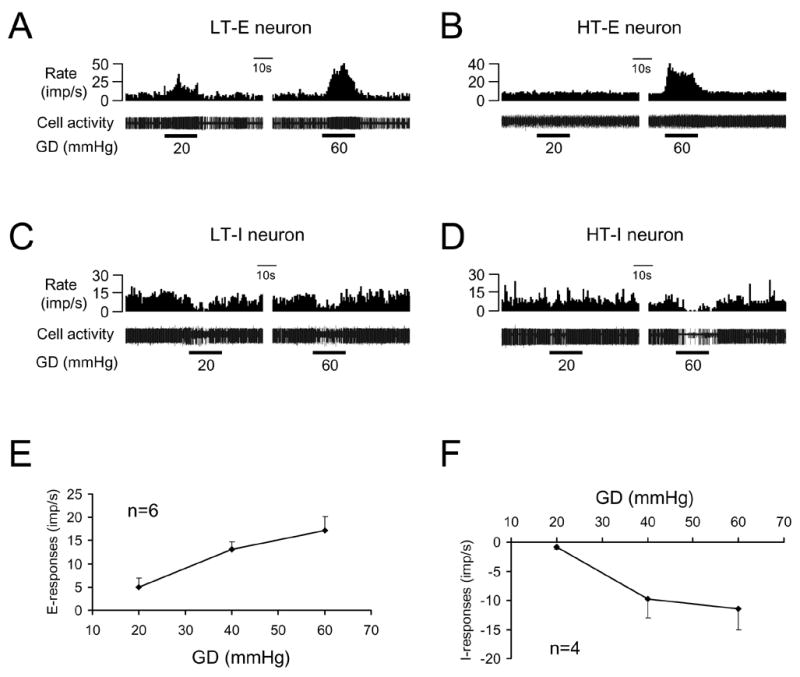
Thoracic spinal neuronal responses to graded gastric distension (GD). A: Low-threshold excitatory (LT-E) responses to GD. B: High-threshold excitatory (HT-E) responses to GD. C: Low-threshold inhibitory (LT-I) responses to GD. D: High-threshold (HT-I) responses to GD. E: Summary for excitatory responses to graded GD. F: Summary for inhibitory responses to graded GD.
Based on the recovery time of neuronal activity to the control level after termination of noxious GD (60 mmHg, 20 s), neurons excited by GD were further divided into the following two subgroups: 16 neurons with recovery time 10 s were classified as short-lasting excitatory (SL-E, Fig. 1A), and 27 neurons with recovery time >10 s were classified as long-lasting excitatory (LL-E, Fig. 1B). Quantitative analyses of spontaneous activity, excitatory responses to GD, duration, and latency of responses in SL-E and LL-E neurons are shown in Table 2.
Table 2.
Short- and long-lasting excitatory responses of thoracic (T9–T10) spinal neurons to gastric distension (60 mmHg, 20s).
| Groups | n | Spontaneous activity (imp/s) | Latency (s) | Responses (imp/s) | Duration (s) |
|---|---|---|---|---|---|
| Short-lasting | 16 | 11.0±2.0 | 1.6±0.3 | 17.8±3.2 | 23.7±0.8 |
| Long-lasting | 27 | 7.0±1.5 | 2.1±0.4 | 20.3±2.4 | 47.0±4.3* |
P<0.05 compared to duration of short-lasting responses.
GES modulation of neuronal activity
GES-A, -B, -C and –D changed activity of 12/33 (36%), 4/31 (13%), 22/29 (76%) and 13/30 (43%) spinal neurons responding to GD, respectively (Table 3). Spinal neuronal activity was affected more frequently with GES-C (an increased pulse width of 3 ms) than with other GES parameters (P<0.05). In contrast, GES-B (a reduced frequency of 14 Hz) was least effective in activating the spinal neurons with gastric input (P<0.05). GES primarily increased activity of most spinal neurons with gastric input, and a few neurons were inhibited by GES (Table 3). Figure 3A-E shows excitatory responses of a spinal neuron to GD and to GES with different parameters. Table 4 quantitatively shows effects of GES with different parameters on spinal neurons excited by GD. Mean excitatory responses to GES-C (16.1±2.8 imp/s) were significantly greater than responses to GES-D (9.6±1.6 imp/s) but not different from those to GES-A or GES-B (Table 4). The same parameters of GES that excited most spinal neurons inhibited some spinal neurons with excitatory responses to GD. Also, some neurons exhibited different responses to different parameters of GES (Table 4). Fig. 3F-J shows an example of a spinal neuron with an excitatory response to GD, which was excited by GES-A, inhibited by GES-C, D, and unaffected by GES-B. Furthermore, GES-A affected activity of 4 SL-E and 6 LL-E neurons; GES-B affected activity of 2 SL-E and 2 LL-E neurons; GES-C affected activity of 11 SL-E and 7 LL-E neurons; GES-B affected activity of 6 SL-E and 4 LL-E neurons. In addition, GES changed activity of spinal neurons with low-threshold responses to GD and also affected high-threshold neurons (Table 5).
Table 3.
