Abstract
p21ras GTPase is the protein product of the most commonly mutated human oncogene and has been identified as a target for reactive oxygen and nitrogen species (ROS/RNS). Post-translational modification of reactive thiols, by reversible S-glutathiolation and S-nitrosation, and potentially also by irreversible oxidation, may have significant effects on p21ras activity. Here we used an isotope-coded affinity tag (ICAT) and mass spectrometry to quantitate the reversible and irreversible oxidative post-translational thiol modifications of p21ras caused by peroxynitrite (ONOO−) or glutathione disulfide (GSSG). The activity of p21ras was significantly increased following exposure to GSSG, but not to ONOO−. The results of LC-MS/MS analysis of tryptic peptides of p21ras treated with ONOO− showed that ICAT labeling of Cys118 was decreased by 47%, whereas Cys80 was not significantly affected and was thereby shown to be less reactive. The extent of S-glutathiolation of Cys118 by GSSG was 53%, and that of the terminal cysteines was 85%, as estimated by the decrease in ICAT labeling. The changes in ICAT labeling caused by GSSG were reversible by chemical reduction, but those caused by peroxynitrite were irreversible. The quantitative changes in thiol modification caused by GSSG associated with increased activity demonstrate the potential importance of redox modulation of p21ras.
Keywords: Isotope-coded affinity tag (ICAT), oxidant stress, post-translational modification, p21ras, thiol, S-glutathiolation, mass spectrometry
Introduction
Human p21ras is a small (186-residue) guanine nucleotide binding protein (G-protein) that is essential for cellular proliferation and differentiation.[1] In addition to its well-characterized regulation by guanine nucleotide exchange factors which release GDP and allow GTP to bind [2,3], and the prenylation of terminal cysteines, which mediates membrane binding [4,5], p21ras activity may be regulated by oxidative post-translational modification of cysteines[6–8]. Of the six cysteine residues of p21ras (Figure 1) four (118, 181, 184 and 186) are surface-exposed, as shown by structural, chemical and mutational studies [9–11]. Although the C-terminal cysteines, Cys181, Cys184 and Cys186, are normally adducted by lipid moieties in intact cells, S-nitroso- or S-glutathione thiol adducts of Cys118 have been implicated as regulating normal and pathological cellular events [6,7,12–14]. Redox modifications of Cys118, which is part of the guanine nucleotide binding site (117KCDL120), have been implicated in activation of p21ras and downstream signaling to Raf-1/Mek/Erk [15–17]. Although it is generally accepted that p21ras participates in redox reactions, the relative extent to which oxidative modification of its thiols directly regulates its functional activity remains poorly understood. Mallis et al.[12] showed that recombinant p21ras can be S-glutathiolated on the C-terminal cysteines by hydrogen peroxide and glutathione or by GSSG, but the effect on activity was not assessed. In a recent study from our lab, we found that direct oxidant-mediated S-glutathiolation of recombinant p21ras increases its activity, and that peroxynitrite formed endogenously can mediate signaling in endothelial cells in response to nitric oxide donors, ONOO−, or stimuli which produce endogenous ONOO−, such as oxidized LDL[17]. In addition, angiotensin II in vascular smooth muscle cells [6], and alpha-adrenergic stimulation [8] or mechanical stretch [18] of cardiac myocytes increase endogenous hydrogen peroxide and initiate MAP kinase and Akt signaling via p21ras S-glutathiolation. In these studies, labeling with biotin-labeled glutathione and mass spectrometry showed that ONOO− or hydrogen peroxide caused S-glutathiolation of p21ras on Cys118. Downstream signaling to ERK and Akt was attenuated by overexpression of a C118S p21ras mutant or of glutaredoxin-1, which is capable of reducing the S-glutathione mixed disulfide , indicating that S-glutathiolation of Cys118 is key to increasing p21ras activity in cells. We have utilized Fourier transform mass spectrometry (FTMS) to provide a complete map of the post-translational modifications of p21ras exposed to oxidants by combining bottom-up and top-down techniques.[19] Consistent with the physiological importance of Cys118 , our FTMS results demonstrated that Cys118 of recombinant p21ras was the primary site of S-glutathiolation by GSSG[19], although this method could not provide quantitation of the degree of modification.
Figure 1.

Amino acid sequence of human p21ras. Six cysteines, Cys51, Cys80, Cys118, Cys181, Cys184 and Cys186 of p21ras are highlighted in bold font and underlined.
