Abstract
We previously reported that an experimental live-attenuated equine infectious anemia virus (EIAV) vaccine, containing a mutated S2 accessory gene, provided protection from disease and detectable infection after virulent virus (EIAVPV) challenge [1,2]. To determine if attenuated EIAV vaccines actually prevent persistent infection by challenge virus, we employed a 14-day dexamethasone treatment of vaccinated horses post-challenge to suppress host immunity and amplify replication levels of any infecting EIAV. At two months post-challenge the horses were all protected from virulent-virus challenge, evidenced by a lack of EIA signs and detectable challenge plasma viral RNA. Upon immune suppression, 6/12 horses displayed clinical EIA. Post-immune suppression characterizations demonstrated that the attenuated vaccine evidently prevented detectable challenge virus infection in 50% of horses. These data highlight the utility of post-challenge immune suppression for evaluating persistent viral vaccine protective efficacy.
Keywords: EIAV, Vaccine, Live-Attenuated, Immune Suppression, Dexamethasone
1. INTRODUCTION
Live-attenuated virus vaccines have been used effectively to control significant viral outbreaks such as smallpox, polio, and measles epidemics [3,4]. However, the use of a live-attenuated human immunodeficiency virus (HIV) vaccine has been controversial due to concerns that have arisen about vaccine safety [5–13]. Thus far the development of vaccines to HIV-1 has relied substantially on the use of animal lentivirus models to evaluate the efficacy of various vaccine strategies.
EIAV, a macrophage-tropic lentivirus, causes a persistent infection in horses and a chronic disseminated disease of worldwide importance in veterinary medicine (reviewed in Montelaro, Ball, et al. 1993). Virus infection utilizes the ELR-1 receptor [14] on target cells and is transmitted via blood-feeding insects or iatrogenic sources such as contaminated syringe needles. Disease occurs in three stages: acute, chronic and inapparent. EIA is characterized during its acute and chronic stages by defined episodes of clinical disease triggered by waves of viremia and distinguished by fever, anemia, thrombocytopenia, edema, and various wasting signs. By 8–12 months post-infection horses typically progress to life-long inapparent carriers, but continue to harbor various steady state levels of viral replication in monocyte-rich tissue reservoirs [15–17]. Stress or immune suppression of EIAV inapparent carriers can induce an increase in viral replication and potentially a recrudescence of disease [17,18]. Among virulent lentiviruses, however, EIAV is unique in that despite aggressive virus replication and associated rapid antigenic variation, greater than 90% of infected animals progress from a chronic disease state to an inapparent carrier stage. This progression to an inapparent stage of disease is achieved by a strict immunologic control over virus replication [17]. The EIAV system therefore serves as a uniquely dynamic model for the natural immunologic control of lentiviral replication and disease. Thus, this model provides a unique and useful lentiviral system for identifying critical immune correlates of protection and ascertaining the potential for developing effective prophylactic lentivirus vaccines.
Over the past 15 years we have evaluated a number of experimental EIAV vaccines based on inactivated whole virus, viral or recombinant envelope subunit vaccines, and various attenuated proviruses [1,2,19–22]. The results of these vaccine trials demonstrate a remarkable breadth of efficacy, ranging from protection from detectable infection and/or disease to severe enhancement of EIAV replication and disease. These observations suggest that vaccine immune responses are a double-edged sword that have beneficial or deleterious effects on the outcome of virus exposure [19,20,22–25].
We have previously reported on a series of studies to evaluate the efficacy of a series of attenuated EIAV proviral vaccines containing various mutations in viral accessory genes[1,2]. Based on these studies, we found that horses inoculated with the EIAVUKΔS2 proviral vaccine for a minimum of six months were uniformly protected from EIA disease and from detectable infection by virulent EIAV challenge. These observations were consistent with the concept that the attenuated EIAV vaccine may have achieved “sterilizing immunity” to prevent the establishment of persistent infection by the challenge virus strain in vaccinated horses.
The feasibility of achieving sterilizing immunity with lentiviral vaccines has been a controversial concept. The efficacy achieved by attenuated lentiviral vaccines has often been used to argue the case for the possibility of vaccine immunity preventing persistent lentiviral infection in humans and animals. In light of the apparent protective efficacy of the attenuated EIAVUKΔS2 vaccine in preventing detectable infection by virulent virus challenge, we sought to design an experiment to more rigorously test the protective efficacy of an experimental lentiviral vaccine. Thus, in the current report we summarize an EIAV vaccine studies in which horses were first immunized with an attenuated EIAV vaccine and shown to be apparently protected from infection by virulent virus challenge, as assayed by detection of plasma viral RNA by RT-PCR assays. These vaccinated horses were then treated with dexamethasone to suppress host immunity and to amplify replication by infecting EIAV strains, vaccine or challenge. The results of these studies reveal novel insights into the protective efficacy of the attenuated EIAV vaccines and demonstrate the utility of chemical immune suppression as a rigorous assay of experimental animal lentivirus vaccine efficacy post-challenge.
2. MATERIALS AND METHODS
2.1 Design and production of nucleotide deleted, attenuated viral vaccine candidates
To minimize the potential for reversion of the attenuated EIAV vaccine strain, we introduced nucleotide deletions into the original EIAVUKΔS2 proviral clone [26] that was produced by the introduction of two stop codons in the S2 gene of the pathogenic molecular clone, EIAVUK [27]. Specific nucleotide deletions were designed to conserve the 2 stop codons (G5 and G18, see Figure 1) and the SpeI diagnostic site within the G5 stop codon that was engineered into the EIAVUKΔS2 and to retain the tat stop codon, env open reading frame, and the second splice donor site located after the tat stop codon (Fig. 1). Specific deletions of 6 and 9 nucleotides, designated EIAVD6 and EIAVD9 respectively, were made via PCR-directed mutagenesis of the proviral genome. EIAVUKΔS2 was used as template for PCR reactions. Nucleotide deletions were generated by two-round PCR mutagenesis methods by utilizing overlapping primer pairs. Standard PCR conditions were employed for each construct. The resultant PCR products containing the mutant S2 gene were gel purified and digested with NcoI and BlpI and cloned back into EIAVUKΔS2 (also digested with NcoI and BlpI). All of the attenuated proviral clones were sequenced to verify the required genetic mutations, and sequencing reactions were performed with the Taq Dye Deoxy Terminator Cycle Sequencer Kit (Applied Biosystems, Foster City, CA) using internal EIAV primers [28]. DNA sequences were resolved with an ABI Prism 373 DNA sequencer (Applied Biosystems, Foster City, CA).
Figure 1. Details of the construction of the nucleotide-deleted live-attenuated vaccine candidate.