Effects of gastric electrical stimulation (GES) with different parameters on the activity of lower thoracic spinal neurons responding to gastric distension (GD) in rats.
| GD-response | GES-A | GES-B | GES-C | GES-D | ||||
|---|---|---|---|---|---|---|---|---|
| R | NR | R | NR | R | NR | R | NR | |
| Excitatory (E) | 7E, 3I | 17 | 4E | 21 | 15E, 3I | 5 | 7E, 3I | 14 |
| Inhibitory (I) | 1E, 1I | 4 | 0 | 6 | 1E, 3I | 2 | 1E, 2I | 3 |
|
| ||||||||
| Total (R/tested) | 12/33 (36%) | 4/31* (13%) | 22/29* (76%) | 13/30 (43%) | ||||
R, response to GES. NR, no response to GES.
P< 0.05 compared to responsive neurons with other GES parameters.
Fig. 3.
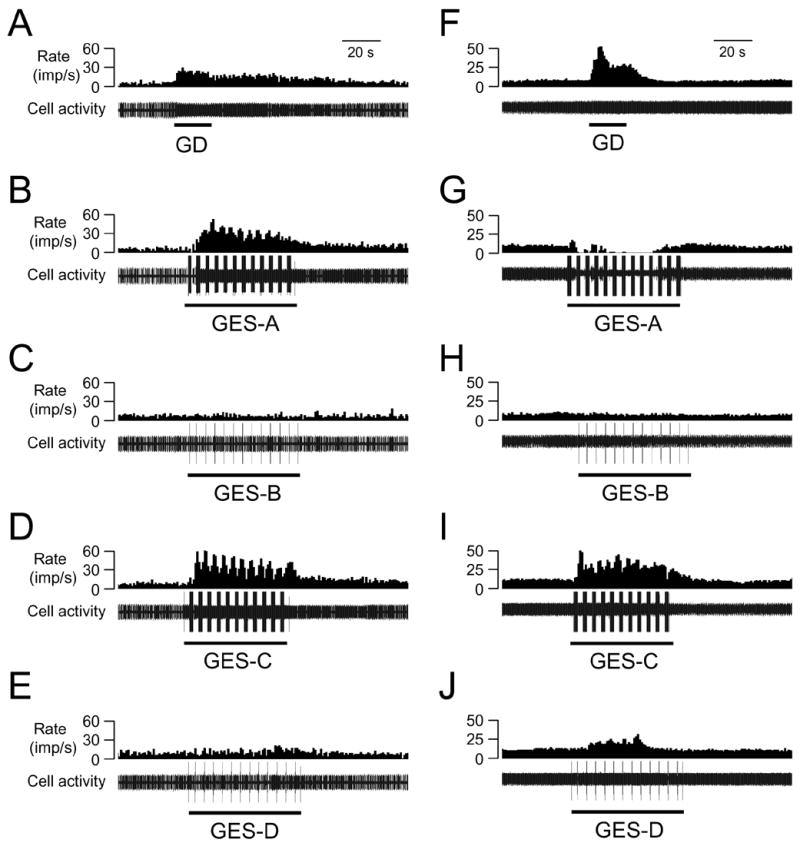
The effects of GES with different parameters on spinal neurons excited by gastric distension (GD). A-E: GES-A, -C, and –D increased and GES-B did not affect activity of a spinal neuron excited by GD (60 mmHg). F-J. GES-A inhibited, GES-C and –D increased, and GES-B did not affect activity of another spinal neuron excited by GD.
Table 4.
Effects of GES with different parameters on T9–T10 spinal neurons excited by GD.
| GES | Effects | n | Spontaneous activity (imp/s) | Latency (s) | E-Response (imp/s) | I-Responses (imp/s) | Duration (s) |
|---|---|---|---|---|---|---|---|
| GES-A | E | 7 | 11.3±2.8 | 4.1±1.2 | 20.0±4.8 | / | 65.1±6.5 |
| I | 3 | 8.5±1.8 | 4.9±2.2 | / | 5.7±2.0 | 78.5±12.4 | |
| GES-B | E | 4 | 10.3±3.2 | 11.3±4.9 | 10.9±4.1 | / | 62.9±10.4 |
| GES-C | E | 15 | 11.9±1.8 | 6.1±1.5 | 16.1±2.8 | / | 59.5±6.4 |
| I | 3 | 9.8±3.2 | 7.8±3.0 | / | 7.8±1.8 | 62.3±9.4 | |
| GES-D | E | 7 | 9.3±2.6 | 3.9±1.3 | 9.6±1.6* | / | 64.7±5.7 |
| I | 3 | 11.0±6.3 | 3.9±0.3 | / | 5.1±2.5 | 59.9±2.5 |
E, excitatory. I, inhibitory.
P<0.05 compared to E-responses to GES-C.
Table 5.