In the present study an isotope-coded affinity tag (ICAT) reagent was used to quantitate the oxidative post-translational modifications of cysteine thiols of p21ras that are responsible for redox modulation of its activity. The ICAT reagent used here is an iodoacetamide (IAM) analogue that has been employed extensively in quantitative proteomics to evaluate the abundance of expressed proteins [20]. The key property of the ICAT reagent is that only free thiols are susceptible to modification by its IAM moiety [21]. To the extent that oxidant-sensitive cysteine thiols have been oxidized, the labeling by the ICAT reagent is decreased. The degree of oxidation is quantified from the decrease in labeling calculated from the ratio of ICAT labeling of control and oxidant-modified samples (Figure 2). The modified cysteine residue is identified from the liquid chromatography (LC) mass spectrometry (MS and MS/MS) analysis of the peptides obtained after proteolysis of the protein(s)[22,23]. For the present experiments we used ONOO− and GSSG which regulate the activity of p21ras by introducing various oxidative modifications [17,19].
Figure 2.

ICAT approach to quantitatively evaluate reversible and irreversible oxidative post-translational thiol modification. Protein samples with free cysteine thiols ■ and less-reactive cysteine thiols □ are exposed to peroxynitrite or GSSG and normal conditions or control buffer before labeling with ICAT reagent. Some of the reactive cysteine thiols are oxidized depending upon the level of the oxidant and oxidized thiols are designated as ▲. Following oxidation, labeling of the free thiols is performed, with light
 and heavy
and heavy
 ICAT reagent. ICAT-labeled samples are mixed and digested with trypsin, followed by purification through HPLC cation exchange and avidin affinity cartridges as outlined in Methods. Affinity-captured peptides are analyzed by LC-MS and MS/MS. As shown for the reactive cysteines, the oxidized cysteines are not susceptible to labeling by ICAT reagent, and hence there is a decreased intensity in the signal from the heavy-labeled peptide in MS of the reactive cysteine-containing peptide. For less reactive cysteines, the peptides in samples prepared under normal and oxidant stress conditions exhibit equivalent signal intensity in MS. From the relative peak intensities of the MS of light and heavy ICAT-labeled peptides, the ratio of oxidized thiols in the samples can be estimated. Identity of the peptide sequences can be derived from MS/MS analysis of these peptides. In order to evaluate whether the oxidation caused by peroxynitrite or GSSG is reversible or irreversible, the oxidized protein samples are treated with TCEP (for disulfide reduction of S-glutathiolation modification) or ascorbate (for reducing S-nitrosation) before labeling with heavy ICAT. From the LC-MS analysis, reversibility of the modifications caused by peroxynitrite or GSSG can be quantitated.
ICAT reagent. ICAT-labeled samples are mixed and digested with trypsin, followed by purification through HPLC cation exchange and avidin affinity cartridges as outlined in Methods. Affinity-captured peptides are analyzed by LC-MS and MS/MS. As shown for the reactive cysteines, the oxidized cysteines are not susceptible to labeling by ICAT reagent, and hence there is a decreased intensity in the signal from the heavy-labeled peptide in MS of the reactive cysteine-containing peptide. For less reactive cysteines, the peptides in samples prepared under normal and oxidant stress conditions exhibit equivalent signal intensity in MS. From the relative peak intensities of the MS of light and heavy ICAT-labeled peptides, the ratio of oxidized thiols in the samples can be estimated. Identity of the peptide sequences can be derived from MS/MS analysis of these peptides. In order to evaluate whether the oxidation caused by peroxynitrite or GSSG is reversible or irreversible, the oxidized protein samples are treated with TCEP (for disulfide reduction of S-glutathiolation modification) or ascorbate (for reducing S-nitrosation) before labeling with heavy ICAT. From the LC-MS analysis, reversibility of the modifications caused by peroxynitrite or GSSG can be quantitated.
Experimental Methods
Recombinant p21 H-ras was obtained from Biomol (Plymouth Meeting, PA) in buffer containing 2 mM Tris (pH 8.0), 45 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol (DTT), and 50% glycerol, and peroxynitrite was obtained from Calbiochem (San Diego, CA). ONOO− concentration was measured before each experiment by measurement of the UV absorption at 302 nm, and the concentration adjusted with NaOH (0.1 M). Mant-GDP was purchased from Molecular Probes (Eugene, OR). GSSG and all chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). Trypsin Gold (MS grade) was purchased from Promega (Madison, WI). The ICAT reagent kit was obtained from Applied Biosystems (Foster City, CA).