Schematic representation of EIAV genome, S2 gene, and mutant clones derived from EIAVUKΔS2. The genomic structure of EIAV provirus is shown at the top. The complete deduced amino acid sequence of the putative S2 protein is shown in single-letter amino acid code at the bottom. The residues in which the original stop codons were introduced into various positions in the EIAV S2 gene are shadowed and underlined. The region where the true nucleotide deletions were generated are indicated below the S2 amino acid sequence. The EIAVUKΔS2 sequence is displayed at the top and the deletion clones are aligned with it. Specific genomic regions considered during development of deletion mutants are indicated above the EIAVUKΔS2 sequence (Materials and Methods). The G5 stop codon in the SpeI diagnostic site (shaded) is boxed.
Viral stocks were prepared by harvesting the supernatant medium from equine dermal (ED) cells (ATCC CRL 6288) transfected with mutant proviral DNA using Lipofectamine (Gibco BRL), as described [26]. Viral stocks were assayed by a micro reverse transcriptase (RT) assay, and stock viral titers were determined in an infectious center assay in fetal equine kidney cells, as described previously [29].
Lipofectamine-mediated transfection (Gibco BRL) of ED cells was performed in 6-well plates with 1 X 106 cells and 5μg DNA of each specific deleted proviral clone, as previously described [26]. Transfected cell medium supernatants were sampled periodically for RT assay determinations of viral replication. Culture and infection of equine monocyte-derived macrophage was performed as previously described [30], utilizing equivalent RT levels of transfected supernatants. Infected cell medium supernatants were sampled periodically for RT assay determinations of viral replication.
2.2. Experimental subjects, clinical evaluation, and sample collection
Fourteen outbred horses of mixed age and gender and documented to be serognegative for EIAV infection were used in the vaccine trial described here. All horses were clinically monitored daily and maintained as described previously [22,31]. Daily rectal temperatures and clinical status were recorded. Samples of whole blood, plasma, and serum were collected from each horse at regular intervals and daily during apparent febrile episodes (>39ºC). Plasma samples were stored at −80ºC for use in quantitative or qualitative RT-PCR assays to determine the levels and identity (vaccine or challenge virus) of plasma viral RNA. Serum samples were stored at −20ºC for serological assays. CBC analysis of whole blood was performed using an IDEXX QBC Vet Autoreader. Hematocrit and platelet numbers were monitored weekly. Isolated PBMC were stored in liquid nitrogen for later evaluation of EIAV-specific cytolytic activity.
2.3. Experimental vaccination and virus challenge procedures
Based on the replication properties observed for the two nucleotide deletion mutants in transfected ED cells and infected equine macrophages, we selected the EIAVD9 virus as the test vaccine strain for the current studies. Thus, the immunogenicity and protective efficacy of the EIAVD9 vaccine construct was examined in three groups of four horses experimentally inoculated at different dosages and routes of the experimental vaccine strain. Each vaccinate was then challenged using a low dose multiple exposure (LDME) challenge to simulate natural exposure by horse fly bites [1]. The test horses were vaccinated two times at 30-day intervals by either intramuscular injection of either 103 TCID50 or 105 TCID50, or by intravenous injection of 103 TCID50 of the D9 vaccine stock [1,2]. Six months following the second vaccine dose, the twelve vaccinated horses and two naïve horses were challenged by the LDME procedure with the reference virulent EIAVPV stock. The LDME protocol consisted of three sequential i.v. inoculations, at two day intervals, of 10 median horse infectious doses (HID50) of the virulent challenge virus, EIAVPV; 1 HID50 is the equivalent of approximately 0.1 TCID50. The horses were monitored daily for clinical symptoms of EIA, and blood was drawn at regular intervals (weekly, daily if febrile) for assays of platelets, viral replication, and virus-specific immune responses [15,22]. The horses were observed for a total of 310 days, at which time they were euthanized.
2.4. Immune suppression procedures
Procedures for immune suppression of the EIAV-inoculated horses were based on protocols described previously by Kono et al. [18] and Tumas et al. [32]and detailed in Craigo et al. [33]. Briefly, dexamethasone (Phoenix Science, Kansas City, MO) was administered intramuscularly for 14 days at a dose of 0.11mg/kg body weight/day. The horses were monitored daily by CBC (complete blood count) and for physical signs of adverse reactions to drug treatment.
Skin tests for delayed-type hypersensitivity (DTH) [34,35] reactions were performed during the pre-immune suppression and immune suppression periods. Skin test sites were prepared by shaving and cleaning small areas on the neck. The horses were intradermally administered (at different sites on the necks) both 50μg of PHA (Sigma, St. Louis, MO.) in 1ml of saline, and 1ml of saline alone. The net increase in skin thickness was determined from measurements made with constant tension calipers 24 hours post-injection of antigen. DTH ratios were calculated as the ratio of antigen (PHA) reaction to control (saline) reaction.
2.5. Quantitative and qualitative serological analyses
Detection of serum antibody reactivity to the EIAV capsid protein p26 was conducted using the ViraCHEK®/EIA kit per the manufacturer’s instructions (Synbiotics Laboratory, Via Frontera, San Diego, CA). Serum samples were also evaluated for seroreactivity by the standard agar gel immunodiffusion (AGID) procedure [36] diagnostic assay for EIA. Serum IgG antibody reactivity to EIAV envelope glycoproteins was assayed quantitatively (end point titer) and qualitatively (avidity index, conformation ratio) using our standard concanavalin A (ConA) ELISA procedures as described previously [15]. Statistical significance of the differences between vaccine groups was determined through a nonparametric One-way ANOVA (Kruskal-Wallis Test) with Dunnett’s post test (GraphPad InStat version 3.0, San Diego, CA). Virus neutralizing activity to the challenge virus strain EIAVPV mediated by immune sera was assessed in an indirect cell-ELISA based infectious center assay using a constant amount of infectious EIAVPV and sequential 2-fold dilutions of serum [31].
2.6. Assays of PBMC cytolytic T-lymphocyte (CTL) activity
EIAV-specific CTL by PBMC from vaccinated and challenged animals was measured in a standard 51Cr release assay as described in Hammond et al. [31] with the following modifications. Briefly, target cells (PBMC) were expanded for seven days with 2.5ug/ml pokeweed mitogen (PWM) and pulsed with 104nM envelope- or gag-specific peptide pools for antigen delivery in labeling media (RPMI) containing 100μCi of 51Cr (Amersham, Arlington Heights, Ill.). Peptide pools consisted of 20-mer peptides overlapping by 10 residues representing the entire Envelope or Gag proteins. EIAV-stimulated PBMC effector cells were added at an effector-to-target-cell ratio of 20:1. Percent specific lysis was calculated as follows: % Specific lysis = [(E−C)/(N−C)] x 100, where E was the average counts per minute from experimental wells containing target and effector cells, C was the average counts per minute from control wells containing target cells only (spontaneous lysis), and N was the average counts per minute from control wells containing target cells in 0.5% Nonidet P-40 (maximal lysis).