Effects of gastric electrical stimulation (GES) with different parameters on the activity of low- and high-threshold (LT, HT) spinal neurons responding to gastric distension (GD).
| GD-response | GES-A | GES-B | GES-C | GES-D | ||||
|---|---|---|---|---|---|---|---|---|
| LT | HT | LT | HT | LT | HT | LT | HT | |
| Excitatory | 5 | 4 | 3 | 1 | 12 | 6 | 5 | 5 |
| Inhibitory | 2 | 0 | 0 | 0 | 0 | 4 | 3 | 0 |
Effects of cervical vagotomy
Bilateral transection of cervical vagus nerves enhanced excitatory responses to noxious GD (60 mmHg, 20s) in 3 neurons, reduced responses in one neuron and did not affects one neuron. Taken together, the mean excitatory GD-responses (34.4±11.8 imp/s, n=5) after vagotomy were not statistically different than before vagotomy (27.5±5.3 imp/s). For 6 neurons excited by GES-C (n=5) and GES-A (n=1), bilateral cervical vagotomy increased responses to GES in 2 neurons, decreased responses to GES in one neuron and did not change responses in 3 neurons. Mean excitatory responses to GES after vagotomy (26.3±7.7 imp/s) were similar to responses before vagotomy (23.7±6.5 imp/s). Fig. 4A and B show an example of the effects of vagotomy on excitatory responses of a spinal neuron to GD and GES.
Fig. 4.
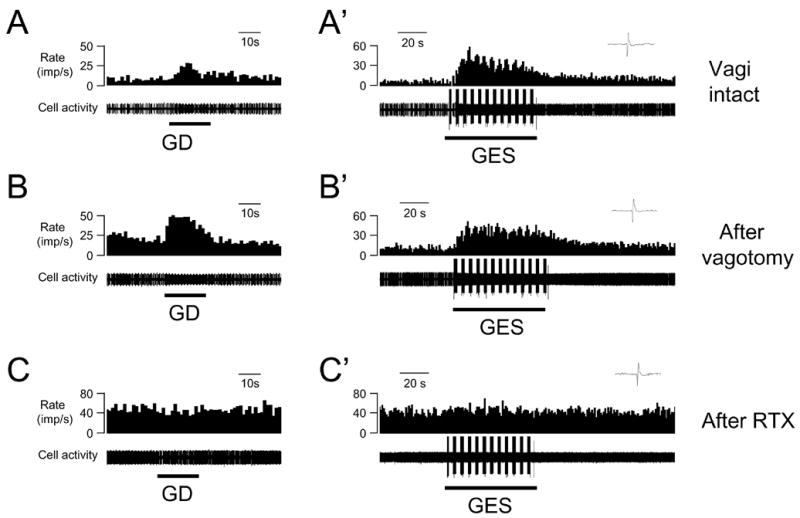
The effects of cervical vagotomy and intravenous RTX on spinal neuronal responses to GD and GES. A, A’: Excitatory responses to GD (60 mmHg) and GES-C in an animals with intact vagi. Upper right panel show an expanded view of a single trace of an action potential. B, B’: Excitatory responses of neuron A to GD and GES-C after bilateral cervical vagotomy. C, C’: Intravenous RTX (2.0 μg/kg) eliminated excitatory responses to both GD and GES-C in the same neuron.
Effect of RTX
To determine whether VR-1 receptors play a role in neuronal responses to gastric spinal afferents, intravenous RTX was used as a pharmacological tool to desensitize spinal neurons containing VR1. Spinal neuronal responses to GD and GES were examined 20 min after RTX in 4 vagotomized rats. Intravenous RTX (2.0 μg/kg) initially increased activity in 3 neurons and reduced activity in one neuron. Twenty minutes later, RTX abolished spinal neuronal excitatory responses to GD (n=4) and GES-C (n=3) or –A (n=1). Fig. 4B and C show an example of excitatory responses of a spinal neuron to GD and GES-C that were eliminated by RTX.
Viscerosomatic convergence
Of 59 GD-responsive neurons examined for somatic mechanical stimulation, 48 (81%) neurons received convergent somatic inputs, which is similar to the proportion (75/95, 79%) of neurons that did not respond to GD. Of GD-responsive spinal neurons with viscerosomatic inputs, 25 neurons were classified as WDR, 23 neurons were HT, and no LT neurons were found. For neurons that did not respond to GD, however, 31 LT, 29 WDR and 15 HT neurons inputs were identified. Somatic receptive fields of GD-responsive spinal neurons were located on the low back, flank, medial/lateral abdominal areas. Fig. 3A-C shows the location and properties of somatic fields of spinal neurons responding to GD. Furthermore, GES-A affected activity of 6 WDR, 3 HT neurons and one neuron in which the somatic receptive field was not found (NF); GES-B affected activity of 4 WDR neurons; GES-C affected activity of 8 WDR, 9 HT neurons and one NF neuron; GES-D affected activity of 5 WDR, 3 HT and 2 NF neurons.
Discussion
The results of this study showed that innocuous and/or noxious gastric distension altered activity in 39% of T9–T10 spinal neurons. The majority (69%) of GD-responsive neurons had excitatory responses, and the remainder of the neurons had inhibitory or biphasic patterns of responses. Therefore, caudal thoracic (T9–T10) spinal neurons might play an important role in processing sensory information from the stomach. In addition, most spinal neurons responsive to GD had nociceptive somatic fields on low back, flank, and medial/lateral abdominal areas. This viscerosomatic convergence likely is the basis of referred pain originating from the stomach. GES with different parameters mainly exerted an excitatory effect on activity of 16–78% of T9–T10 spinal neurons responding to gastric distension. The degree of neuronal excitation with GES depended upon stimulation parameters; effects of GES on neuronal activity were enhanced with stimulation at an increased pulse width and frequency. Cervical vagotomy did not significantly abolish excitatory spinal neuronal responses to GD and GES. In contrast, intravenous RTX abolished all spinal neuronal responses to GD and GES. We concluded, therefore, that spinal neuronal activation by GD and GES depended on afferent impulses traveling in thoracic spinal (sympathetic) fibers expressing VR-1.