Immediately prior to experiments recombinant p21 H-ras (10–20 μg) was dialyzed to remove commercial buffer components including DTT and was then treated for 5 min at 37 °C with ONOO− (10–100 μM) or with GSSG (10–100 μM) in phosphate buffer (0.1 M, pH 7.0).
p21ras activity assay
The assay was adapted from previously described protocols. [17,24,25] Recombinant H-p21ras (2 μg) was loaded with a fluorescent nucleotide analogue N-methylanthraniloyl GDP (Mant-GDP), which exhibits fluorescence only when bound to the protein. The p21ras was incubated 1 h at 37°C with an excess of Mant-GDP in a binding buffer (potassium phosphate buffer (50 mM), NaCl (50 mM), MgCl2 (5 mM) and EDTA (5 mM)) and the protein-Mant-GDP complex was purified on a PL-6 spin column. The p21ras-Mant-GDP complex was then diluted to 1 μM in 100 μL of binding buffer containing GTP (200 μM) and treated with ONOO− (10–100 μM) or oxidized glutathione (GSSG, 10–100 μM) for 5 min. The decrease in fluorescence was quantified with a fluorimeter with an excitation wavelength of 355 nm and an emission wavelength of 460 nm. The results were normalized by the fluorescence before addition of peroxynitrite or GSSG as described[17].
ICAT labeling of p21ras pretreated or not with peroxynitrite or GSSG
ONOO− or GSSG-treated and -untreated p21ras samples were first dialyzed against the phosphate (0.1M, pH 7.4) buffer, and then two times against Tris (50 mM, pH 8.5) buffer containing 0.1 % SDS, which was added to allow more complete labeling by the ICAT reagent of the unfolded protein. The p21 H-ras (10 μg) was then incubated at 37 °C with the acid-cleavable 12C (light) or 13C (heavy) ICAT reagent using the protocol supplied by the manufacturer. After 2 h, the light and heavy ICAT labeled protein samples were mixed, and the mixture was subjected to digestion with trypsin by incubating at 37 °C for 10–14 h. The tryptic peptides were purified using a cation exchange cartridge to remove excess labeling reagent. The desalted peptides were affinity purified using the avidin affinity cartridge provided and were then dried and suspended in the cleavage reagent to release the peptides from the acid-cleavable linker by incubating at 37 °C for 2 h. The acid-cleaved peptides were dried and then suspended in 1% formic acid for capillary LC-electrospray ionization (ESI) MS/MS experiments. To determine the reversibility of the modifications introduced by ONOO− and GSSG, (tris(2-carboxyethyl) phosphine (TCEP, 5 mM), a non-thiol based reducing agent, or sodium ascorbate (1 mM) was added for 30 min at 50 °C prior to the ICAT labeling.
Capillary High-Performance LC (HPLC)-ESI MS/MS of ICAT-labeled Peptides
Capillary HPLC with electrospray tandem mass spectrometry was performed using a CapLCTM system (Waters Corp., Bedford, MA) coupled to a quadrupole orthogonal time-of-flight mass spectrometer (Q-TOF API US; Micromass/Waters Corp.) equipped with a Pico Tip Sprayer, NanoLockSpray,TM and Z-SprayTM source. Affinity-purified ICAT-labeled peptides were dissolved in 1% formic acid at a concentration of ca. 1 pmol/μl; injection volumes were 1 μl. Sample concentration and desalting were performed on-line using a peptide trap cartridge (Michrom BioResources, Inc., Auburn, CA) in line with the auto-sampler and column. Separation was on a 300-μm x 15-cm capillary column packed with Vydac C18 phase (5 μm, 300 Å). A linear gradient was used to elute peptides into the mass spectrometer at 2 μl min−1: 5–65% B over 55 min (A: 95% H2O, 5% acetonitrile (ACN), 0.1% formic acid, 0.001% TFA; B: 5% H2O, 85% ACN, 10% 2-propanol, 0.1% formic acid, 0.001% TFA). Columns were washed and re-equilibrated between LC experiments. ESI was carried out at 2.8 kV, with the ion source temperature at 80 °C, and 22 V cone voltage. Mass spectra were acquired in the positive-ion mode over the range m/z 400–1600. NanoLockSprayTM was performed by constant infusion of renin substrate hexadecapeptide (Sigma) 10−6 M in 60% ACN at 0.2 μl min−1. Mass accuracy was within 10 ppm and resolution was above 1:10,000 (fwhm). MS/MS spectra were acquired for the three most-abundant peaks in the MS spectrum if these had signal intensities >25 counts. MS/MS collision energies were dependent on the mass and charge of the precursor and ranged from 16 to 40 volts. Argon was the collision gas at 2 x 10−5 Torr in the cell. MS/MS spectra were acquired for 4 x 1-s scans over the range m/z 100–1600. Mass Lynx 4.0 and Protein Lynx Global Server 2.2 (Micromass/Waters Corp.) were used for data analysis. Targeted and advanced shotgun expression analyses were used for quantitation and identification of ICAT-labeled peptides. In both cases, LC-MS data were used for quantitative analysis. The quantitation of ICAT pairs was accomplished by estimating the peak areas for the chromatograms of the monoisotopic ion clusters reconstructed from the mass spectra recorded during the elution of the respective ICAT-labeled peptides. The precision of the peak area measurement was estimated by measuring the MS peak areas for the pairs of peptides in an experiment in which light and heavy ICAT labeling was performed in identical samples without treating with either peroxynitrite or GSSG. A 95% confidence interval (2 times the SD) was calculated for the change in peak area for each peptide pair. This value equaled ±12 %. The ICAT labeling experiments were repeated twice and the error of estimation for any two identical experiments were within this ±12 % limit.