2.7. Quantitative and Qualitative RT-PCR analysis of plasma virus RNA
Plasma samples from all animals were analyzed for the levels of viral RNA per milliliter of plasma using a previously described quantitative real-time multiplex RT-PCR assay based on gag-specific amplification primers [37]. The standard RNA curve was linear in the range of 101 molecules as a lower limit and 108 molecules as an upper limit. To differentiate the virion genomic RNA of the EIAV vaccine strain from the challenge virus (EIAVPV), virion-associated genomic RNA was extracted from plasma samples and then characterized by a nested RT-PCR and restriction digestion analysis that differentiated parental and mutated S2 gene sequences [1]. The nested RT-PCR is highly specific and provides detection levels down to 20 RNA copies of S2 [2]. Analyses were confirmed by sequencing of the same PCR products. Diagnostic analyses were performed during all 3 periods of observation: pre-challenge, post-challenge, and during immune suppression.
3. RESULTS
3.1. Construction of a nucleotide deleted EIAVUKΔS2 proviral vaccine strain
To minimize the potential for reversion of the two stop codons introduced into the S2 gene of our previously characterized EIAVUKΔS2 proviral vaccine strain, we sought to engineer S2 gene nucleotide deletions in addition to the existing stop codons in the EIAVUKΔS2 provirus. As detailed in Materials and Methods and Figure 1, the specific nucleotide deletions were designed to conserve known overlapping reading frames, splice acceptor sites, and the engineered stop codons and diagnostic restriction site contained in the EIAVUKΔS2 provirus. Initial studies revealed that proviral mutants containing relatively large S2 deletions of 14bp and 25bp were replication defective, indicating a critical role for S2 gene sequences in undefined EIAV proviral functions (data not shown). However, S2 gene deletions of 6bp (designated EIAVD6) and 9bp (designated EIAVD9) were found to be replication competent in transfected ED cells. Specifically, the EIAVD9 provirus displayed similar replication properties to the parental EIAVUKΔS2 and the wild-type EIAVUK constructs, whereas the EIAVD6 replication was approximately 100-fold lower than the other three virus constructs (data not shown). These replication data highlight the relatively unpredictable impact of various nucleotide deletions on viral replication properties and identify the EIAVD9 mutant as the optimal mutant strain for further evaluation in vaccine trials, as described below.
3.2. Experimental vaccination with attenuated EIAVD9, challenge with virulent EIAV, and immune suppression with dexamethasone
The current vaccine trial was designed to achieve two objectives. First, the studies were designed to test whether experimental immunization with the EIAVD9 vaccine at different doses and different routes of administration displayed similar immunogenicity and protection as previously described for the EIAVUKΔS2 vaccine [1,2]. Second, the trial was designed to include a second assay for protection from challenge virus infection by using a dexamethasone treatment to immune suppress and amplify infecting viral replication in vaccinated horses that were apparently protected from virulent EIAVPV challenge, as determined by quantitative and diagnostic PCR assays of viral plasma RNA.
3.2.1. Pre-challenge profiles of EIAVD9 vaccinated horses
Three groups of four horses were inoculated with the EIAVD9 vaccine strain as follows: 103 TCID50 intramuscularly (I.M.), 103 TCID50 intravenously (I.V.), or 105 TCID50 I.M, as detailed in Materials and Methods. As summarized in Figures 2, all EIAVD9 vaccinated animals remained asymptomatic for signs of EIA. There was a single 1-day febrile episode noted in one horse (#266, Figure 2D) from during the 210 day pre-challenge observation period. However, platelet reduction (thrombocytopenia) or other typical clinical signs of EIA disease did not accompany the increase in temperature, suggesting that EIAV was not responsible for the fever. The lack of clinical signs in the vaccinates was associated with a relatively low level of EIAVD9 replication during the first six months post-inoculation. Viral replication levels were similar in all vaccinates, averaging 103–104 RNA copies/ml plasma. In addition, no increase in EIAV plasma RNA levels were observed during the single febrile episode in horse #266 further suggesting that the fever was not caused by the EIAVD9 vaccine strain infection. Thus, these data demonstrate that the EIAVD9 vaccine strain achieved similar levels of replication in inoculated horses regardless of the route (I.V. or I.M.) or dose (103 or 105 TCID50) used for immunization. Moreover, the EIAVD9 replication levels in vivo were similar to those previously observed with the EIAVUKΔS2 vaccine strain, consistent with the similar in vitro replication kinetics observed for these two vaccine strains in the preceding studies.
Figure 2. Clinical and virological profiles of EIAVD9 vaccinated horses.

Twelve horses (Panels A-D) were inoculated with either 103 TCID50 EIAVD9 I.V., with 103 TCID50 EIAVD9 I.M. (Panels E-H), or with 105 TCID50 EIAVD9 I.M. (Panels I-L) as described in Materials and Methods (⬇Vax⬇). Rectal temperature (
 , right Y axis) and platelet counts (
, right Y axis) and platelet counts (
 , first left Y axis) were followed daily for up to 300 days (X-axis) after the first vaccine dose. Quantification of the virus load (
, first left Y axis) were followed daily for up to 300 days (X-axis) after the first vaccine dose. Quantification of the virus load (
 , second left Y axis) was performed on viral RNA extracted from plasma at periodic time points prior to and after virulent virus challenge using the LDME protocol (DOC,↓↓↓). The period of dexamethasone-induced immune suppression is demarcated by a pink shaded box. Two naïve control animals (Panels M-N) were also challenged with the LDME protocol (DOC,↓↓↓). Febrile episodes were defined by a rectal temperature above 39°C (
, second left Y axis) was performed on viral RNA extracted from plasma at periodic time points prior to and after virulent virus challenge using the LDME protocol (DOC,↓↓↓). The period of dexamethasone-induced immune suppression is demarcated by a pink shaded box. Two naïve control animals (Panels M-N) were also challenged with the LDME protocol (DOC,↓↓↓). Febrile episodes were defined by a rectal temperature above 39°C (
 ) in conjunction with thrombocytopenia (platelets ≤100,000/μl of whole blood) and other clinical symptoms of EIA. S2 diagnostic results for each animal are indicated in each respective panel above the date of analysis by either a V (vaccine strain) or a C (challenge strain).
) in conjunction with thrombocytopenia (platelets ≤100,000/μl of whole blood) and other clinical symptoms of EIA. S2 diagnostic results for each animal are indicated in each respective panel above the date of analysis by either a V (vaccine strain) or a C (challenge strain).