Locations of neurons
In the present study, T9–T10 spinal segments were searched for spinal neurons that responded to GD because these segments receive primary sympathetic afferent fibers in the splanchnic nerve innervating the stomach in rats. Neuroanatomic studies demonstrate that most splanchnic nerve afferent fibers from the stomach enter the spinal cord via the upper thoracic (T3–T6) through upper lumbar (L1–L3) dorsal roots, but the most numerous cell bodies of primary gastric sensory neurons are located in T8–T11 dorsal root ganglia (Brtva et al. 1989; Green and Dockray 1988; Neurhuber and Niedrle 1979; Ozaki and Gebhart 2001; Sharkey et al. 1984). Although we searched for spinal neurons throughout the entire extent of the dorsal horn and intermediate zone of gray matter, the sites of most responsive neurons were located in laminae I, II, V, and VII. Neurons excited by gastric distension were located in both superficial and deeper lamina, whereas the majority of the neurons inhibited by gastric distension were found in deeper lamina. This intraspinal regional distribution of GD-responsive neurons is similar to a previous report, in which repeated noxious gastric distensions (80 mmHg) induce minor c-Fos expression in superficial laminae and the intermediolateral nucleus of the T8–T10 spinal cord in rats (Traub 1996). However, acid applied either to the mucosa or serosa of the stomach causes a small stimulation of c-fos transcription in laminae I and II but not in the deeper laminae of the caudal thoracic spinal cord. In contrast to this finding, application of formalin to the serosal, but not mucosal, leads to an increase in c-fos mRNA positive neurons in laminae I-IV (Schuligoi et al. 1996). Intragastric infusion of capsaicin also causes an increase in c-fos mRNA expression in lamina I that is associated with a significant decrease in c-fos mRNA expression in laminae III and IV of the T8–T12 thoracic spinal cord (Holzer et al. 2005). The differences in the laminar distribution of spinal neurons responding to stimulation of the stomach observed in these studies might underline the assumption that mechanical and chemical sensitive afferents innervating the stomach have different intraspinal signaling and processing mechanisms for gastric afferent information.
Neuronal responses to GD
The percentage (39%) of T9–T10 spinal neurons that responded to noxious GD (60 mmHg) in the present study is higher than the proportion (16%) of C1–C2 spinal neurons responsive to the same stimulation (Qin et al. 2003). Furthermore, 70% of T9–T10 spinal neurons responding to GD were excited; 19% were inhibited and the other neurons had biphasic patterns. In contrast, about half of C1–C2 neurons were excited and half were inhibited by GD (Qin et al. 2003). These differences might be due to spinal segmental viscerotopic processing of afferent information from the stomach. The T9–T10 spinal segments receive primary afferent fibers from the splanchnic nerve innervating the stomach, whereas upper cervical segments have secondary responses to gastric input transmitted by vagal and intraspinal pathways (Qin et al. 2003). Another interesting observation is that only 31/997 (3%) rat splanchnic nerve afferent fibers respond to GD (Ozaki and Gebhart 2001), which is significantly lower than 39% of T9–T10 spinal neurons that responded to GD in the present study. These results suggested that the sympathetic afferent fibers may have numerous branches within the gray matter because those afferent fibers have a wide rostrocaudal distribution that can innervate spinal neurons in several segments (Sugiura et al. 1989).
The present study also identified separate populations of LT and HT mechanosensitive spinal neurons that received gastric input. Seventy-one percent of spinal neurons responding to GD were identified as LT (≤20 mmHg) and 29% were HT (≥40 mmHg). These results are similar to previous observations on afferent fibers in the splanchnic nerve innervating the rat stomach, in which 78% LT and 22% HT (≥30 mmHg) fibers responding to GD were reported (Ozaki and Gebhart. 2001). The HT spinal neurons are likely important for intraspinal visceronociceptive transmission and processing associated with acute stomach pain. LT spinal neurons, on the other hand, might relate to nonpainful sensations (e.g., fullness, bloating, nausea) that arise from the stomach. Based on the duration of the neuronal responses, the present study also identified two groups of spinal neurons with noxious gastric input: 37% SL and 63% LL response patterns. One explanation is that SL and LL responses might be associated with afferent activity of Aδ and C fibers from the stomach, respectively. However, of 31 splanchnic afferent fibers responding to GD in rats, 6 (19%) were C-fibers and 25 (81%) were Aδ-fibers (Ozaki and Gebhart 2001). Thus, SL and LL responses in this study might not be associated with activity of different types of gastric afferent fibers. On the other hand, in the present study, a majority of excitatory neuronal responses exhibited slowly adapting pattern; whereas the rest of the neurons exhibited sustained response pattern during GD; no neuron with rapidly adapting pattern (on-off response) to GD was found. These results were generally consistent with a previous observation made from rat splanchnic nerve recordings (Ozaki et al. 2001), in which an increase in activity of 30/31 single afferent fibers exhibit slowly adapting pattern during GD. One fiber had a nonadapting and sustained responses during GD. No fiber had a rapidly adapting on-off response. However, the actual relationship between afferent fiber activity and spinal neuronal responses encoding gastric stimulation needs to be explored.