Results and Discussion
S-Glutathiolation of p21ras promotes guanine nucleotide exchange
p21ras can be S-glutathiolated by GSSG in vitro [12,17], and S-glutathiolation mediates the activation of p21ras by oxidants in endothelial[17] and smooth muscle [6] cells. To confirm the ability of S-glutathiolation to activate p21ras under the conditions of the present studies, the nucleotide exchange rate of recombinant p21ras protein was measured by the release of fluorescent-labeled GDP.[17] The guanine nucleotide exchange activity of recombinant p21ras was significantly increased by GSSG (10–100 μM, Figure 3), in a concentration-dependent manner. However, there was no significant effect of ONOO− (10–100 μM).
Figure 3.
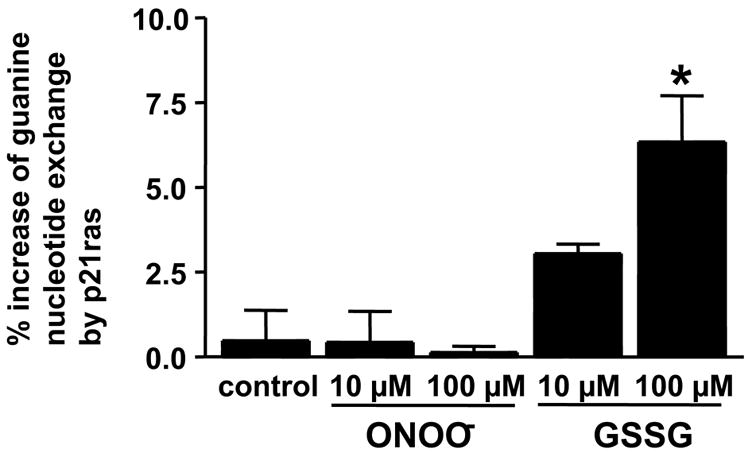
S-Glutathiolation, but not ONOO−, promotes p21ras guanine nucleotide exchange activity. The guanine nucleotide exchange activity of recombinant p21ras was measured by monitoring the fluorescence of p21ras bound Mant-GDP complexes. The protein was either untreated (control) or treated with peroxynitrite (ONOO−, 10 μM or 100 μM) or GSSG (10 or 100 μM). The bar graph shows the percentage of the guanine nucleotide exchange activity of p21ras (i.e., the unbound mant-GDP expressed as a percent of the total bound) in control, peroxynitrite and GSSG-treated samples 5 min after treatment. * p <0.05; n=3.
ICAT-based quantification of oxidative modifications in peroxynitrite-treated p21ras
To measure the thiol modifications of p21ras that can modulate its activity, ICAT labeling was carried out on p21ras treated under the conditions used above. The free cysteines in the unoxidized protein were labeled with light (12C) ICAT reagent, and protein treated with either GSSG or ONOO− was labeled with heavy (13C) ICAT reagent. After mixing the light and heavy labeled protein mixture, the protein was digested with trypsin and ICAT-labeled peptides were separated by avidin affinity capture. Thiol oxidation by ONOO− or GSSG decreases the heavy ICAT labeling of the affected cysteines compared to that obtained with the light ICAT reagent. The extent of the labeling is quantitated by LC-MS and the ICAT-labeled peptides can be identified by tandem MS (Figure 2). As an example, the total ion chromatogram obtained from one LC-MS/MS analysis of ICAT-labeled tryptic peptides is shown in Figure 4a. The summed mass spectrum of peptides eluting between 46.9 and 47.9 min is shown in Figure 4b. The region that contains the [M+H]+ signals from the peptide with the less-reactive Cys80 (m/z 920.85 and m/z 925.38, z=2) is shown in Figure 4c. The molecular ion regions in the mass spectra of this cysteine containing peptide obtained before and after exposure to ONOO−, are shown in Figure 5a. Tandem MS analysis was performed on this peptide that contains the less-reactive Cys80 and confirmed the peptide sequence (Fig. S1). The ratio between unmodified and modified cysteines in this peptide from control and ONOO− -treated samples was measured from the peak areas of the chromatograms for the monoisotopic ion clusters in the mass spectra (Fig. 5a,b). From the peak areas of the light and heavy ICAT-labeled peptides, it was determined that 13% of Cys80 was oxidized when treated with ONOO− (100 μM). In contrast, the intensity of the MS peaks of the heavy ICAT-labeled peptide containing Cys118 showed a 47 % decrease in intensity after treatment with ONOO− (Fig. 5b).