3.2.2. Post-challenge clinical and virologic profiles of EIAVD9 vaccinated horses
As summarized in Figure 2M and 2N, the two EIAV-naïve control horses upon LDME challenge with EIAVPV developed classic EIA disease signs within the first two months post-challenge (#D64 by 20dpc, #D58 by 51dpc), as observed in previous experimental infections [1,2]. The control horses concurrent fevers and platelet declines were accompanied by peaks in viral loads of 106–108 RNA copies/ml plasma. In contrast, the twelve EIAVD9 vaccinated animals remained asymptomatic for EIA following challenge with EIAVPV, as monitored by a lack of clinical signs, including increases in rectal temperature and decreases in platelet levels (Figure 2A-L). There were no significant increases in viral load in 11 of the 12 vaccinated horses after LDME challenge; viral loads remained around 104–105 RNA copies/ml plasma. Only horse #266 (Figure 2D,103 I.V. vaccine group) displayed a spike in viral load to approximately 107 RNA copies/ml plasma around 40 days post-challenge, at which time there was also a subclinical increase in temperature that coincided with a drop in platelets to 132,000/ml, (EIA thrombocytopenia ≤100,000/ml). To test for the presence of infecting challenge virus, viral RNA species (wild type and mutant S2 sequences) contained in pre-challenge and post-challenge plasma samples were assayed by our diagnostic RT-PCR/enzyme digestion and sequencing (see Material and Methods). The results of these RT-PCR diagnostic assays detected only the presence of the EIAVD9 vaccine strain RNA in all twelve vaccinated horses at multiple time points over the 9-month observation period; there was no evidence of the EIAVPV challenge virus RNA within the limits of detection of this PCR diagnostic assay (Figures 2A-L). In contrast, the diagnostic RT-PCR assay readily detected only EIAVPV RNA in the two infected control horses after the LDME inoculations (Figure 2M-N). Taken together, these data demonstrate that the EIAVD9 vaccine achieved protection from apparent disease and infection by virulent virus challenge, as observed previously with the EIAVUKΔS2 vaccine trials [1,2].
3.2.3. Post-immune suppression clinical and virologic profiles of EIAVD9 vaccinated horses
Having established that all twelve vaccinated horses were protected from apparent disease and infection by the challenge virus, we initiated at 63 days post-challenge a regimen of dexamethasone treatment to suppress host immunity and to amplify infecting EIAV strains, vaccine or challenge. Prior to immune suppression, delayed type hypersensitivity (DTH) tests were performed to establish baseline levels of immune reactivity (Figure 3). Immune suppression of the vaccinates was carried out by I.M. administration of dexamethasone at 0.11mg/kg for a period of 14 days. Blood samples were taken at regular intervals for measurements of platelets, plasma virus (twice weekly), and EIAV-specific antibody responses. The DTH assays indicated an effective suppression of host immunity by days 9–10 of the drug treatment (Figure 3), as observed previously [2,33]. Concomitant with the evident immune suppression, the vaccinated and challenged horses experienced increased viral loads that were 102–104 fold higher than the pre-immune suppression level, evidently reflecting the loss of immune control of EIAV infection and documenting the expected amplification in viral replication levels. Interestingly, 6 of the 12 horses exhibited clinical signs of EIA (fever and thrombocytopenia) as a result of the dexamethasone immune suppression. Horses #266, #C16, #C62, #C66, #C55, and #B81 (Figure 2D, F–H, & K–L) all experienced concurrent fever and thrombocytopenia as a result of the immune suppression. These clinical signs were associated with viremia levels that increased from approximately 104-105 RNA copies/ml plasma to 106–108 RNA copies/ml plasma (Figure 2D, F–H, & K–L).
Figure 3. Delayed-type hypersensitivity analysis of EIAVD9 vaccinated, immune suppressed animals.

Immune status of dexamethasone treated animals was monitored through a delayed-type hypersensitivity assay. DTH responses were determined by subcutaneous injection of 50μg of PHA in 1ml of saline and 1ml of saline alone into the necks of the horses. Twenty-four hours post-injection the diameter of the reaction was measured using constant tension calipers. The control reaction (saline alone) was divided into the PHA reaction to yield the DTH ratio (Y axis). PIS, Pre-Immune suppression; IS, Immune suppression; DPV, days post vaccination.
To complement the quantitative measurements of virus replication after immune suppression, we also performed our standard RT-PCR S2 diagnostic assays of plasma viral RNA to detect the presence of either the vaccine EAIVD9 or challenge EIAVPV strains present after immune suppression. As summarized for each animal in Figure 2, the RT-PCR diagnostic assays detected only EIAVPV challenge virus RNA in five of the twelve vaccinates, including horses from each of the three vaccine groups (Horses #B61, #C16, #C62, #C15, and #C55). Challenge and vaccine virus RNA was detected in one horse, #C22. In contrast, only the EIAVD9 vaccine strain was detected in the other six horses (Horses #C23, #266, #C9, #C66, #C13, and #B81) after immune suppression. Thus, these results based on experimental immune suppression demonstrate that the attenuated EIAV vaccine was able to prevent detectable infection by challenge virus in about 50% of the experimental horses. This is in marked contrast to the apparent 100% protection from detectable challenge virus infection observed by standard RT-PCR assays of plasma RNA, as described in the preceding section and previous vaccine trials (Table 1). Thus, these data demonstrate that immune suppression can provide a more rigorous analysis of vaccine protective efficacy and that the attenuated EIAV vaccines appear to limit, rather than prevent, infection by challenge virus in at least 50% of experimental horses. Moreover, these data demonstrate for the first time that the attenuated EIAVD9 vaccine may be associated with disease in immune-compromised horses (e.g., #266, #C66, #B81) persistently infected with the vaccine strain of virus. However, the current data cannot definitively exclude a possible involvement of undetectable challenge virus in the observed disease.
Table 1.
Summary of the Efficacy of EIAVD9 Vaccine/Immune Suppression Trial
| Protection (%)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Post-Challenge
|
Immune Suppression
|
|||||||
| EIAV strain/dose | Route | Plasma viral RNA levels (DOC) | Time to Challenge (months) | Time to Immune Suppression (months) | Disease | Infection | Disease | Infection |
| EIAVD9/103 | IV | 2.6 X 104 ± 1.4 X 104 | 7 | 9 | 4/4 (100%) | 4/4 (100%) | *1/4 (25%) | 2/4 (50%) |
| EIAVD9/103 | IM | 8.1 X 104 ± 3.7 X 104 | 7 | 9 | 4/4 (100%) | 4/4 (100%) | *3/4 (75%) | 2/4 (50%) |
| EIAVD9/105 | IM | 1.5 X 10 5 ± 3.3 X 104 | 7 | 9 | 4/4 (100%) | 4/4 (100%) | *2/4 (50%) | 2/4 (50%) |
|
| ||||||||
| Control | NA | NA | NA | NA | 0/2 (0%) | 0/2 (0%) | NA | |
D9 strain induced disease in one horse
3.3. Characterization of humoral and cellular immune responses to EIAVD9 vaccination and EIAVPV challenge
In light of the variation in protection from challenge-virus infection observed among the various vaccinates, we sought next to compare the host immune responses to evaluate potential correlates of vaccine protective efficacy.
3.3.1. Commercial Diagnostics
The immunogenicity of the vaccine strain was first characterized by reactivity testing in standard USDA-approved commercial diagnostic assays for EIAV infection based on detecting antibody to the viral capsid protein, p26. These diagnostic assays included the USDA reference agar gel immunodiffusion (AGID) test [36] and the ELISA-based ViraCHEK® assay (Materials and Methods) [1]. All experimentally vaccinated horses were seropositive in the ViraCHEK® diagnostic assay at the day of challenge except for horses #C23 and #C15, 210 days post vaccination (data not shown). The immune serum samples for horses #C22, #C23, #C9, #C16, and #C15 were negative in the less sensitive AGID tests (data not shown) at the day of challenge. These results illustrated that majority of the horses inoculated with EIAVD9 become seropositive in reference diagnostic assays that are the basis of USDA and other national regulatory policies to control EIAV infection, as previously demonstrated with the parental vaccine strain EIAVUKΔS2 [1,2].