Effects of GES
Clinically and experimentally, various parameters of GES have been applied for inducing gastric sensory and motor effects (Abell and Minocha 2002; Familoni et al. 1997; Forster et al. 2001; Lin et al. 2002; McCallum et al. 1998; Sobocki et al. 2003). The majority of these studies seem to indicate that GES with low frequency and high energy is able to entrain gastric slow waves and improve gastric emptying; whereas, GES with high frequency and low energy improves gastroparetic symptoms of nausea and vomiting without obvious improvement in gastric motility (Lin et al. 2004). GES with an increased pulse frequency appears to be more effective than GES with increased pulse amplitudes in altering gastric motor function and reversing vasopressin-induced functional gastroparesis in rats (Blanc-Louvry et al. 2002; Nowak et al. 2004). Based on the pulse width, GES can be classified into three categories: long-pulse, short-pulse and train of short pulses (Chen et al. 2003). Long-pulse stimulation is capable of preventing dysrhythmias and uncoupling the gastric slow waves, whereas the short-pulse stimulation and electroacupuncture significantly reduces vomiting, a symptom of nausea, via the vagal-mediated pathway in dogs and rats (Chen et al. 2003; Liu et al. 2004). Long-pulse field electrical stimulation has delayed effects on gastric emptying of liquid, gastric contractility and vagal activity (Ouyang et al. 2003). In the present study, we used four sets of stimulation parameters and the selection of these parameters was based on the following: Parameter A is the set of parameters commonly used for the treatment of obesity (D’Argent et al. 2002); in parameter B, the frequency is reduced to 14 Hz which is commonly used in treating nausea and vomiting in patients with gastroparesis (Abell et al and Forster et al. 2002); Parameter C is similar to Parameter A except that the pulse width is increased to 3ms. The reason behind this selection is that this set of parameters was known more effective in activating GD-responsive neurons in the paraventricular nucleus (Tang et al. 2006). Parameter D is typically used to alter gastric motility functions (Ouyang et al 2005). In the present study, the four sets of GES parameters mainly induced excitatory responses in most spinal neurons with gastric input. GES-C, with pulse trains of increased pulse width, was most effective and GES-B, with decreased frequency, was least effective in eliciting neuronal responses in the spinal cord. Moreover, GES-D, with single long pulses, elicited smaller excitatory responses than those produced by GES-C, which used shorter pulses at higher frequency than GES-D. These results suggested that both the frequency and width of the stimulation pulse play an important role in the modulation of thoracic spinal neuronal activity. In general, results are similar to observations on the effects of GES on neuronal activity in the nucleus of the solitary tract and in the paraventricular nucleus of hypothalamus in rats (Qin et al. 2005; Tang et al. 2006).
Afferent pathways
The stomach receives dual innervation from parasympathetic (vagal) and thoracic spinal sympathetic (splanchnic). After bilateral vagotomy, all T9–T10 tested spinal neurons still responded to GD in the present study, indicating a dependence on the sympathetic afferent pathway. This observation is consistent with a previous study, in which no changes were observed in the total number and regional distribution pattern in the thoracic spinal cord for c-fos expression to noxious GD after vagotomy (Traub 1996). However, bilateral cervical vagotomy can eliminate afferent effects of GD on activity of 50% C1–C2 spinal neurons; effects on the remaining GD-responsive neurons involve spinal visceral (sympathetic) afferent pathways (Qin et al. 2003). This segmental difference for spinal neuronal responses to GD represents intraspinal integrative processing for vagal and sympathetic (splanchnic) afferent inputs from the stomach. Although bilateral cervical vagotomy did not eliminate T9–T10 spinal neuronal responses to GD in the present study, vagotomy enhanced, reduced or did not affect responses to GD in different spinal neurons. This finding suggested that vagal afferents might tonically modulate the activity of individual thoracic spinal neurons receiving gastric inputs, although vagal input was not necessary to initially elicit spinal neuronal responses to GD. Generally, it is believed that vagal afferents from the stomach play a role in conveying information about autonomic regulatory function (e.g. absorption, secretion, storage, emptying) and conscious sensations (e.g., satiety, nausea), whereas gastric nociceptive signaling travels via spinal sympathetic afferents. However, the induction of c-Fos in the nucleus of the solitary tract by repeated noxious GD is significantly greater than in the viscerotopic segments of the spinal cord (Traub 1996). Unmyelinated, capsaicin-sensitive vagal afferents are essential for the pseudoaffective cardiovascular response to noxious GD in rats (Tougas and Wang, 1999). It is possible that nociceptive input through the vagus nerve is important for the emotional-affective and autonomic response component of gastric pain; whereas spinal afferent pathways might relate to the sensory-discriminative aspect. The present study showed that T9–T10 spinal neurons encoded gastric inputs at non-noxious and noxious ranges of graded intensities of GD. This finding is consistent with properties of sympathetic afferent fibers that respond to both innocuous and noxious distension of stomach (Ozaki and Gebhart 2001). It thus is possible, in addition to gastric pain, spinal sympathetic afferents also signal non-painful mechanical sensations such as satiety and nausea.