Figure 4.
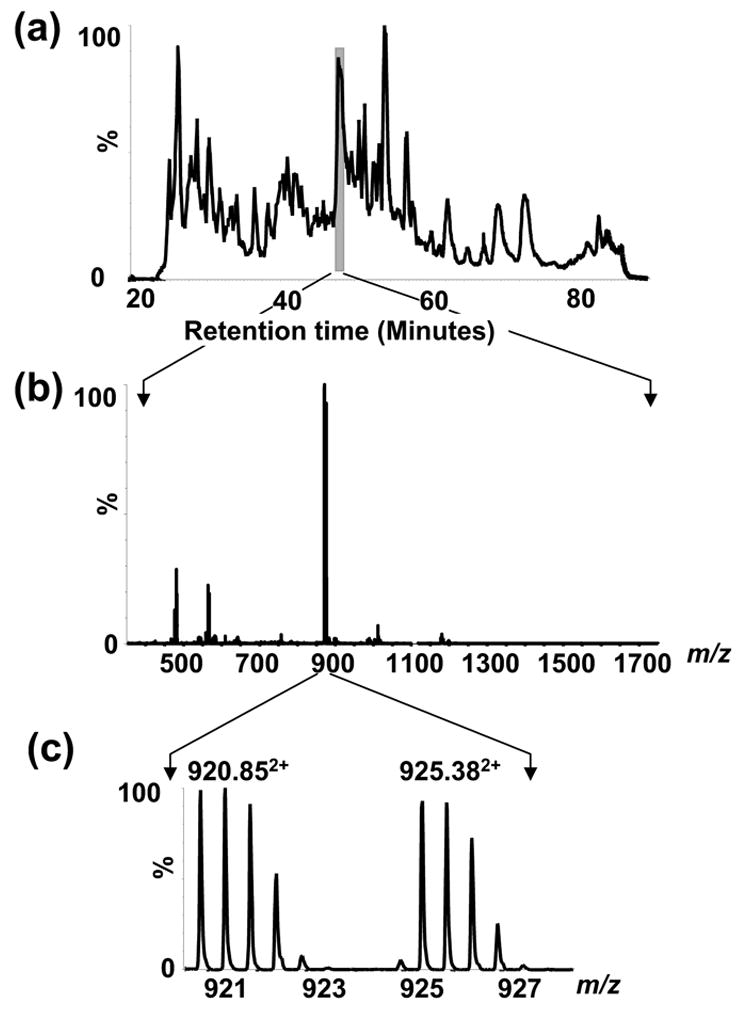
LC-MS/MS of ICAT-labeled tryptic peptides of p21ras. (a) Total ion chromatogram for LC-ESI-MS/MS of ICAT-labeled tryptic peptides of p21ras. (b) The m/z 400–1800 region of the mass spectrum of the peptides eluting between 46.9 and 47.9 min. c) Expanded view of the mass spectrum around m/z 920 showing the pair of light (12C) and heavy (13C) ICAT-labeled p21ras peptide, Thr74 – Lys88.
Figure 5.
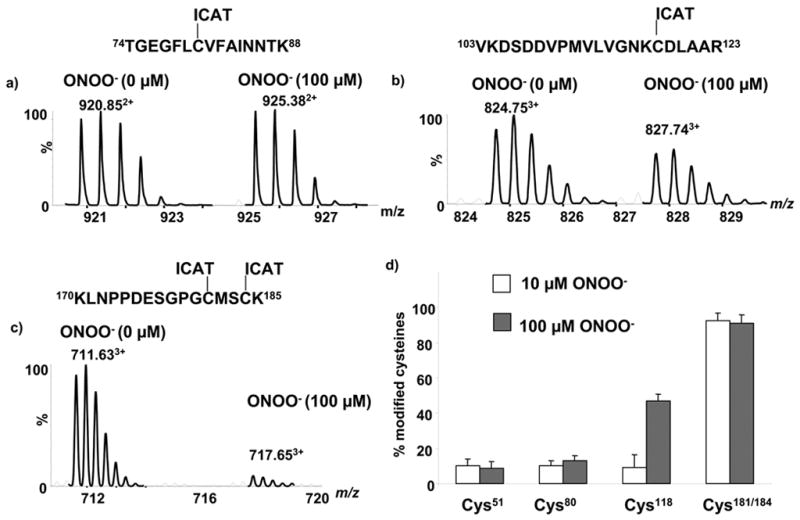
Effect of ONOO− on ICAT-labeling of cysteines of p21ras. a) MS of the ICAT-labeled peptide pair (Thr74 – Lys88) of p21ras containing less-reactive Cys80 (b) MS of the ICAT-labeled peptide pair Val103 –Arg123 of p21ras containing reactive Cys118 and (c) MS of the ICAT-labeled peptide pair Lys170-Lys185 of p21ras containing the terminal Cys181 and Cys184 d) Summary of ICAT labeling experiments quantitating individual cysteine modifications of p21ras treated with peroxynitrite.