3.3.2. Quantitative and Qualitative Serological Responses of the Vaccinates
We have previously characterized a complex and lengthy maturation of immune responses to viral envelope proteins during the first six to eight months post-infection that appears to be a distinctive feature of lentiviral infections as steady state infection and host immunity levels are established [31,38–40]. In the case of EIAV, we have developed a panel of quantitative and qualitative serological assays that define a characteristic maturation of EIAV envelope-specific serum antibodies in experimentally infected horses and that correlates with the development of protective vaccine immunity [1,2,15,25]. Therefore, we next utilized this standard panel of quantitative (titer) and qualitative (avidity and conformation) assays to examine and compare the maturation of EIAV envelope-specific antibody responses between vaccinates that were protected vs. not protected from infection as detected upon chemical immune suppression (Figure 4). These data revealed a similar evolution of envelope-specific antibody responses in all animals, regardless of status of protection, with the exception of the conformational dependence.
Figure 4. Development of envelope-specific antibody responses to the EIAVD9 vaccine.
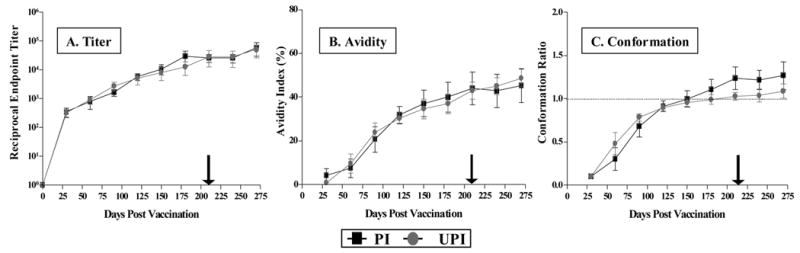
Longitudinal characterization of the quantitative and qualitative properties of induced EIAV envelope-specific antibodies were conducted in ConA ELISA assays of (A) endpoint titer, (B) avidity, and (C) conformational dependence as described in Materials and Methods. (A) Mean serum antibody titers for each time point are presented as the log10 of the highest reciprocal dilution yielding reactivity two standard deviations above background. (B) Mean avidity index measurements are presented as percentages of the antibody-antigen complexes resistant to disruption with 8M urea. (C) Mean conformation dependence values are calculated as the ratio of serum antibody reactivity with native envelope compared to denatured envelope antigen. Conformation ratios greater than 1.0 indicate predominant antibody specificity for conformational determinants, while ratios less than 1.0 indicate predominant antibody specificity for linear envelope determinants. PI, protected from infection (■); UPI, unprotected from infection (
 ).
).
There were no remarkable differences in the endpoint titer of EIAV envelope-specific IgG between the vaccinates (Figure 4A). All animals revealed a rise in titer over the pre-challenge period that reached a steady state of approximately 104.5. There was no evidence of an anamnestic response after the LDME challenge of vaccinated horses, consistent with no or limited infection by the challenge virus. The qualitative serological assays of antibody avidity demonstrated similar envelope-specific antibody responses among the different vaccinates. In general, avidity values progressively increased to steady state values of about 40–50% among the vaccinates (Figure 4B), indicative of a mature, protective antibody response. Similarly, serological assays for antibody conformation ratios demonstrated a progressive increase in values suggesting a switching of predominant antibody specificity from linear to conformational epitopes by the DOC for all horses (Figure 4C). However, the animals which were infected with the challenge strain were characterized by a less pronounced evolution of antibody conformational dependence. The animals protected from infection averaged steady state ratios of 1.3 (Figure 4C), while the unprotected animals ratios averaged modestly lower at 1.0. While these ratios were not statistically significant in their respective differences, it did indicate an association of protection with the recognition of more conformational epitopes, similar to observations from our previous trials [2]. These quantitative and qualitative serological assays indicate essentially similar profiles for envelope-specific antibody responses elicited by the EIAVD9 vaccine in protected and unprotected horses.
3.3.3. Neutralizing Antibody Responses of the Vaccinates
We previously reported a relatively slow development of serum neutralizing antibodies over a several month period following experimental EIAV infection with either virulent or avirulent strains, with average maximum titers averaging 1:300 [15]. To examine the ability of the EIAVD9 attenuated EIAV strains to elicit serum neutralizing antibody and the role of neutralizing antibodies in protection, we tested immune serum samples taken at six months post vaccination for their ability to inactivate the virulent challenge EIAVPV infectivity [31], as summarized in Figure 5A. These data demonstrated that the EIAVD9 attenuated candidate generated serum antibody neutralizing titers that ranged from undetectable (#C15) to 1:167 (#B61), averaging 1:37 (±12) at six months post inoculation. Day of challenge reciprocal neutralizing antibody titers were above background in 83% of horses protected from infection as detected upon immune suppression with dexamethasone. Similarly, 67% of horse not protected from infection displayed DOC titers above background. Thus, these data do not indicate a statistically significant correlation between the levels of neutralizing antibodies and protection from disease or infection.
Figure 5. Characterization of virus-specific serum neutralization and cytolytic T-cell activity in horses vaccinated with EIAVD9.

(A) The mean reciprocal dilutions of serum from vaccinated horses which neutralized 50% of input EIAVPV as measured in an infectious center assay are presented for serum samples collected at the day of challenge and four weeks post challenge, as described in Materials and Methods. The line (–) denotes the cut off (≥15) value for valid 50% neutralization titers. (B) EIAV Env- and Gag-specific CTL activity elicited by experimental immunization and challenge was measured using fresh PBMC from experimental horses at the day of challenge. The EIAV Env- and Gag-specific CTL activity is presented as percent specific lysis of target cells. The line (–) denotes the cut off (≥10) value for valid specific lysis. *, designates animals protected from disease upon chemical immune suppression.
3.3.4. Cytolytic T Cell Responses of the Vaccinates
Finally, to examine the ability of the EIAVD9 attenuated EIAV strain to elicit viral-specific CTL activity and the role of CTL in protection, we assessed the virus-specific CTL responses elicited after the six month pre-challenge period of observation. EIAVD9-generated Env- and Gag-specific reactivity of isolated PBMC were measured in our peptide CTL assay system (Materials and Methods) as summarized in Figure 5B. In general, vaccinated horses with and without detectable challenge virus infection displayed equivalent Env and Gag reactivity. The overall level of viral-specific CTL activity ranged from undetectable to approximately 36% specific lysis (#C62). Three animals did not display reactivities above background (#C23, #266, and #C9). Overall there did not appear to be an obvious correlation between the apparent levels of CTL activity and protection from disease or infection.