Several previous studies have examined the pathways involved in GES effects. These results suggest that the effects of GES on gastric motility involve vagal-mediated neural mechanisms. For example, vagotomy eliminates the anti-emetic effects of short-pulse GES on nausea and vomiting induced by vasopressin (Chen et al. 2003). Using an advanced spectral analysis of heart rate variability for the assessment of vagal activity, GES is found to be involved with increased vagal activity and accelerated gastric emptying in dogs and rats (Liu et al. 2004; Ouyang et al. 2003). Additionally, GES is reported to produce an increase in the firing rate of vagal single afferent fibers associated with antral contractions of the stomach in rats (Peles et al. 2003), and GES mainly exerts excitatory effects on NTS neuronal activity (Qin et al. 2005). However, some investigators noted a role of sympathetic pathways in the effects of GES on gastrointestinal functions. The intravenous administration of guanethidine, an adrenergic blockade, prevents the inhibitory effect of GES on antral motility and rectal tone, indicating that GES effects are mediated via sympathetic adrenergic nerve activation (Zhu and Chen 2005; Liu et al. 2005). Long-pulse retrograde GES at a tachygastrial frequency suppresses postprandial antral contractions via alpha- and beta-adrenergic pathways (Ouyang et al. 2005). In the present study, examination of spinal neuronal activation by GES indicated that effects of GES on gastric function might primarily involve spinal sympathetic afferent pathways. It is reasonable to suggest that effects of GES on gastric motility occur not only via a long loop of vagal afferents-supraspinal sites-spinal preganglionic neurons-sympathetic efferents–stomach, but also might involve a short loop of spinal sympathetic afferents-thoracic spinal neurons-sympathetic efferents-stomach.
Vanilloid receptor-1
Capsaicin stimulates afferent neurons by gating transient receptor potential ion channels of vanilloid type-1 (VR-1 or TRPV1) receptors, which are expressed by both vagal and spinal afferent neurons innervating the rat stomach (Ward et al. 2003; Horie et al. 2004; Schicho et al. 2004). Retrograde tracing has shown that 70–80% of the nodose and dorsal root ganglion neurons supplying the rat stomach, respectively, express VR-1 (Schicho et al. 2004). When directly applied to the somata, capsaicin excites 90 % of the dorsal root ganglion neurons and 60 % of the nodose ganglion neurons projecting to the rat stomach (Sugiura et al. 2005). VR-1-like fibers appear to be predominantly spinal in origin, but a few vagal VR1-like fibers exist in the stomach (Patterson et al. 2003; Ward et al. 2003). Capsaicin administered into the rat stomach caused neurons in the superficial lamina I of the dorsal horn to express c-fos mRNA, which indicates that intragastric capsaicin activates sensory neurons that project primarily to lamina I of the spinal cord (Holzer et al. 2005). Noxious acid challenge of the stomach increased TRPV1 protein in spinal but not vagal or intrinsic sensory afferents (Schicho et al. 2004). Noxious gastric stimulation with acid also induces release of glutamate, SP, and CGRP from capsaicin-sensitive sensory afferents in the dorsal horn of the spinal cord (Schicho et al. 2005). The present study showed that intravenous RTX abolished spinal neuronal responses to GD and GES in rats with bilateral cervical vagotomy, indicating a dependence on the spinal (sympathetic) afferent fibers expressing VR-1. Thus, VR-1 contained in spinal visceral afferents might be a key molecule in the transduction of gastric nociception and might play an important role in gastric pain and hypersensitivity.
Summary
Results of this study showed that GD and GES altered activity of T9–T10 spinal neurons in superficial and deeper laminae of gray matter of spinal cord. Afferent impulses evoked by GES with different parameters modulated to different degrees the activity of thoracic spinal neurons receiving inputs from the stomach. Afferent effects of gastric mechanical and electrical stimulation on spinal neurons were mainly dependent on thoracic spinal (sympathetic) afferent fibers containing VR-1, but some responses were modulated by vagal afferent pathways. These data provide a possible spinal mechanism that could help explain how GES with different parameters is effective in the regulation of autonomic function of the stomach to treat gastric motor disorders or morbid obesity.
Fig. 5.
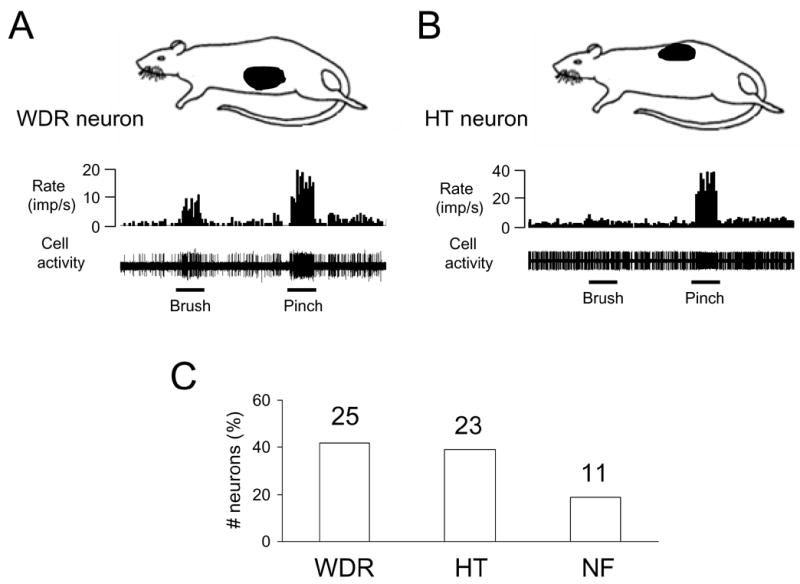
Characteristics of somatic receptive fields of lower thoracic (T9–T10) spinal neurons. A: Location of somatic receptive field and responses of a wide dynamic range (WDR) neuron to somatic mechanical stimulation. B: Location of somatic receptive field and responses of a high-threshold (HT) neuron to somatic stimulation. C: Summary for somatic field properties of spinal neurons responding to GD. NF, somatic field not found.