The CID MSMS spectrum (Fig. S2) recorded from the doubly charged precursor ion with m/z 824.75 was used to determine the sequence of the redox-sensitive Cys118 containing peptide. The b and y ions in the MSMS spectrum showed that this peptide spans residues Val103–Arg123 of p21ras. When treated with the reducing agent, TCEP (5 mM), labeling of Cys118 was still decreased by 51% (data not shown). This is similar to the change seen without TCEP in Fig. 5b, indicating that peroxynitrite irreversibly modifies the Cys118 thiol. Also consistent with this, the modification was not changed by sodium ascorbate (1 mM, data not shown) ruling out significant S-nitroso modification of the cysteines of p21ras by ONOO−. Our previous FTMS analysis showed that p21ras Cys118 is modified to cysteine sulfonic acid by ONOO− (100 μM) [19], explaining why ICAT labeling was not reversed by treatment by TCEP. The terminal cysteines Cys181 and Cys184 were nearly completely and irreversibly modified (>90 %) by ONOO− as shown by the MS of the ICAT-labeled peptides shown in figure 5c. There is an 18-Da mass difference in the [M+3H]3+ ions observed at m/z 711.6 and 717.6 corresponding to the peptide Lys170– Lys185 in which both Cys181 and Cys184 were labeled with ICAT reagent. Therefore, oxidation of one or the other or both of the cysteines led to a change in mass and loss from the spectrum of the MS peak for the heavy isotope ICAT labeled peptide. Although this prevents us from quantitating oxidation of the individual thiols, both were shown to be oxidized to sulfonic acid by ONOO− previously [19].
The extent of modification of different cysteines of p21ras with different ONOO− concentrations (10 or 100 μM) is shown in Figure 5d. There is no significant modification of Cys118 by ONOO− (10 μM) whereas ~47 % modification was found with 100 μM. At both 10 and 100 μM ONOO−, Cys181/184 were found to be more than 90 % modified. However, even after treatment with ONOO− (100 μM), Cys51 and Cys80 were unaffected. This shows that the cysteines of p21ras are differentially reactive to ONOO−.
ICAT-based quantification of reversible S-glutathiolation of p21ras
In order to quantitatively evaluate the extent of S-glutathiolation that increases p21ras activity as shown in figure 2, p21ras treated with GSSG (100 μM) was subjected to ICAT labeling (Fig. 6a). This concentration of GSSG caused a considerable decrease in the labeling of the heavy isotope-labeled peptide that contains Cys118, whereas there was no appreciable change in the intensity of the heavy isotope-labeled peptide that contains the less reactive thiol on Cys80 (Fig. 6b). From the change in intensity of the MS peaks, 53% of the Cys118 thiol was modified by GSSG. When treated with TCEP (5 mM), the labeling by the heavy isotope ICAT reagent was restored completely (Fig. 6d and S3) consistent with the reversible nature of S-glutathiolation. In contrast, ascorbate (X mM) did not significantly affect labeling of Cys118 after treatment with GSSG consistent with stability of the dithiol under these conditions (data not shown).
Figure 6.