4. DISCUSSION
We previously reported that a live-attenuated virus vaccine, designated EIAVUKΔS2, provided protection from detectable disease and infection by virulent virus challenge, suggesting, but not proving, the achievement of apparently “sterilizing” vaccine immunity [1,2]. The current investigation was designed to employ chemical immune suppression treatments to rigorously further examine these previous conclusions. Hence the goal of these studies was to explore whether attenuated EIAV vaccine-produced immunity actually prevented the establishment of persistent infection by challenge virus or limited such infection to levels below the sensitivity of the RT-PCR assays used to detect challenge virus RNA in plasma.
Towards this goal, we first developed a second generation attenuated proviral vaccine, designated EIAVD9, in which nucleotide deletions were introduced into the viral S2 gene in combination with the previously introduced stop codons in the parental EIAVUKΔS2 vaccine strain. Screening of a number of different deletion mutants revealed a marked and unpredictable replication phenotype for the various S2 deletion mutants, suggesting that these gene sequences perform critical structural or regulatory functions for EIAV replication in addition to encoding the accessory S2 protein. Experimental immunization of twelve horses with the EIAVD9 vaccine was shown to elicit 100% protection from disease by experimental virulent virus challenge, as evidenced by a lack of detectable clinical signs indicative of EIA. In addition, diagnostic RT-PCR assays to distinguish vaccine and challenge virus S2 genes present in plasma of challenged vaccinates detected only vaccine virus in all twelve horses, indicating a lack of detectable infection by these PCR assays. Thus, the current observations clearly demonstrate that immunization with the EIAVD9 vaccine strain elicited vaccine immunity that was able to provide protection from detectable disease and infection in challenged vaccinates, as previously reported for the EIAVUKΔS2 vaccine strain [1,2].
To more rigorously assess the protective efficacy of the EIAVD9 vaccine, the challenged vaccinates were treated at two months post-challenge with dexamethasone to suppress host immunity, thereby allowing amplification of any infection, either by vaccine or challenge virus. While RT-PCR assays of infecting virus in plasma prior to immune suppression indicated only the vaccine EIAVD9 virus in all twelve challenged vaccinated horses, the same assays detected only challenge virus in five horses, only vaccine virus in six horses, and a combination of both virus strains in one horse. Thus, these data indicated an apparent 50% protection from challenge virus infection, in contrast to the 100% protection indicated by RT-PCR assays in the absence of immune suppression (Table 1). Moreover, these studies demonstrate the utility of the chemical immune suppression as a rigorous assay that should be considered for use in other animal persistent lentivirus vaccine trials to detect viral reservoirs and assess the protective efficacy of experimental immunization strategies. Claims of “sterilizing” protection need to be rigorously evaluated even when cellular viral reservoirs have been assessed. Previous to this study we demonstrated in assays which detected down to 20 copies of viral RNA that an apparent “sterile” protection of vaccinated animals from EIAV challenge both in the periphery and in lymphoid tissues [1,2]. In a very recent study, dexamethasone immune suppression validation of vaccine safety and efficacy was employed to validate influenza vaccine safety in the ferret animal model [41].
It is interesting to note here that the immune suppression assays revealed the presence of only the vaccine or the challenge EIAV strain in eleven of twelve experimental horses; the presence of both strains was detected in only one horse. These observations seem to suggest a tendency for there to be some exclusion mechanism between the established persistent vaccine virus infection and an introduced challenge virus infection. These observations with EIAV are reminiscent of similar observations reported by Narayan and colleagues [42] that identified the presence of only the attenuated SHIV vaccine strain or the challenge SHIV strain in experimentally immunized and challenged monkeys after CD8 T-cell depletions. The mechanisms responsible for this apparent exclusion between established lentivirus vaccine strains and challenge virus strains remain to be defined.
Assuming that the current observations with a limited number of horses can be extrapolated to a large population, the observation of 50% protection from detectable infection by challenge virus warrants further consideration. Is the glass half full or half empty? Our perspective is that the protective efficacy observed with the EIAVD9 vaccine provides the most compelling data to date from any animal lentivirus system that a vaccine can not only elicit immunity to prevent clinical disease by controlling challenge virus infection, but that vaccine immunity can actually prevent the establishment of detectable persistent infection by challenge virus. Therefore, while attenuated lentiviral vaccines may not be practical for commercial use for obvious safety and regulatory concerns, they continue to provide a unique model in which to examine the correlates of the most protective vaccine immunity achieved to date.
Towards this objective of identifying differences in vaccine immunity in horses with and without detectable infection by challenge virus, we characterized quantitative and qualitative EIAV envelope-specific antibody responses that have previously been used to define the progression from immature non-protective vaccine immunity to mature protective vaccine immunity by attenuated lentiviral vaccines, including EIAV [1,2,39,43]. The results of these comparative serological assays including serum antibody titer, avidity, and conformation revealed a similar characteristic evolution of EIAV envelope-specific antibody responses in protected and unprotected horses during the first six months post inoculation with the EIAVD9 and during the following several months post-infection. As observed previously, EIAV envelope-specific antibodies progressed from being relatively low titer, low avidity, and directed primarily to linear epitopes during the first few months post inoculation to steady state values after six months that were characterized by high titer, high avidity antibodies directed more to conformational epitopes. Importantly, there was no significant changes in quantitative and qualitative antibody properties after challenge of the vaccinates with virulent EIAV, indicating a lack of anamnestic responses and consistent with either the prevention or strict limitation of challenge virus infection by host vaccine immune responses. There was however an association observed between the development of higher conformational dependence ratios and protection from infection, indicative of a particular reliance of protective immunity on the recognition of conformational epitopes. This correlate of protection has been observed in previous trials [2] which we plan to further explore and incorporate into our studies of humoral protective efficacy. Neutralizing antibody and CTL measurements failed in this trial to indicate an incontrovertible correlate of protection from both disease and infection. On the contrary, previous studies from our laboratory have demonstrated neutralizing antibodies were associated with increased levels of protection from virulent challenge strain infection [2]. These observations highlight the need for innovative immunological assays to identify new potential immune correlates of protective vaccine immunity in animal lentivirus systems.
The prevalence of lentivirus infections in domestic animals such as horses, cats, goats, and sheep presents a need and opportunity for the development of a commercial vaccine to control these infections that constitute a worldwide problem in veterinary medicine. Attenuated lentiviral vaccines appear to offer an efficacious method for the development of commercial lentivirus vaccines that could be widely applied in veterinary medicine. In this regard, a highly cell-adapted attenuated EIAV vaccine was used in millions of horses in China during the 1980s, reportedly greatly reducing the prevalence of EIA in that country [44]. However, the potential of attenuated EIAV vaccines in veterinary medicine in most countries faces two problems. The first is that live-attenuated EIAV vaccines cause immunized horses to become seropositive in USDA-approved diagnostic assays that are the basis of regulatory policies to control EIAV infections in the USA and a number of other countries, where identified seropositive horses must be strictly controlled or destroyed. Second, there is the concern about the possible virulence of the attenuated viral vaccine, either by genetic reversion or by an immune suppression of the vaccinated horse due to stress or other infections. In the current studies, we observed clinical EIA in six of the twelve vaccinated horses upon immune suppression with dexamethasone, apparently correlating with the increased levels of EIAV replication in the presence of compromised host immunity. Unexpectedly, however, three of the six horses displaying disease were found to contain only the EIAVD9 vaccine strain in their plasma during the disease episode. Thus, these observations may suggest that the EIAVD9 vaccine strain can cause disease in immune compromised horses, in marked contrast to its highly attenuated avirulent phenotype in mature, immuno-competent horses. However, additional experiments are required to exclude the possible role of challenge virus in the observed disease.