Acknowledgments
The authors would like to thank Dr. M. J. Chandler for helpful comments and D. Holston for her excellent technical assistance. We also appreciate Dr. S. H. Liu for histological examination of recording sites in spinal cord. This study was partially supported by a grant from National Institutes of Health (1R43DK066709-01, Dr. J.D.Z. Chen; HL075524, Dr. R.D. Foreman).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell TL, Minocha A. Gastroparesis and the gastric pacemaker: a revolutionary treatment for an old disease. J Miss State Med Assoc. 2002;43:369–375. [PubMed] [Google Scholar]
- Blanc-Louvry IL, Guerre F, Songne B, Ducrotte P. Gastric stimulation: influence of electrical parameters on gastric emptying in control and diabetic rats. BMC Surgery. 2002:2. doi: 10.1186/1471-2482-2-5. http://www.Biomedcentral.com/1471–2482/2/5. [DOI] [PMC free article] [PubMed]
- Chen JDZ, Qian L, Quyang H, Yin J Enteric neuromuscular disorders and pain group. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterol. 2003;124:401–409. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- D'Argent J. Gastric electrical stimulation as therapy of morbid obesity: preliminary results from the French study. Obes Surg. 2002;12 (Suppl 1):21S–25S. doi: 10.1381/096089202762552638. [DOI] [PubMed] [Google Scholar]
- Familoni BO, Abell TL, Nemoto D, Voeller G, Johnson B. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892–897. doi: 10.1023/a:1018804128695. [DOI] [PubMed] [Google Scholar]
- Forster J, Sarosiek I, Delcore R, Lin Z, Raju GS, McCallum RW. Gastric pacing is a new surgical treatment for gastroparesis. Am J Surg. 2001;182:676–681. doi: 10.1016/s0002-9610(01)00802-9. [DOI] [PubMed] [Google Scholar]
- Green T, Dockray GJ. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea-pig. Neuroscience. 1988;25:181–93. doi: 10.1016/0306-4522(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Greenstein RJ, Belachew M. Implantable gastric stimulation (IGS) as therapy for human morbid obesity: report from the 2001 IFSO symposium in Crete. Obes Surg. 2002;(Suppl 1):3S–5S. doi: 10.1007/BF03342139. [DOI] [PubMed] [Google Scholar]
- Grundfest-Bronaltowski S, Davies CR, Olsen E. Electrical control of gastric emptying in denervated and reinnervated canine stomach: a pilot study. Artif Organs. 1990;14:254–259. doi: 10.1111/j.1525-1594.1990.tb02966.x. [DOI] [PubMed] [Google Scholar]
- Holzer P, Painsipp E, Schuligoi R. Differential effects of intragastric acid and capsaicin on gastric emptying and afferent input to the rat spinal cord and brainstem. BMC Neurosci. 2005;6:60. doi: 10.1186/1471-2202-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie S, Yamamoto H, Michael GJ, Uchida M, Belai A, Watanabe K, Priestley JV, Murayama T. Protective role of vanilloid receptor type 1 in HCl-induced gastric mucosal lesions in rats. Scand J Gastroenterol. 2004;39:303–12. doi: 10.1080/00365520310008647. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources Commission on Life Sciences and National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Lin Z, Chen JD. Advances in gastrointestinal electrical stimulation. Crit Rev Biomed Eng. 2002;30:419–57. doi: 10.1615/critrevbiomedeng.v30.i456.70. [DOI] [PubMed] [Google Scholar]
- Lin Z, Sarosiek FI, McCallum RW. Effects of high-frequency gastric electrical stimulation on gastric myoelectric activity in gastroparetic patients. Neurogastroenterol Motil. 2004;16:205–212. doi: 10.1111/j.1365-2982.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Qiao X, Chen JDZ. Vagal afferents involved in short-pulse gastric electrical stimulation in rats. Dig Dis Sci. 2004;49:729–737. doi: 10.1023/b:ddas.0000030081.91006.86. [DOI] [PubMed] [Google Scholar]
- Liu J, Hou X, Song G, Cha H, Yang B, Chen JD. Gastric electrical stimulation using endoscopically placed mucosal electrodes reduces food intake in humans. Am J Gastroenterol. 2006;101:798–803. doi: 10.1111/j.1572-0241.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230:133–41. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Neuhuber W, Niederle B. Spinal ganglion cells innervating the stomach of the rat as demonstrated by somatopetal transport of horseradish peroxidase (HRP) Anat Embryol. 1979;155:355–362. doi: 10.1007/BF00317648. [DOI] [PubMed] [Google Scholar]
- Nowak L, Krolczyk G, Sobocki J, Zurowski D, Thor PJ. Gastric stimulation is effective in reversing vasopressin induced gastroparesis. Folia Med Cracov. 2004;45:71–9. [PubMed] [Google Scholar]