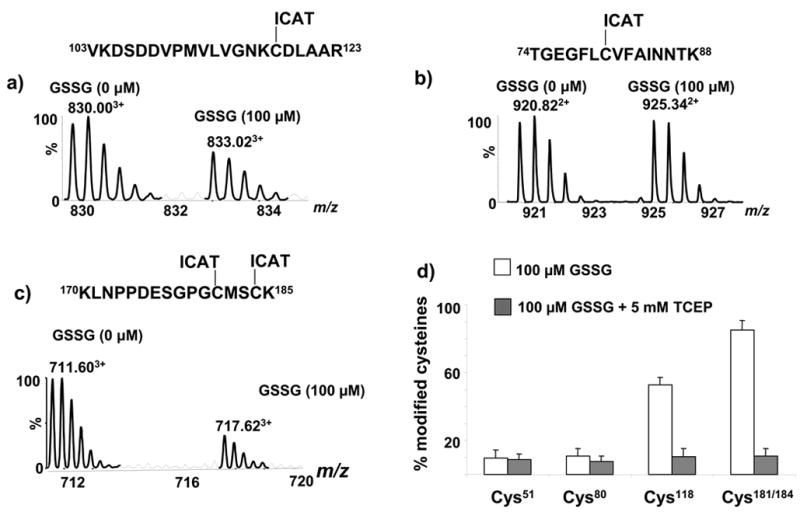
Effect of GSSG on ICAT labeling of cysteines of p21ras. a) MS of ICAT labeled peptide pair (Val103 –Arg123) containing reactive Cys118 in which the methionine is in oxidized form, c) MS of the ICAT-labeled peptide pair (Thr74 – Lys88) of p21ras containing less-reactive Cys80 and e) MS of the ICAT-labeled peptide pair (Lys170–Lys185) of p21ras containing terminal cysteines Cys181 and Cys184 in which both cysteines were ICAT-labeled and the methionine is in oxidized form. d) Summary of ICAT labeling of p21ras to quantitate cysteine modifications caused by GSSG.
We also quantified S-glutathiolation of the terminal cysteines Cys181 and Cys184 following treatment with GSSG and showed that the modification was reversible (Fig. 6c, d). We estimated that in 85% of p21ras one or the other or both of these cysteines were reversibly S-glutathiolated by GSSG (100 μM, Figure 6c). The histogram (Fig. 6d) summarizes the extent of modification of the detected cysteines in p21ras by GSSG, showing that both Cys51 and Cys80 were minimally modified. As with ONOO− above, because we detected only the doubly ICAT-labeled peptide, the results indicate that in 85% of the protein one of the two labeled terminal cysteines or both were modified. In our previous study using FTMS analysis of S-glutathiolated p21ras, we found that each of the three terminal cysteines can be individually or multiply S-glutathiolated by GSSG[19]. Thus, in agreement with earlier studies (Mallis et al.), oxidative modification of recombinant p21ras terminal cysteines can be quantitatively large.
Conclusions
We describe here the quantification of reversible and irreversible oxidative modifications of p21ras that regulate its function by applying an ICAT-based mass spectrometric method. Cys118 and terminal cysteines were found to be targets for reversible modification by GSSG and irreversible modification by ONOO−. The extensive reversible S-glutathiolation of Cys118 by GSSG under conditions in which p21ras activity is increased indicates the large degree to which this reactive thiol can be modified. A similar degree of modification was observed in endothelial cells treated with ONOO− by using a semi-quantitative method which labeled cell proteins with biotin-tagged glutathione [17]. Our results also demonstrate that different cysteines on p21ras have very different sensitivities towards modifications by oxidants. In vivo, because the terminal cysteines are largely modified by lipid, Cys118 is the prime target for oxidants and regulates p21ras activity as indicated by the fact that both S-glutathiolation and stimulation of activity by oxidants is mostly prevented in cells transfected with a Cys118 mutant [6,14,17].
Supplementary Material
Acknowledgments
This project has been funded in whole or in part with funds from the National Institutes of Health, the National Center for Research Resources (Grant No. P41RR10888-6) and the National Heart, Lung, and Blood Institute sponsored Boston University Cardiovascular Proteomics Center (Contract No. N01-HV-28178). M.S., N.C. and RAC were supported by P01 HL081738 and R01 AG 027080. M.S. was supported in part by an NIH Postdoctoral Research Fellowship (No. T32/HL07224).