Based on these studies, it is evident that the goal of veterinary lentivirus vaccine development must be to achieve the mature protective immunity elicited with attenuated virus vaccines with alternative vaccine strategies that are consistent with established regulatory policies for the particular lentivirus disease. The primary role for attenuated lentiviral vaccines is to serve as a readily accessible model of protective vaccine immunity that can be used to dissect the critical correlates of protection that can then be used to guide the development of alternative immunization strategies using DNA or viral vector-based vaccines. Ultimately, novel measures of accurate vaccine protective efficacy including the utilization of chemical immune suppression should be considered for inclusion in further lentiviral vaccine trials.
Acknowledgments
This work was supported by NIH/NIAID Grant RO1 AI25850 and by funds from the Lucille P. Markey Charitable Trust, the University of Kentucky Agricultural Experiment Station, and Intervet Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li F, Craigo JK, Howe L, Steckbeck JD, Cook S, Issel C, et al. A Live Attenuated Equine Infectious Anemia Virus Proviral Vaccine with a Modified S2 Gene Provides Protection from Detectable Infection by Intravenous Virulent Virus Challenge of Experimentally Inoculated Horses. The Journal of Virology. 2003;77(13):7244–7253. doi: 10.1128/JVI.77.13.7244-7253.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craigo JK, Li F, Steckbeck JD, Durkin S, Howe L, Cook SJ, et al. Discerning an Effective Balance between Equine Infectious Anemia Virus Attenuation and Vaccine Efficacy. The Journal of Virology. 2005;79(5):2666–2677. doi: 10.1128/JVI.79.5.2666-2677.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mims CA. The Pathogenesis of Infectious Diseases. 3. SanDiego: Academic; 1987. [Google Scholar]
- 4.Melink JA. Vaccines. 2. SanDiego: Academic; 1994. [Google Scholar]
- 5.Baba TW, Liska V, Khimani AH, Ray NB, Dailey PJ, Penninck D, et al. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- 6.Das AT, Baldwin CE, Vink M, Berkhout B. Improving the Safety of a Conditional-Live Human Immunodeficiency Virus Type 1 Vaccine by Controlling both Gene Expression and Cell Entry. The Journal of Virology. 2005;79(6):3855–3858. doi: 10.1128/JVI.79.6.3855-3858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkhout B, Verhoef K, van Wamel JLáB, Back NKáT. Genetic Instability of Live-Attenuated Human Immunodeficiency Virus Type 1 Vaccine Strains. The Journal of Virology. 1999;73(2):1138–1145. doi: 10.1128/jvi.73.2.1138-1145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desrosiers RC. Prospects for live attenuated HIV. Nat Med. 1998;4:982. doi: 10.1038/1949. [DOI] [PubMed] [Google Scholar]
- 9.Almond N, Stott J. Live attenuated SIV--a model of a vaccine for AIDS. Immunology Letters. 1999;66(1–3):167–170. doi: 10.1016/s0165-2478(98)00153-9. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RP. Live attenuated AIDS vaccines: Hazards and hopes. Nat Med. 1999;5:154–155. doi: 10.1038/5515. [DOI] [PubMed] [Google Scholar]
- 11.Blower SM, Koelle K, Kirschner DE, Mills J. Live attenuated HIV vaccines: Predicting the tradeoff between efficacy and safety. PNAS. 2001;98(6):3618. doi: 10.1073/pnas.061029998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulder PJR, Walker BD. HIV-1 Superinfection - A Word of Caution. N Engl J Med. 2002;347:756–758. doi: 10.1056/NEJMe020091. [DOI] [PubMed] [Google Scholar]
- 13.Learmont JC, Geczy AF, Mills J, Ashton LJ, Raynes-Greenow CH, Garsia RJ, et al. Immunologic and Virologic Status after 14 to 18 Years of Infection with an Attenuated Strain of HIV-1 -- A Report from the Sydney Blood Bank Cohort. N Engl J Med. 1999;340(22):1715. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Jin S, Jin J, Li F, Montelaro RC. A tumor necrosis factor receptor family protein serves as a cellular receptor for the macrophage-tropic equine lentivirus. Proc Natl Acad Sci U S A. 2005;102(28):9918–9923. doi: 10.1073/pnas.0501560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond SA, Li F, McKeon BM, Sr, Cook SJ, Issel CJ, Montelaro RC. Immune responses and viral replication in long-term inapparent carrier ponies inoculated with equine infectious anemia virus. J Virol. 2000;74(13):5968–5981. doi: 10.1128/jvi.74.13.5968-5981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrold SM, Cook SJ, Cook RF, Rushlow KE, Issel CJ, Montelaro RC. Tissue sites of persistent infection and active replication of equine infectious anemia virus during acute disease and asymptomatic infection in experimentally infected equids. J Virol. 2000;74(7):3112–3121. doi: 10.1128/jvi.74.7.3112-3121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montelaro RC, Ball JM, Rushlow K. Equine retroviruses. In: Levy JA, editor. The Retroviridae. Vol. 1993. New York, N.Y.: Plenum Press; pp. 257–360. [Google Scholar]
- 18.Kono Y, hirasawa K, Fukunaga Y, Taniguchi T. Recrudesence of equine infectious anemia by treatment with immunosuppressive drugs. Nat Inst Anim Hlth Quart. 1976;16:8–15. [PubMed] [Google Scholar]
- 19.Hammond SA, Cook SJ, Falo LD, Jr, Issel CJ, Montelaro RC. A particulate viral protein vaccine reduces viral load and delays progression to disease in immunized ponies challenged with equine infectious anemia virus. Virology. 1999;254:37–49. doi: 10.1006/viro.1998.9550. [DOI] [PubMed] [Google Scholar]
- 20.Raabe ML, Issel CJ, Cook SJ, Cook RF, Woodson B, Montelaro RC. Immunization with a recombinant envelope protein (rgp90) of EIAV produces a spectrum of vaccine efficacy ranging from lack of clinical disease to severe enhancement. Virology. 1998;245:151–162. doi: 10.1006/viro.1998.9142. [DOI] [PubMed] [Google Scholar]
- 21.Wang SZ, Rushlow KE, Issel CJ, Cook RF, Cook SJ, Raabe ML, et al. Enhancement of EIAV replication and disease by immunization with a baculovirus-expressed recombinant envelope surface glycoprotein. Virology. 1994;199:247–251. doi: 10.1006/viro.1994.1120. [DOI] [PubMed] [Google Scholar]