- Ouyang H, Yin J, Zhu H, Xu X, Chen JDZ. Effects of gastric electrical field stimulation with long pulses on gastric emptying in dogs. Neurogastroenterol Motil. 2003;15:109–416. doi: 10.1046/j.1365-2982.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- Ouyang H, Xing J, Chen JD. Tachygastria induced by gastric electrical stimulation is mediated via alpha- and beta-adrenergic pathway and inhibits antral motility in dogs. Neurogastroenterol Motil. 2005;17:846–53. doi: 10.1111/j.1365-2982.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol. 2001;281:G1449–G1459. doi: 10.1152/ajpgi.2001.281.6.G1449. [DOI] [PubMed] [Google Scholar]
- Pan HL, Khan GM, Alloway KD, Chen SR. Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci. 2003;23:2911–9. doi: 10.1523/JNEUROSCI.23-07-02911.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson LM, Zheng H, Ward SM, Berthoud HR. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277–87. doi: 10.1007/s00441-002-0682-0. [DOI] [PubMed] [Google Scholar]
- Peles S, Petersen J, Aviv R, Policker S, Abu-Hatoum O, Ben-Haim SA, Gutterman DD, Sengupta JN. Enhancement of antral contractions and vagal afferent signaling with synchronized electrical stimulation. Am J Physiol Gastrointest Liver Physiol. 2003;285:G577–85. doi: 10.1152/ajpgi.00109.2003. [DOI] [PubMed] [Google Scholar]
- Qin C, Sun Y, Chen JD, Foreman RD. Gastric electrical stimulation modulates neuronal activity in nucleus tractus solitarii in rats. Auton Neurosci. 2005;119:1–8. doi: 10.1016/j.autneu.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Qin C, Chandler MJ, Miller KE, Foreman RD. Responses and afferent pathways of C1–C2 spinal neurons to gastric distension in rats. Auton Neurosci. 2003;104:128–136. doi: 10.1016/S1566-0702(03)00002-X. [DOI] [PubMed] [Google Scholar]
- Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on thoracic spinal neurons receiving input from stomach in rats. Experimental Biology Meeting. 2006 doi: 10.1016/j.neures.2006.09.003. #772.7 abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KA, Williams RG, Dockray GJ. Sensory substance P innervation of the stomach and pancreas. Demonstration of capsaicin-sensitive sensory neurons in the rat by combined immunohistochemistry and retrograde tracing. Gastroenterology. 1984;87:914–21. [PubMed] [Google Scholar]
- Schicho R, Florian W, Liebmann I, Holzer P, Lippe IT. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci. 2004;19:1811–8. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- Schicho R, Donnerer J, Liebmann I, Lippe IT. Nociceptive transmitter release in the dorsal spinal cord by capsaicin-sensitive fibers after noxious gastric stimulation. Brain Res. 2005;1039:108–15. doi: 10.1016/j.brainres.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Herzeg G, Wachter C, Jocic M, Holzer P. Differential expression of c-fos messenger RNA in the rat spinal cord after mucosal and serosal irritation of the stomach. Neuroscience. 1996;72:535–44. doi: 10.1016/0306-4522(95)00552-8. [DOI] [PubMed] [Google Scholar]
- Sobocki J, Thor PJ, Krolczyk G. High frequency electrical stimulation of the stomach is more effective than low frequency pacing for the treatment of postoperative functional gastric stasis in humans. Neuromodulation. 2003;6:254–257. doi: 10.1046/j.1525-1403.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–20. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Terui N, Hosoya Y. Difference in distribution of central terminals between visceral and somatic unmyelinated (C) primary afferent fibers. J Neurophysiol. 1989;62:834–40. doi: 10.1152/jn.1989.62.4.834. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–27. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Joo F, Blumberg PM. Duration of desensitization and ultrastructural changes in dorsal root ganglia in rats treated with resiniferatoxin, an ultrapotent capsaicin analog. Brain Res. 1989;503:68–72. doi: 10.1016/0006-8993(89)91705-8. [DOI] [PubMed] [Google Scholar]
- Tang M, Zhang J, Chen JD. Central mechanisms of gastric electrical stimulation involving neurons in the paraventricular nucleus of the hypothalamus in rats. Obes Surg. 2006;16:344–52. doi: 10.1381/096089206776116372. [DOI] [PubMed] [Google Scholar]
- Tougas G, Wang L. Pseudoaffective cardioautonomic responses to gastric distension in rats. Am J Physiol. 1999;277:R272–R278. doi: 10.1152/ajpregu.1999.277.1.R272. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distension in the rat. Neuroscience. 1996;74:873–884. doi: 10.1016/0306-4522(96)00173-x. [DOI] [PubMed] [Google Scholar]
- Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121–35. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- Zhu Hongbing, Chen Jiande DZ. Implantable gastric stimulation inhibits gastric motility via sympathetic pathway in dogs. Obes Surg. 2005;15:95–100. doi: 10.1381/0960892052993549. [DOI] [PubMed] [Google Scholar]