Abbreviations
- ONOO−
peroxynitrite
- GSSG
glutathione disulfide
- IAM
iodoacetamide
- ICAT
isotope-coded affinity tag, ACN, acetonitrile
- TFA
trifluoroacetic acid
- DTT
dithiothreitol
- TCEP
(tris(2-carboxyethyl) phosphine
- LC
liquid chromatography
- HPLC
high performance LC
- capLC
capillary LC
- MS
mass spectrometry
- MS/MS
tandem MS
- ESI
electrospray ionization
- TOF
time-of-flight
- qTOF
quadrupole TOF
- TIC
total ion chromatogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Vojtek AB, Der CJ. Increasing complexity of the Ras signaling pathway. J Biol Chem. 1998;273:19925–19928. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- 2.Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, Kuriyan J. The structural basis of the activation of Ras by Sos. Nature. 1998;394:337–343. doi: 10.1038/28548. [DOI] [PubMed] [Google Scholar]
- 3.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 4.Hancock JF. Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 5.Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PI. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 6.Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 7.Heo J, Campbell SL. Mechanism of p21Ras S-nitrosylation and kinetics of nitric oxide-mediated guanine nucleotide exchange. Biochemistry. 2004;43:2314–2322. doi: 10.1021/bi035275g. [DOI] [PubMed] [Google Scholar]
- 8.Kuster GM, Pimentel DR, Adachi T, Ido Y, Brenner DA, Cohen RA, Liao R, Siwik DA, Colucci WS. Alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes is mediated via thioredoxin-1-sensitive oxidative modification of thiols on Ras. Circ. 2005;111:1192–1198. doi: 10.1161/01.CIR.0000157148.59308.F5. [DOI] [PubMed] [Google Scholar]
- 9.Pai EF, Krengel U, Petsko GA, Goody RS, Kabsch W, Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 A resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990;9:2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock JF, Magee AI, Childs JE, Marshall CJ. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 11.Lander HM, Milbank AJ, Tauras JM, Hajjar DP, Hempstead BL, Schwartz GD, Kraemer RT, Mirza UA, Chalt BT, Burk SC, Quilliam LA. Redox regulation of cell signalling. Nature. 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 12.Mallis RJ, Buss JE, Thomas JA. Oxidative modification of H-ras: S-thiolation and S-nitrosylation of reactive cysteines. Biochem J. 2001;355:145–153. doi: 10.1042/0264-6021:3550145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lander HM, Ogiste JS, Pearce SFA, Levi R, Novogrodsky A. Nitric oxide-stimulated guanine nucleotide exchange on p21ras. The Journal of Biological Chemistry. 1995;270:7017–7020. doi: 10.1074/jbc.270.13.7017. [DOI] [PubMed] [Google Scholar]
- 14.Clavreul N, Bachschmid MM, Hou X, Shi C, Idrizovic A, Ido Y, Pimentel D, Cohen RA. S-glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol. 2006;26:2454–2461. doi: 10.1161/01.ATV.0000242791.28953.4c. [DOI] [PubMed] [Google Scholar]
- 15.Lander HM, Hajjar DP, Hempstead BL, Mirza UA, Chait BT, Campbell S, Quilliam LA. A molecular redox switch on p21(ras). Structural basis for the nitric oxide-p21(ras) interaction. J Biol Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 16.Finkel T. Redox-dependent signal transduction. FEBS Letters. 2000;476:52. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- 17.Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J. 2006;20:518–520. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- 18.Pimentel DR, Adachi T, Ido Y, Heibeck T, JBLYMJCRC WS. Strain-stimulated hypertrophy in cardiac myocytes is mediated by reactive oxygen species-dependent Ras S-glutathiolation. J Mol Cell Cardiol 2006. 2006;0000 doi: 10.1016/j.yjmcc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Zhao C, Sethuraman M, Clavreul N, Kaur P, Cohen RA, O’connor PB. Detailed Map of Oxidative Post-Translational Modifications of Human P21Ras Using Fourier Transform Mass Spectrometry. Anal Chem. 2006;78:5134–5142. doi: 10.1021/ac060525v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 21.Kim JR, Yoon HW, Kwon KS, Lee SR, Rhee SG. Identification of proteins containing cysteine residues that are sensitive to oxidation by hydrogen peroxide at neutral pH. Anal Biochem. 2000;283:214–221. doi: 10.1006/abio.2000.4623. [DOI] [PubMed] [Google Scholar]
- 22.Sethuraman M, McComb ME, Huang H, Huang S, Heibeck T, Costello CE, Cohen RA. Isotope-coded affinity tag (ICAT) approach to redox proteomics: identification and quantitation of oxidant-sensitive cysteine thiols in complex protein mixtures. J Proteome Res. 2004;3:1228–1233. doi: 10.1021/pr049887e. [DOI] [PubMed] [Google Scholar]
- 23.Sethuraman M, McComb ME, Heibeck T, Costello CE, Cohen RA. Isotope-coded affinity tag approach to identify and quantify oxidant-sensitive protein thiols. Mol Cell Proteomics. 2004;3:273–278. doi: 10.1074/mcp.T300011-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Lenzen C, Cool RH, Wittinghofer A. Analysis of intrinsic and CDC25-stimulated guanine nucleotide exchange of p21ras-nucleotide complexes by fluorescence measurements. Methods Enzymol. 1995;255:95–109. doi: 10.1016/s0076-6879(95)55012-7. [DOI] [PubMed] [Google Scholar]
- 25.Lenzen C, Cool RH, Prinz H, Kuhlmann J, Wittinghofer A. Kinetic analysis by fluorescence of the interaction between Ras and the catalytic domain of the guanine nucleotide exchange factor Cdc25Mm. Biochemistry. 1998;37:7420–7430. doi: 10.1021/bi972621j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