- 22.Issel CJ, Horohov DW, Lea DF, Adams WV, Jr, Hagius SD, McManus JM, et al. Efficacy of inactivated whole-virus and subunit vaccines in preventing infection and disease caused by equine infectious anemia virus. J Virol. 1992;66:3398–3408. doi: 10.1128/jvi.66.6.3398-3408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montelaro RC, Grund CH, Raabe MR, Woodson B, Cook RF, Cook SJ, et al. Characterization of protective and enhancing immune responses to equine infectious anemia virus resulting from experimental vaccines. AIDS Res Hum Retroviruses. 1996;12:413–415. doi: 10.1089/aid.1996.12.413. [DOI] [PubMed] [Google Scholar]
- 24.Raabe ML, Issel CJ, Montelaro RC. In vitro antibody-dependent enhancement assays are insensitive indicators of in vivo vaccine enhancement of equine infectious anemia virus. Virology. 1999;259:416–427. doi: 10.1006/viro.1999.9772. [DOI] [PubMed] [Google Scholar]
- 25.Hammond SA, Raabe ML, Issel CJ, Montelaro RC. Evaluation of antibody parameters as potential correlates of protection or enhancement by experimental vaccines to equine infectious anemia virus. Virology. 1999;262:416–430. doi: 10.1006/viro.1999.9939. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Puffer BA, Montelaro RC. The S2 gene of equine infectious anemia virus is dispensable for viral replication in vitro. J Virol. 1998;72:8344–8348. doi: 10.1128/jvi.72.10.8344-8348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook RF, Leroux C, Cook SJ, Berger SL, Lichtenstein DL, Ghabrial NN, et al. Development and characterization of an in vivo pathogenic molecular clone of equine infectious anemia virus. J Virol. 1998;72:1383–1393. doi: 10.1128/jvi.72.2.1383-1393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leroux C, Issel C, Montelaro RC. Novel and dynamic evolution of equine infectious anemia virus genomic quasispecies associated with sequential disease cycles in an experimentally infected pony. J Virol. 1997;71(12):9627–9639. doi: 10.1128/jvi.71.12.9627-9639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grund CH, Lechman ER, Issel CJ, Montelaro RC, Rushlow KE. Lentivirus cross-reactive determinants present in the capsid protein of equine infectious anaemia virus. J Gen Virol. 1994;75 ( Pt 3):657–662. doi: 10.1099/0022-1317-75-3-657. [DOI] [PubMed] [Google Scholar]
- 30.Raabe MR, Issel CJ, Montelaro RC. Equine monocyte-derived macrophage cultures and their applications for infectivity and neutralization studies of equine infectious anemia virus. J Virol Methods. 1998;71:87–104. doi: 10.1016/s0166-0934(97)00204-8. [DOI] [PubMed] [Google Scholar]
- 31.Hammond SA, Cook SJ, Lichtenstein DL, Issel CJ, Montelaro RC. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J Virol. 1997;71(5):3840–3852. doi: 10.1128/jvi.71.5.3840-3852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumas DB, Hines MT, Perryman LE, Davis WC, McGuire TC. Corticosteroid immunosuppression and monoclonal antibody-mediated CD5+ T lymphocyte depletion in normal and equine infectious anemia virus-carrier horses. J Gen Virol. 1994;75:959–968. doi: 10.1099/0022-1317-75-5-959. [DOI] [PubMed] [Google Scholar]
- 33.Craigo JK, Leroux C, Howe L, Steckbeck JD, Cook SJ, Issel CJ, et al. Transient immune suppression of inapparent carriers infected with a principal neutralizing domain-deficient equine infectious anaemia virus induces neutralizing antibodies and lowers steady-state virus replication. J Gen Virol. 2002;83(6):1353–1359. doi: 10.1099/0022-1317-83-6-1353. [DOI] [PubMed] [Google Scholar]
- 34.Baus E, Andris F, Dubois PM, Urbain J, Leo O. Dexamethasone inhibits the early steps of antigen receptor signaling in activated T lymphocytes. Journal of Immunology. 1996;156(12):4555–4561. [PubMed] [Google Scholar]
- 35.Hodgin EC, McGuire TC, Perryman LE, Grant BD. Evaluation of delayed hypersensitivity responses in normal horses and immunodeficient foals. Am J Vet Res. 1978;39(7):1161–1167. [PubMed] [Google Scholar]
- 36.Coggins L, Norcross NL. Immunodiffusion reaction in equine infectious anemia. Cornell Vet. 1970;60:330–335. [PubMed] [Google Scholar]
- 37.Cook RF, Cook SJ, Li FL, Montelaro RC, Issel CJ. Development of a multiplex real-time reverse transcriptase-polymerase chain reaction for equine infectious anemia virus (EIAV) J Virol Methods. 2002;105(1):171–179. doi: 10.1016/s0166-0934(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 38.Montelaro RC, Cole KS, Hammond SA. Maturation of immune responses to lentivirus infection: implications for AIDS vaccine development. AIDS Res Hum Retroviruses. 1998;14 (Suppl 3):S255–S259. [PubMed] [Google Scholar]
- 39.Cole KS, Rowles JL, Jagerski BA, Murphey-Corb M, Unangst T, Clements JE, et al. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. The Journal of Virology. 1997;71(7):5069–5079. doi: 10.1128/jvi.71.7.5069-5079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole KS, Murphey-Corb M, Narayan O, Joag SV, Shaw GM, Montelaro RC. Common Themes of Antibody Maturation to Simian Immunodeficiency Virus, Simian-Human Immunodeficiency Virus, and Human Immunodeficiency Virus Type 1áInfections. The Journal of Virology. 1998;72(10):7852–7859. doi: 10.1128/jvi.72.10.7852-7859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber VC, McCullers JA. Live attenuated influenza vaccine is safe and immunogenic in immunocompromised ferrets. J Infect Dis. 2006;193(5):677–684. doi: 10.1086/500247. [DOI] [PubMed] [Google Scholar]
- 42.Mackay GA, Liu Z, Singh DK, Smith MS, Mukherjee S, Sheffer D, et al. Protection Against Late-Onset AIDS in Macaques Prophylactically Immunized with a Live Simian HIV Vaccine Was Dependent on Persistence of the Vaccine Virus. J Immunol. 2004;173(6):4100–4107. doi: 10.4049/jimmunol.173.6.4100. [DOI] [PubMed] [Google Scholar]
- 43.Cole KS, Rowles JL, Murphey-Corb M, Clements JE, Robinson J, Montelaro RC. A model for the maturation of protective antibody responses to SIV envelope proteins in experimentally immunized monkeys. J Med Primatol. 1997;26:51–58. doi: 10.1111/j.1600-0684.1997.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 44.Shen R-XaWZ. Development and use of an equine infectious anemia donkey leucocyte attenuated vaccine. EIAV: A National Review of Policies, Programs, and Future Objectives. 1985 [Google Scholar]


